Effect of Hot-Dip Galvanizing Process on Selective Oxidation and Galvanizability of Medium Manganese Steel for Automotive Application
Abstract
1. Introduction
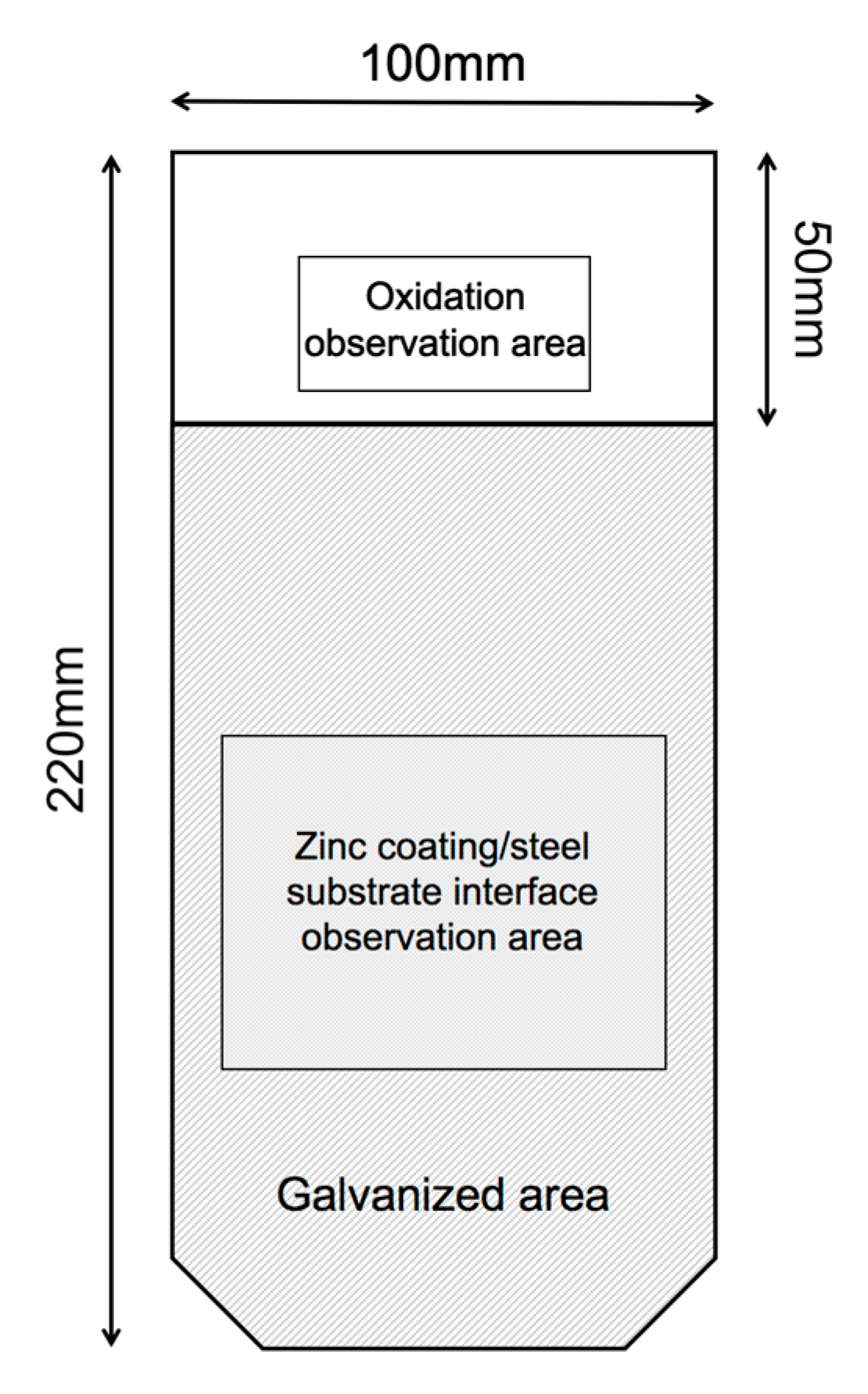
2. Materials and Experiments
3. Experimental Results
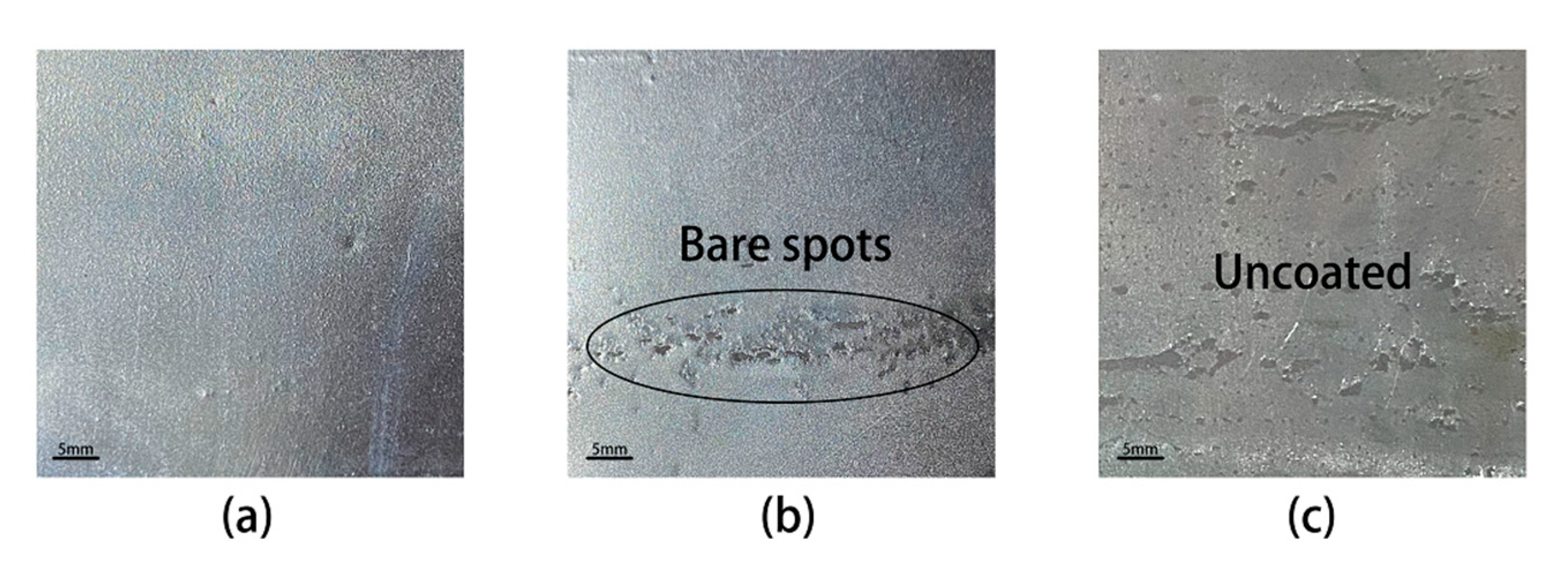
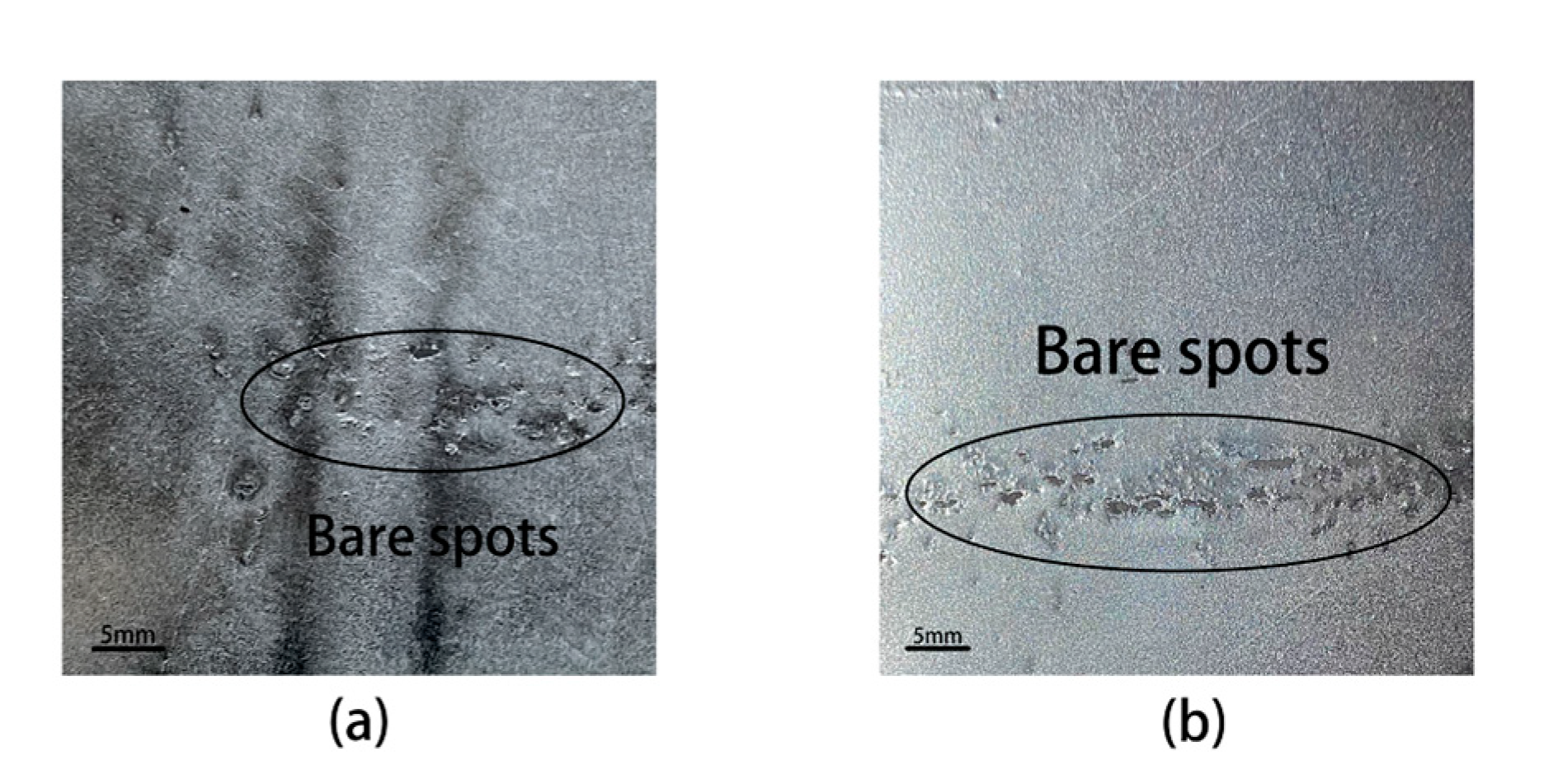
3.1. Surface Structure of Samples Annealed at Different Annealing Temperatures
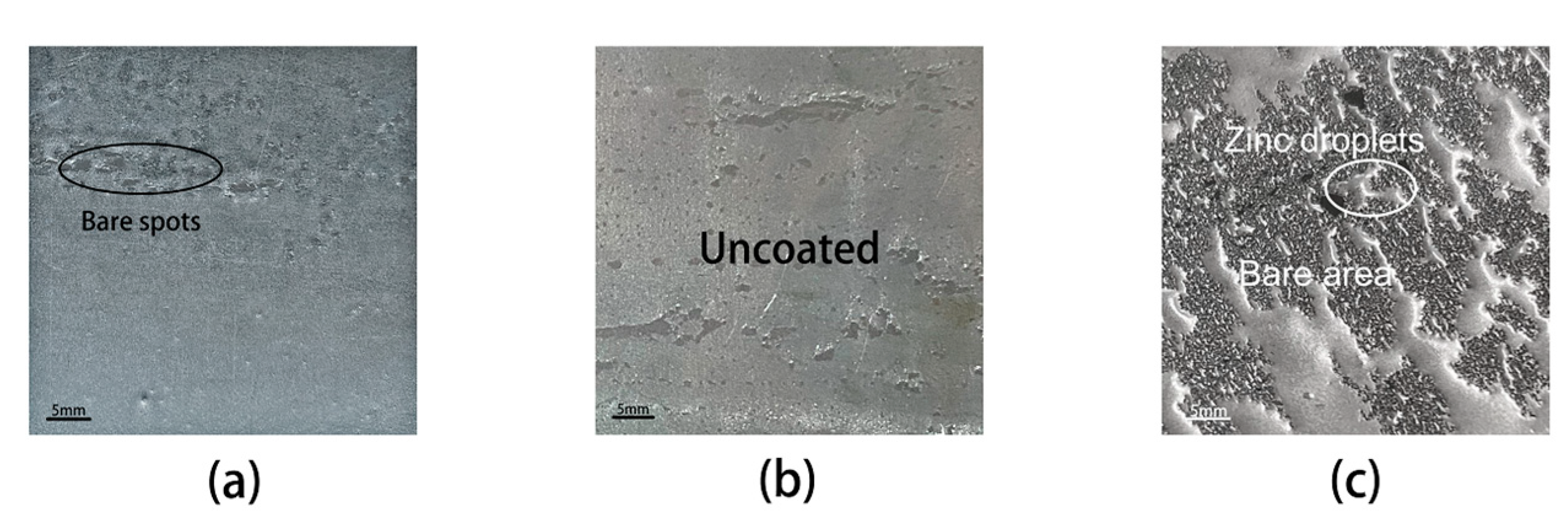
3.2. Surface Structure of Samples Annealed at Different Dew Points
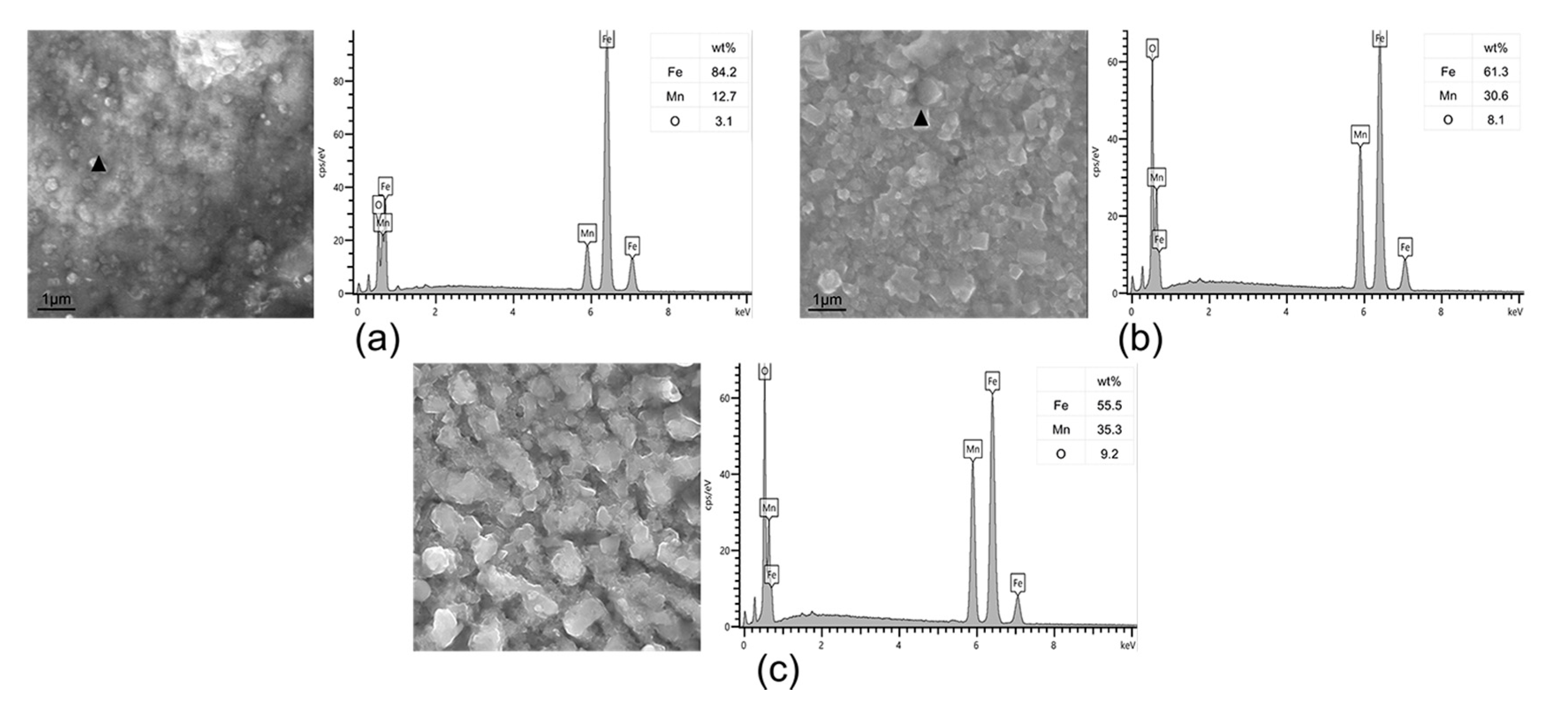
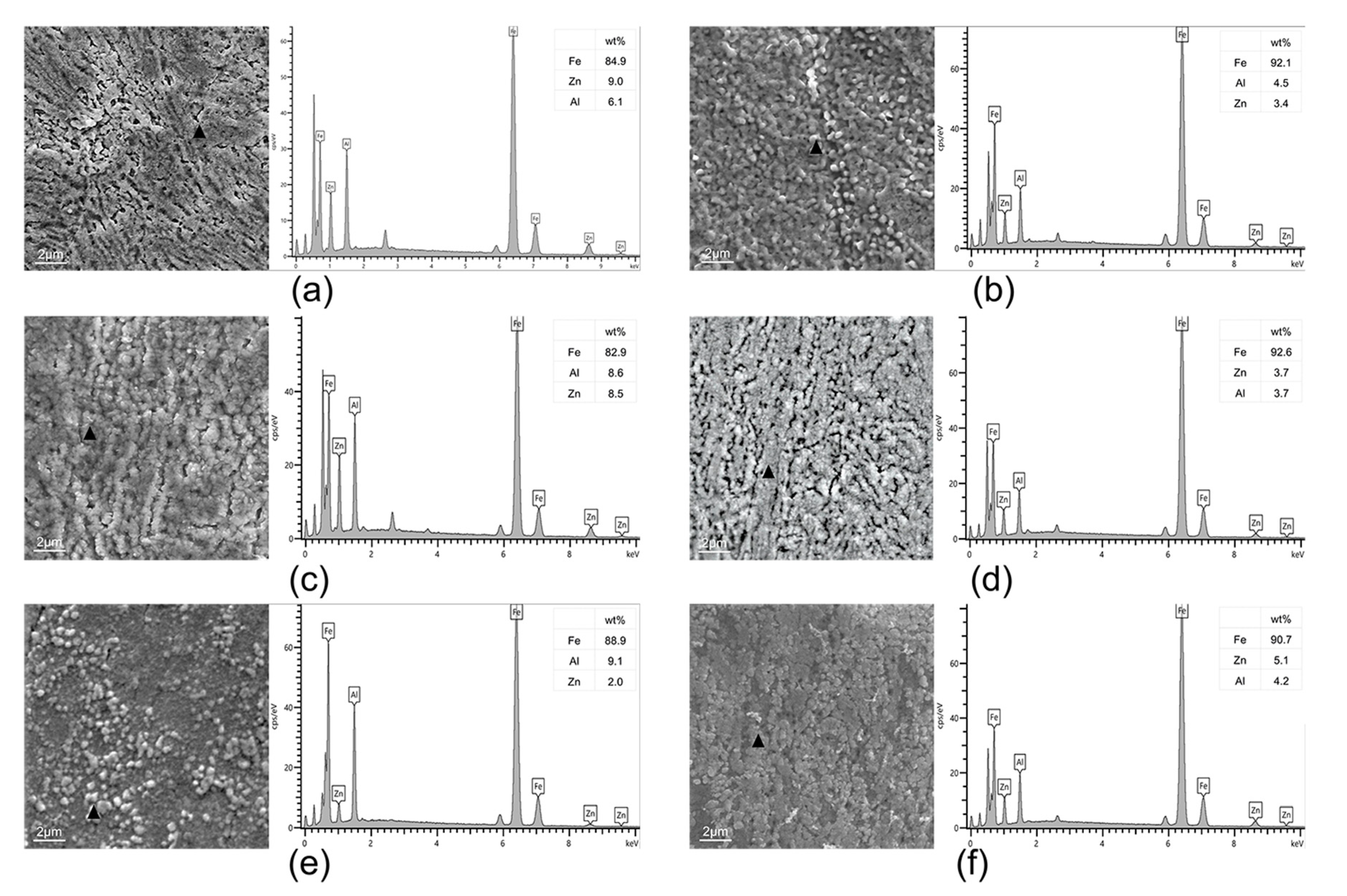
3.3. Surface Microstructure in Different H2 Content Atmospheres
4. Discussion
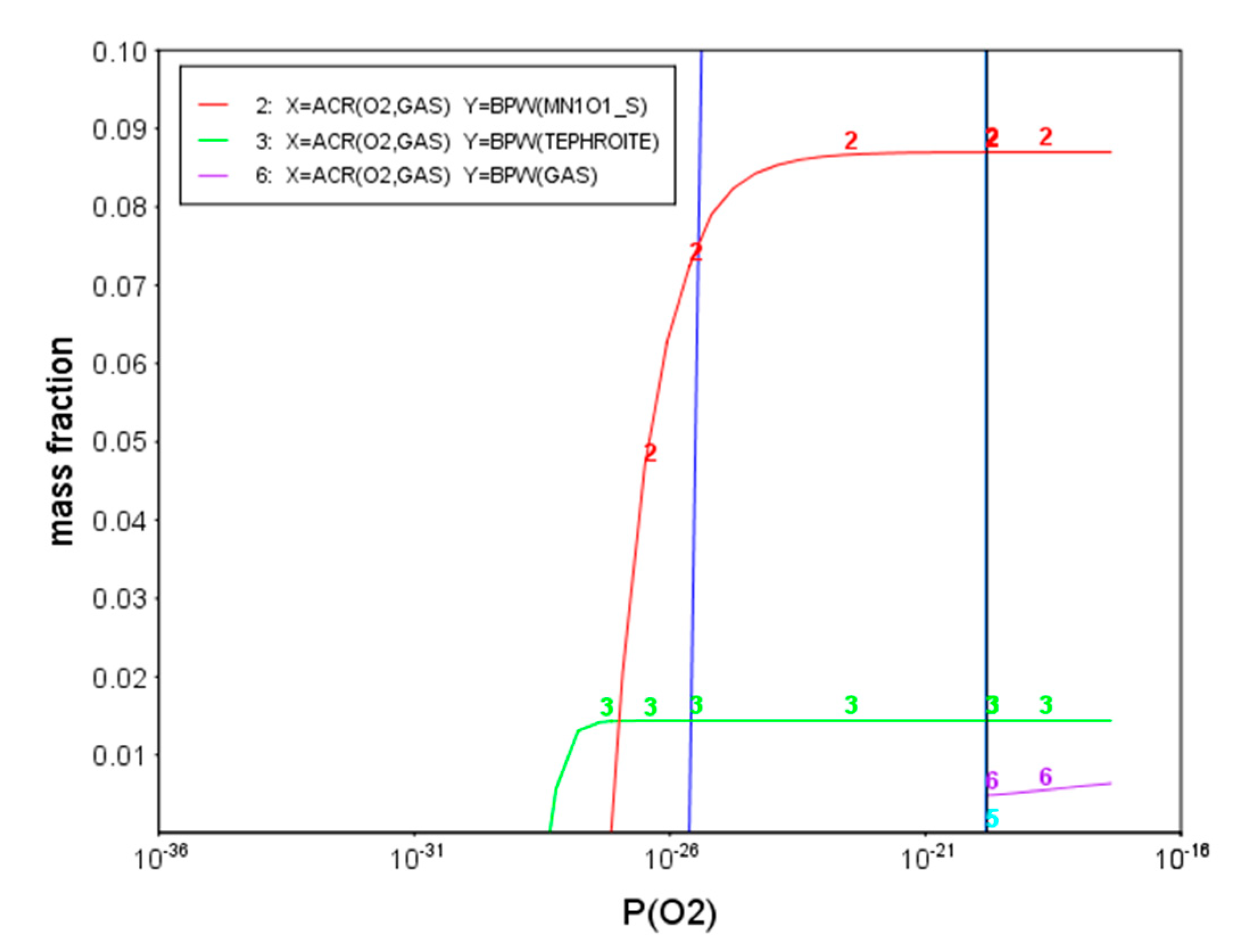
4.1. Thermodynamic Analysis on the Oxide Formation of Experimental Steel during Annealing
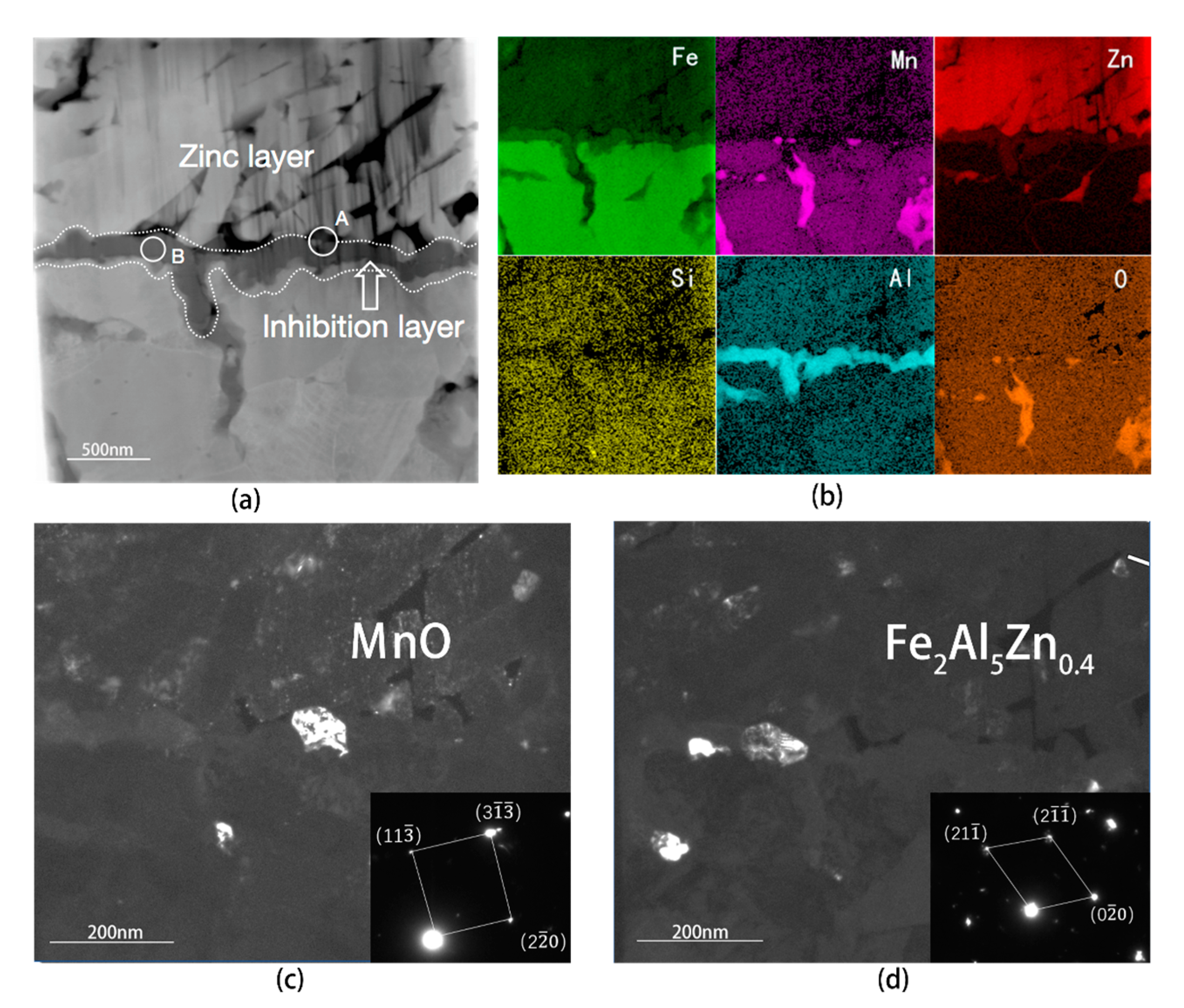
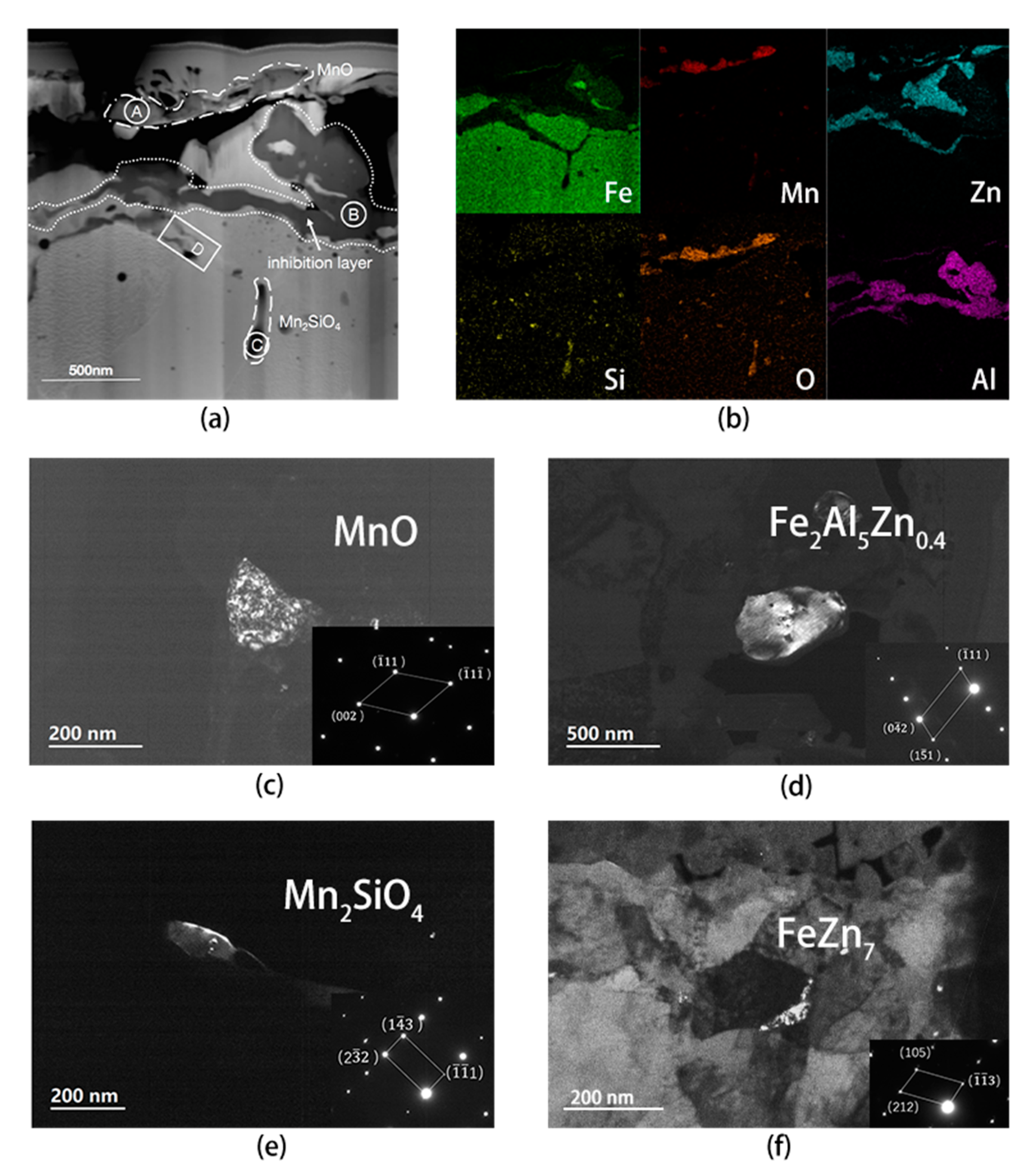
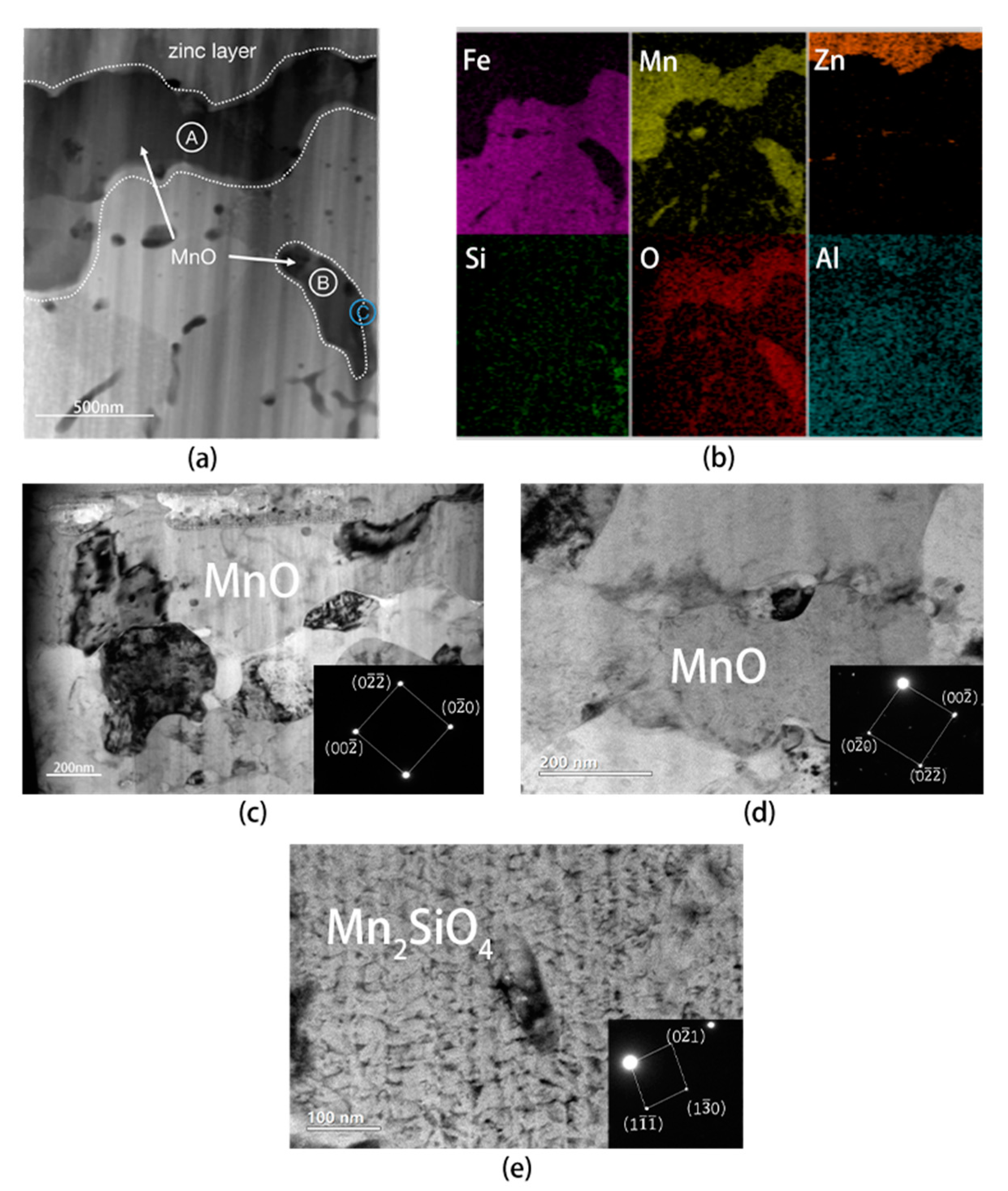
4.2. Analysis of Interfacial Microstructure of Experimental Steel
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Luo, H.W.; Shi, J.; Wang, C. Experimental and numerical analysis on formation of stable austenite during the intercritical annealing of 5Mn steel. Acta Mater. 2011, 5, 4002–4014. [Google Scholar] [CrossRef]
- Xu, H.-F.; Zhao, J.; Cao, W.; Shi, J.; Wang, C.; Li, J.; Dong, H.; Wang, C. Heat treatment effects on the microstructure and mechanical properties of a medium manganese steel (0.2C–5Mn). Mater. Sci. Eng. A 2012, 532, 435–442. [Google Scholar] [CrossRef]
- Wang, C.; Cao, W.Q.; Hang, Y. Influences of austenization temperature and annealing time on duplex ultrafine microstructure properties of medium Mn steel. J. Iron Steel Res. Int. 2015, 22, 42–47. [Google Scholar] [CrossRef]
- Cho, L.; Jung, G.S.; Cooman, B.C.D. On the transition of internal to external selective oxidation on CMnSi TRIP steel. Metall. Mater. Trans. A 2014, 45, 5158–5172. [Google Scholar] [CrossRef]
- Seyed Mousavi, G.; Mcdermid, J.R. Selective oxidation of a C–2Mn–1.3Si (wt pct) advanced high-strength steel during continuous galvanizing heat treatments. Metall. Mater. Trans. A 2018, 49, 1–15. [Google Scholar] [CrossRef]
- Samanta, S.; Halder, A.K.; Deo, Y. Effect of Mn and Cr on the selective oxidation, surface segregation and hot-dip Zn coatability. Surf. Coat. Technol. 2019, 377, 1–9. [Google Scholar] [CrossRef]
- Yonggang, D.; Hongshuang, D.; Misra, R.D.K. Influence of dew point on the selective oxidation, microstructure and mechanical properties of a high-Al low-Si dual phase steel during hot-dip galvanizing process. Prot. Met. Phys. Chem. Surf. 2018, 54, 496–502. [Google Scholar]
- Staudte, J.; Mataigne, J.M.; Del Frate, F. Optimizing the manganese and silicon content for hot dip galvanizing of 3rd generation advanced high strength steels. Metall. Res. Technol. 2014, 111, 17–23. [Google Scholar] [CrossRef]
- Maderthaner, M.; Jarosik, A.; Angeli, G.; Haubner, R. Effect of dew point on the selective oxidation of advanced high strength steels. Mater. Sci. Forum 2017, 891, 292–297. [Google Scholar] [CrossRef]
- Mousavi, G.S.; Mcdermid, J.R. Effect of dew point on the reactive wetting of a C–2Mn–1.3Si(wt%) advanced high strength steel during continuous galvanizing. Surf. Coat. Technol. 2018, 351, 11–20. [Google Scholar] [CrossRef]
- Pourmajidian, M.; Mcdermid, J.R. Selective oxidation of a 0.1C–6Mn–2Si third generation advanced high-strength steel during dew-point controlled annealing. Metall. Mater. Trans. A 2018, 49, 1795–1808. [Google Scholar] [CrossRef]
- Pourmajidian, M.; Mcdermid, J.R. Effect of annealing temperature on the selective oxidation and reactive wetting of a 0.1C–6Mn–2Si advanced high strength steel during continuous galvanizing heat treatments. ISIJ Int. 2018, 58, 1635–1643. [Google Scholar] [CrossRef]
- Pourmajidian, M.; McDermid, J.R. On the reactive wetting of a medium-Mn advanced high-strength steel during continuous galvanizing. Surf. Coat. Technol. 2019, 357, 418–426. [Google Scholar] [CrossRef]
- Yang, T.; He, Y.; Chen, Z.; Zheng, W.; Wang, H.; Li, L. Effect of dew point and alloy composition on reactive wetting of hot dip galvanized medium manganese lightweight steel. Coatings 2020, 10, 37. [Google Scholar] [CrossRef]
- Bellhouse, E.M.; McDermid, J.R. Analysis of the Fe–Zn interface of galvanized high Al–low Si TRIP steels. Mater. Sci. Eng. A 2008, 491, 39–46. [Google Scholar] [CrossRef]
- Suzuki, Y.; Sugimoto, Y.; Yamashita, T.; Fujita, S.; Yamaguchi, S. Thermodynamic analysis of selective oxidation behavior of Si and Mn-added steel during recrystallization annealing. ISIJ Int. 2009, 49, 564–573. [Google Scholar] [CrossRef]
- Huachu, L.; Yanlin, H.; Lin, L. Application of thermodynamics and Wagner model on two problems in continuous hot-dip galvanizing. Appl. Surf. Sci. 2009, 256, 1399–1403. [Google Scholar]
- Alibeigi, S.; Kavitha, R.; Meguerian, R.J.; McDermid, J.R. Reactive wetting of high Mn steels during continuous hot-dip galvanizing. Acta Mater. 2011, 59, 3537–3549. [Google Scholar] [CrossRef]
- Kim, Y.; Shin, K.-S.; Jeon, S.-H.; Chin, K.-G.; Lee, J. The influence of the dew point on the wettability of twinning-induced-plasticity steels by liquid Zn–0.23-wt% Al. Corros. Sci. 2014, 85, 364–371. [Google Scholar] [CrossRef]
- Khondker, R.; Mertens, A.; McDermid, J.R. Effect of annealing atmosphere on the galvanizing behavior of a dual-phase steel. Mater. Sci. Eng. A 2007, 463, 157–165. [Google Scholar] [CrossRef]




| No. | Annealing Temperature/K | Dew Point/°C | H2 Content |
|---|---|---|---|
| 1# | 873 | −10 | 20% |
| 2# | 923 | −10 | 5% |
| 3# | 20% | ||
| 4# | 1093 | −50 | 20% |
| 5# | −10 | ||
| 6# | +10 |
| No. | P(O2)/atm |
|---|---|
| 1# | 1.72 × 10−28 |
| 3# | 7.24 × 10−27 |
| 4# | 4.28 × 10−26 |
| 2# | 1.16 × 10−25 |
| 5# | 1.86 × 10−22 |
| 6# | 4.16 × 10−21 |
| Sample No. | P(O2)/atm | Mangnese Content/wt.% |
|---|---|---|
| 1# | 1.72 × 10−28 | 5.5 |
| 3# | 7.24 × 10−27 | 11.7 |
| 4# | 4.28 × 10−26 | 12.7 |
| 2# | 1.16 × 10−25 | 23.3 |
| 5# | 1.86 × 10−22 | 30.6 |
| 6# | 4.16 × 10−21 | 35.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; He, Y.; Zheng, W.; Wang, H.; Zhang, Y.; Li, L. Effect of Hot-Dip Galvanizing Process on Selective Oxidation and Galvanizability of Medium Manganese Steel for Automotive Application. Coatings 2020, 10, 1265. https://doi.org/10.3390/coatings10121265
Chen Z, He Y, Zheng W, Wang H, Zhang Y, Li L. Effect of Hot-Dip Galvanizing Process on Selective Oxidation and Galvanizability of Medium Manganese Steel for Automotive Application. Coatings. 2020; 10(12):1265. https://doi.org/10.3390/coatings10121265
Chicago/Turabian StyleChen, Zhang, Yanlin He, Weisen Zheng, Hua Wang, Yu Zhang, and Lin Li. 2020. "Effect of Hot-Dip Galvanizing Process on Selective Oxidation and Galvanizability of Medium Manganese Steel for Automotive Application" Coatings 10, no. 12: 1265. https://doi.org/10.3390/coatings10121265
APA StyleChen, Z., He, Y., Zheng, W., Wang, H., Zhang, Y., & Li, L. (2020). Effect of Hot-Dip Galvanizing Process on Selective Oxidation and Galvanizability of Medium Manganese Steel for Automotive Application. Coatings, 10(12), 1265. https://doi.org/10.3390/coatings10121265





