Peroxide Post-Treatment of Wood Impregnated with Micronized Basic Copper Carbonate
Abstract
1. Introduction
2. Materials and Methods
2.1. Accelerated Photo-Degradation
2.2. Black-Stain Fungal Resistance
2.3. Copper Leaching
2.4. Copper Characterization
3. Results
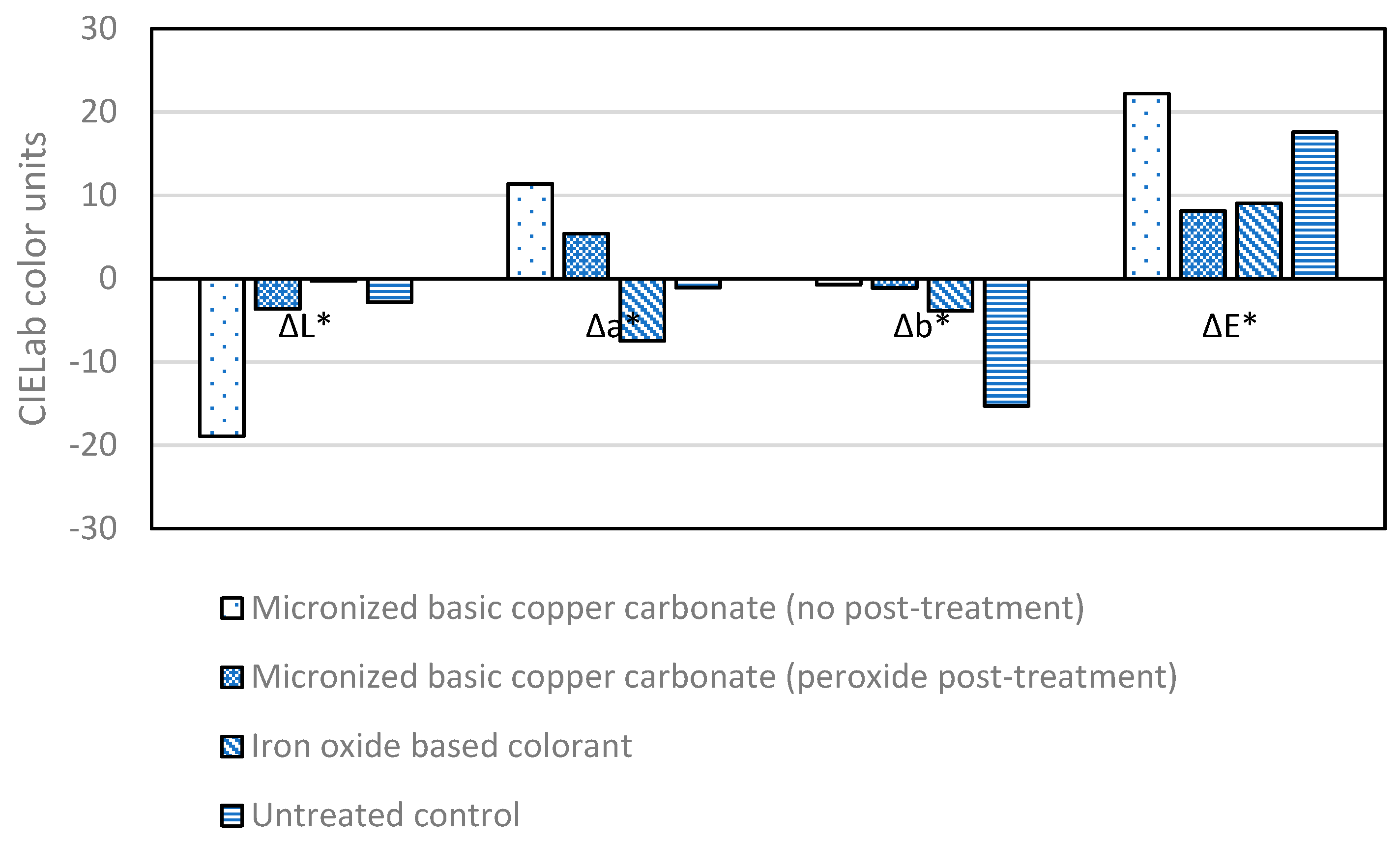
3.1. Accelerated Photo-Degradation
3.2. Black-Stain Fungal Resistance
3.3. Copper Leaching
3.4. Copper Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Peroxide Treatment Optimization






References
- Sandak, A.; Sandak, J.; Dimitriou, A.; Burud, I.; Thiis, T.K.; Gobakken, L.R.; Ormondroyd, G.A.; Kraniotis, D. Assessment and monitoring of aesthetic appearance of building biomaterials during the service life. WIT Trans. Ecol. Environ. 2017, 226, 527–536. [Google Scholar] [CrossRef]
- Evans, P.; Chowdhury, M.J.; Mathews, B.; Schmalzl, K.; Ayer, S.; Kiguchi, M.; Kataoka, Y. Weathering and surface protection of wood. In Handbook of Environmental Degradation of Materials; Kutz, M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2005; pp. 277–297. [Google Scholar] [CrossRef]
- Feist, W.C. Outdoor wood weathering and protection. In Archaeological Wood: Properties, Chemistry, and Preservation; Advances in Chemistry; Rowell, R.M., Barbour, R.J., Eds.; American Chemical Society: Washington, DC, USA, 1990; Volume 225, pp. 263–298. [Google Scholar] [CrossRef]
- Blanchard, V.; Blanchet, P. Color stability for wood products during use: Effects of inorganic nanoparticles. BioResources 2011, 6, 1219–1229. [Google Scholar]
- Jin, L.; Archer, K.; Preston, A. Surface Characteristics of Wood Treated with Various AAC, ACQ and CCA Formulations After Weathering. International Research Group on Wood Preservation. Document No. IRG/WP/2369. IRG Secretariat, Stockholm Sweden. 1991. Available online: https://www.irg-wp.com/search-irg-docs.html (accessed on 20 November 2020).
- Liu, R.; Ruddick, J.N.R.; Jin, L. The Influence of Copper (II) Chemicals on the Weathering of Treated Wood, 1: ACQ Treatment of Wood on Weathering. International Research Group on Wood Preservation. Document No. IRG/WP/94-30040. IRG Secretariat, Stockholm, Sweden. 1994. Available online: https://www.irg-wp.com/search-irg-docs.html (accessed on 20 November 2020).
- Zhang, J.; Kamdem, D.P.; Temiz, A. Weathering of copper-amine treated wood. Appl. Surf. Sci. 2009, 256, 842–846. [Google Scholar] [CrossRef]
- Ozgenc, O.; Hiziroglu, S.; Yildiz, U.C. Weathering properties of wood species treated with different coating applications. BioResources 2012, 7, 4875–4888. [Google Scholar] [CrossRef]
- Zhang, J.; Ziobro, R. Micronized Pigment Technology for Pressure Treatment. Proceedings Canadian Wood Preservation Association, 34, 2013. Available online: https://www.cwpa.ca/publications/ (accessed on 20 November 2020).
- Archer, K.; Sturdivant, R.; Schauwecker, C. Pressure Treatment of Lumber with Colorant Additives. Proceedings Canadian Wood Preservation Association, 34, 2013. Available online: https://www.cwpa.ca/publications/ (accessed on 20 November 2020).
- Grelier, S.; Castellan, A.; Kamdem, D.P. Photoprotection of copper-amine-treated pine. Wood Fiber Sci. 2000, 32, 196–202. [Google Scholar]
- Auger, S.B. Mineral Stains for Wood and Other Substrates. US Patent 2007/0011820 A1, 12 September 2007. [Google Scholar]
- Stirling, R.; Drummond, J.; Zhang, J.; Ziobro, R.J. Micro-Distribution of Micronized Copper in Southern Pine. International Research Group on Wood Protection. Document No. IRG/WP/08-30479. IRG Secretariat, Stockholm, Sweden. 2008. Available online: https://www.irg-wp.com/search-irg-docs.html (accessed on 20 November 2020).
- Matsunaga, H.; Kiguchi, M.; Evans, P.D. Microdistribution of copper-carbonate and iron oxide nanoparticles in treated wood. J. Nanoparticle Res. 2009, 11, 1087–1098. [Google Scholar] [CrossRef]
- Xue, W.; Kennepohl, P.; Ruddick, J.N.R. Investigation of copper solubilization and reaction in micronized copper treated wood by electron paramagnetic resonance (EPR) spectroscopy. Holzforschung 2012, 66, 889–895. [Google Scholar] [CrossRef]
- ASTM G-155 Standard Practice for Operating Xenon Arc Light Apparatus for Exposure of Non-Metallic Materials; ASTM International: West Conshohocken, PA, USA, 2013.
- Stirling, R.; Uzunovic, A.; Morris, P.I. Control of black stain fungi with biocides in semitransparent wood coatings. For. Prod. J. 2011, 61, 359–364. [Google Scholar] [CrossRef]
- AWPA E24-16 Laboratory Method for Evaluating the Mold Resistance of Wood-Based Materials: Mold Chamber Test; American Wood Protection Association: Birmingham, AL, USA, 2016.
- OECD Guidance on the Estimation of Emissions from Wood Preservative—Treated Wood to the Environment: For Wood held in Storage After Treatment and for Wooden Commodities that are not covered and are not in Contact with Ground. Organization for Economic Co-operation and Development, Paris, France. 2008. Available online: https://echa.europa.eu/documents/10162/16908203/pt8_oecd_guidance_emission_estimation_treated_Wood_en.pdf/97bc4a98-abf6-4a3a-a448-88681a17dd52 (accessed on 23 July 2009).
- AWPA A9-16. Standard Method for Analysis of Treated Wood and Treating Solutions by X-ray Spectroscopy; American Wood Protection Association: Birmingham, AL, USA, 2016. [Google Scholar]
- Xue, W.; Kennepohl, P.; Ruddick, J.N.R. Quantification of mobilized copper (II) levels in micronized copper-treated wood by electron paramagnetic resonance (EPR) spectroscopy. Holzforschung 2013, 67, 815–823. [Google Scholar] [CrossRef]
- Deka, M.; Humar, M.; Rep, G.; Kričej, B.; Šentjurc, M.; Petrič, M. Effects of UV light irradiation on colour stability of thermally modified, copper ethanolamine treated and non-modified wood: EPR and DRIFT spectroscopic studies. Wood Sci. Technol. 2008, 42, 5–20. [Google Scholar] [CrossRef]
- Stirling, R.; Wong, D. The Impact of Coatings on the Service Life of Wood Decking. International Research Group on Wood Protection. Document No. IRG/WP/18-20635. IRG Secretariat, Stockholm, Sweden. 2008. Available online: https://www.irg-wp.com/search-irg-docs.html (accessed on 20 November 2020).
- Zhang, J.; Ziobro, R.J. Micronized Copper Preservative Systems: Observations on the Release of Cupric ion (Cu2+) from Treated Wood and Performance against Wood Decay Fungi. International Research Group on Wood Protection. Document No. IRG/WP/09-30519. IRG Secretariat, Stockholm, Sweden. 2009. Available online: https://www.irg-wp.com/search-irg-docs.html (accessed on 20 November 2020).
- Xue, W.; Kennepohl, P.; Ruddick, J.N.R. Reacted copper (II) concentrations in earlywood and latewood of micronized copper-treated Canadian softwood species. Holzforschung 2015, 69, 509–512. [Google Scholar] [CrossRef]
- Kadla, J.F.; Chang, H.M. The reactions of peroxides with lignin and lignin model compounds. In Oxidative Delignification Chemistry, ACS Symposium Series 785; American Chemical Society: Washington DC, USA, 2001; pp. 108–129. [Google Scholar] [CrossRef]
- Gascón-Garrido, P.; Thévenon, M.F.; Mainusch, N.; Militz, H.; Viöl, W.; Mai, C. Siloxane-treated and copper-plasma-coated wood: Resistance to the blue stain fungus Aureobasidium pullulans and the termite Reticulitermes flavipes. Int. Biodeterior. Biodegrad. 2017, 120, 84–90. [Google Scholar] [CrossRef]
- Gadd, G.M. Effect of copper on Aureobasidium pullulans in solid medium: Adaptation not necessary for tolerant behaviour. Trans. Br. Mycol. Soc. 1984, 82, 546–549. [Google Scholar] [CrossRef]
- Gress, J.; De Oliveira, L.M.; Da Silva, E.B.; Lessl, J.M.; Wilson, P.C.; Townsend, T.; Ma, L.Q. Cleaning-induced arsenic mobilization and chromium oxidation from CCA-wood deck: Potential risk to children. Environ. Int. 2015, 82, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Cappellazzi, J.; Konkler, M.J.; Morrell, J.J. Effect of Multiple Leaching Cycles on Decay Resistance of Micronized Copper Azole–Treated Southern Pine Sapwood. For. Prod. J. 2020, 70, 178–181. [Google Scholar] [CrossRef]
- Xue, W.; Ruddick, J.N.R.; Kennepohl, P. Solubilisation and chemical fixation of copper(II) in micronized copper treated wood. Dalton Trans. 2016, 45, 3679–3686. [Google Scholar] [CrossRef] [PubMed]
- Peisach, J.; Blumberg, W.E. Structural implications derived from the analysis of electron paramagnetic resonance spectra of natural and artificial copper proteins. Arch. Biochem. Biophys. 1974, 165, 691–708. [Google Scholar] [CrossRef]






| Treatment | Total Copper (mg/g) | Reacted Copper (mg/g) |
|---|---|---|
| No post-treatment | 5.57 | 2.62 |
| Peroxide post-treatment | 5.04 | 4.24 |
| Treatment | gx | gy | gz | Ax | Ay | Az |
|---|---|---|---|---|---|---|
| No post-treatment | 2.078 | 2.084 | 2.373 | 5 | 45 | 131 |
| Peroxide post-treatment | 2.076 | 2.084 | 2.375 | 5 | 46 | 133 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stirling, R.; Boivin, G.; Uzunovic, A.; Kus, S.; Ruddick, J.N.R. Peroxide Post-Treatment of Wood Impregnated with Micronized Basic Copper Carbonate. Coatings 2020, 10, 1213. https://doi.org/10.3390/coatings10121213
Stirling R, Boivin G, Uzunovic A, Kus S, Ruddick JNR. Peroxide Post-Treatment of Wood Impregnated with Micronized Basic Copper Carbonate. Coatings. 2020; 10(12):1213. https://doi.org/10.3390/coatings10121213
Chicago/Turabian StyleStirling, Rod, Gabrielle Boivin, Adnan Uzunovic, Stacey Kus, and John N. R. Ruddick. 2020. "Peroxide Post-Treatment of Wood Impregnated with Micronized Basic Copper Carbonate" Coatings 10, no. 12: 1213. https://doi.org/10.3390/coatings10121213
APA StyleStirling, R., Boivin, G., Uzunovic, A., Kus, S., & Ruddick, J. N. R. (2020). Peroxide Post-Treatment of Wood Impregnated with Micronized Basic Copper Carbonate. Coatings, 10(12), 1213. https://doi.org/10.3390/coatings10121213






