Investigation of the Influence of Microdroplets on the Coatings Nanolayer Structure
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
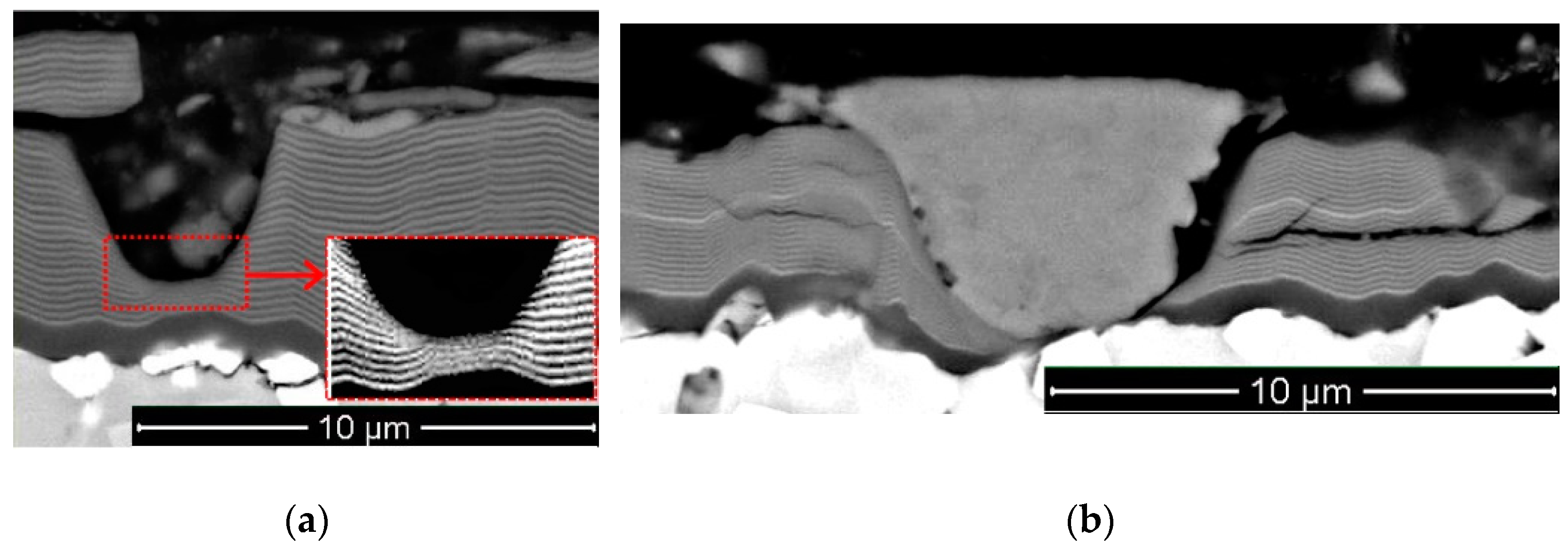
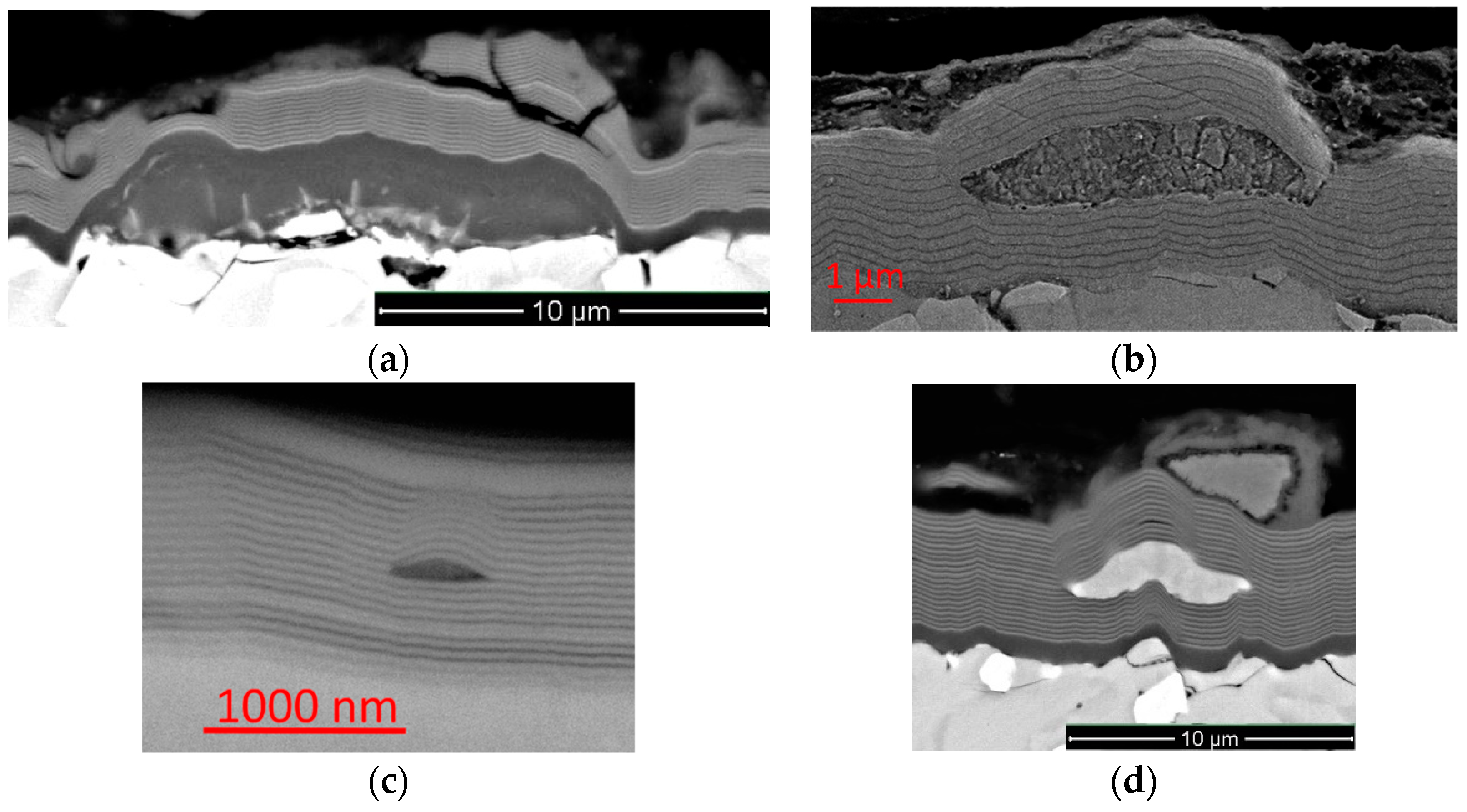
- In the shape of a sphere with a secondary multilayered structure formed around the microdroplet core, and the sizes of such microdroplet cores are within the range of 100–1000 nm. The formation of such microdroplets can also be associated with the high thermal conductivity of the metals forming them;
- In the shape of an ellipse or a tear, when a secondary structure is not typical, and the microdroplet sizes usually stay within 3–5 µm;
- In the shape of a lens, without a surrounding secondary structure, with either large (7–10 µm) or smaller sizes. The formation of such microdroplets can also be associated with the high thermal conductivity of the metals forming them, as well as the formation of nitride compounds that are more refractory compared to pure metal. Due to the above, the surface of the microdroplet solidifies when the microdroplet moves from the cathode to the deposition surface.
Author Contributions
Funding
Conflicts of Interest
References
- Boxman, R.; Goldsmith, S. Macroparticle contamination in cathodic arc coatings: Generation, transport and control. Surf. Coat. Technol. 1992, 52, 39–50. [Google Scholar] [CrossRef]
- Steffens, H.-D.; Mack, M.; Moehwald, K.; Reichel, K. Reduction of droplet emission in random arc technology. Surf. Coat. Technol. 1991, 46, 65–74. [Google Scholar] [CrossRef]
- Akari, K.; Tamagaki, H.; Kumakiri, T.; Tsuji, K.; Koh, E.; Tai, C. Reduction in macroparticles during the deposition of TiN films prepared by arc ion plating. Surf. Coat. Technol. 1990, 1990, 312–323. [Google Scholar] [CrossRef]
- Sanders, D.M. Ion beam self-sputtering using a cathodic arc ion source. J. Vac. Sci. Technol. A 1988, 6, 1929–1933. [Google Scholar] [CrossRef]
- Vereshchaka, A.A.; Mgaloblishvili, O.; Morgan, M.; Batako, A.D.L. Nano-scale multilayered-composite coatings for the cutting tools. Int. J. Adv. Manuf. Technol. 2014, 72, 303–317. [Google Scholar] [CrossRef]
- Michalski, A. Structure and properties of coatings composed of TiN–Ti obtained by the reactive pulse plasma method. J. Mater. Sci. Lett. 1984, 3, 505–508. [Google Scholar] [CrossRef]
- Aksenov, I.I.; Bren’, V.G.; Osipov, V.A.; Padalka, V.G.; Khoroshikh, V.M. Plasma in a stationary vacuum-arc discharge. I. Plasma flux formation. High Temp. 1983, 21, 160–164. [Google Scholar]
- Metel, A.; Bolbukov, V.; Volosova, M.; Grigoriev, S.; Melnik, Y. Equipment for deposition of thin metallic films bombarded by fast argon atoms. Instrum. Exp. Tech. 2014, 57, 345–351. [Google Scholar] [CrossRef]
- Sobol’, O.V.; Andreev, A.A.; Grigoriev, S.N.; Stolbovoy, V.A. See more physical characteristics, structure and stress state of vacuum-arc tin coating, deposition on the substrate when applying high-voltage pulse during the deposition. Probl. At. Sci. Technol. 2011, 4, 174–177. [Google Scholar]
- Metel, A.S.; Grigoriev, S.N.; Melnik, Y.A.; Bolbukov, V.P. Broad beam sources of fast molecules with segmented cold cathodes and emissive grids. Instrum. Exp. Tech. 2012, 55, 122–130. [Google Scholar] [CrossRef]
- Metel, A.S.; Bolbukov, V.P.; Volosova, M.A.; Grigoriev, S.N.; Melnik, Y.A. Source of metal atoms and fast gas molecules for coating deposition on complex shaped dielectric products. Surf. Coat. Technol. 2013, 225, 34–39. [Google Scholar] [CrossRef]
- Metel, A.S.; Melnik, Y.; Metel, A. Broad fast neutral molecule beam sources for industrial-scale beam-assisted deposition. Surf. Coat. Technol. 2002, 156, 44–49. [Google Scholar] [CrossRef]
- Miernik, K.; Walkowicz, J. Spatial distribution of microdroplets generated in the cathode spots of vacuum arcs. Surf. Coat. Technol. 2000, 125, 161–166. [Google Scholar] [CrossRef]
- Anders, S.; Anders, A.; Yu, K.M.; Yao, X.; Brown, I. On the macroparticle flux from vacuum arc cathode spots. IEEE Trans. Plasma Sci. 1993, 21, 440–446. [Google Scholar] [CrossRef]
- Baouchi, A.W.; Perry, A.J. A study of the macroparticle distribution in cathodic-arc-evaporated TiN films. Surf. Coat. Technol. 1991, 41, 253–257. [Google Scholar] [CrossRef]
- Utsumi, T.; English, J.H. Study of electrode products emitted by vacuum arcs in form of molten metal particles. J. Appl. Phys. 1975, 46, 126. [Google Scholar] [CrossRef]
- Vyskočil, J.; Musil, J. Arc evaporation of hard coatings: Process and film properties. Surf. Coat. Technol. 1990, 43–44, 299–311. [Google Scholar] [CrossRef]
- Devia, A.; Benavides, V.; Restrepo, E.; Arias, D.; Ospina, R. Influence substrate temperature on structural properties of TiN/TiC bilayers produced by pulsed arc techniques. Vacuum 2006, 81, 378–384. [Google Scholar] [CrossRef]
- Zhao, Z.; Poulikakos, D.; Fukai, J. Heat transfer and fluid dynamics during the collision of a liquid droplet on a substrate: I—Modeling. Int. J. Heat Mass Transf. 1996, 39, 2771–2789. [Google Scholar] [CrossRef]
- Fukai, J.; Zhao, Z.; Poulikakos, D.; Megaridis, C.M.; Miyatake, O. Modeling of the deformation of a liquid droplet impinging upon a flat surface. Phys. Fluids A 1993, 5, 2588–2599. [Google Scholar] [CrossRef]
- Waldvogel, J.M.; Poulikakos, D.; Wallace, D.B.; Marušák, R. Transport phenomena in picoliter size solder droplet dispension. J. Heat Transf. 1996, 118, 148–156. [Google Scholar] [CrossRef]
- Waldvogel, J.; Poulikakos, D. Solidification phenomena in picoliter size solder droplet deposition on a composite substrate. Int. J. Heat Mass Transf. 1997, 40, 295–309. [Google Scholar] [CrossRef]
- Liu, W.; Wang, G.X.; Matthys, E.F. Determination of the thermal contact coefficient for a molten droplet impinging on a substrate. Transp. Phenom Mater. Process. Manuf. ASME HTD 1992, 196, 111–118. [Google Scholar]
- Attinger, D.; Haferl, S.; Zhao, Z.; Poulikakos, D. Transport phenomena in the impact of a molten droplet on a surface: Macroscopic phenomenology and microscopic considerations. Part II: Heat transferand solidification. Ann. Rev. Heat Transf. 2000, 11, 65–143. [Google Scholar]
- Bennett, T.; Poulikakos, D. Heat transfer aspects of splat-quench solidification: Modelling and experiment. J. Mater. Sci. 1994, 29, 2025–2039. [Google Scholar] [CrossRef]
- Pasandideh-Fard, M.; Mostaghimi, J. On the spreading and solidification of molten particles in a plasma spray process effect of thermal contact resistance. Plasma Chem. Plasma Process. 1995, 16, S83–S98. [Google Scholar] [CrossRef]
- Xiong, B.; Megaridis, C.M.; Poulikakos, D.; Hoang, H. An investigation of key factors affecting solder microdroplet deposition. J. Heat Transf. 1998, 120, 259–270. [Google Scholar] [CrossRef]
- Attinger, D.; Poulikakos, D. On quantifying interfacial thermal and surface energy during molten microdroplet surface deposition. J. Atom. Spray 2003, 13, 309–319. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Longtin, J.P.; Attinger, D. A numerical investigation on the influence of liquid properties and interfacial heat transfer during microdroplet deposition onto a glass substrate. Int. J. Heat Mass Transf. 2007, 50, 2912–2923. [Google Scholar] [CrossRef][Green Version]
- Kumar, A.; Gu, S. Modelling impingement of hollow metal droplets onto a flat surface International. Int. J. Heat Fluid Flow 2012, 37, 189–195. [Google Scholar] [CrossRef]
- Du, J.; Wei, Z.; Wu, H.; Zhao, G.; Wang, X. Numerical simulation of pileup process in metal microdroplet deposition manufacture. J. Enhanc. Heat Transf. 2016, 23, 413–430. [Google Scholar] [CrossRef]
- Muboyadzhyan, S.A.; Budinovskii, S.A.; Gorlov, D.S.; Doronin, O.N. Structure and microporosity of ion-plasma condensed coatings deposited from a two-phase vacuum-arc discharge plasma flow containing evaporated material microdroplets. Russ. Met. 2019, 2019, 52–62. [Google Scholar] [CrossRef]
- Kashkarov, E.B.; Nikitenkov, N.; Syrtanov, M.S.; Sutygina, A.N.; Gvozdyakov, D.V. Effect of bias potential on the structure and distribution of elements in titanium-nitride coatings obtained by cathodic-arc deposition. J. Surf. Investig. 2016, 10, 648–651. [Google Scholar] [CrossRef]
- Anders, A. Cathodic Arcs; Springer Science Business Media, LLC: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, L.; Dong, L.; Gu, H.; Wan, R. Influence of sputtering power on the structure and mechanical properties of Zr–Nb–N nanocomposite coatings prepared by multi-target magnetron Co-sputtering. Key Eng. Mater. 2013, 537, 307–310. [Google Scholar] [CrossRef]
- Klostermann, H.; Fietzke, F.; Labitzke, R.; Modes, T.; Zywitzki, O. Zr–Nb–N hard coatings deposited by high power pulsed sputtering using different pulse modes. Surf. Coat. Technol. 2009, 204, 1076–1080. [Google Scholar] [CrossRef]
- Blinkov, I.V.; Volkhonskii, A.O. The effect of deposition parameters of multilayered nanostructure Ti–Al–N/Zr–Nb–N/Cr–N coatings obtained by the arc-PVD method on their structure and composition. Russ. J. Non-Ferrous Met. 2012, 53, 163–168. [Google Scholar] [CrossRef]
- Makino, Y.; Saito, K.; Murakami, Y.; Asami, K. Phase change of Zr–Al–N and Nb–Al–N films prepared by magnetron sputtering method. Solid State Phenom. 2007, 127, 195–200. [Google Scholar] [CrossRef]
- Yang, G.; Etchessahar, E.; Bars, J.; Portier, R.; Debuigne, J. A miscibility gap in the FCC δ-nitride region of the ternary system titanium-zirconium-nitrogen. Scr. Met. Mater. 1994, 31, 903–908. [Google Scholar] [CrossRef]
- Duwez, P.; Odell, F. Phase Relationships in the Binary Systems of Nitrides and Carbides of Zirconium, Columbium, Titanium, and Vanadium. J. Electrochem. Soc. 1950, 97, 299. [Google Scholar] [CrossRef]
- Sridar, S.; Kumar, R.; Kumar, K.H. Thermodynamic modelling of Ti–Zr–N system. Calphad 2017, 56, 102–107. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Chang, C.-L.; Hsu, C.-H.; Lin, H.-N. Synthesis of (Ti,Zr)N hard coatings by unbalanced magnetron sputtering. Surf. Coat. Technol. 2000, 130, 64–68. [Google Scholar] [CrossRef]
- Uglov, V.V.; Anishchik, V.M.; Zlotski, S.V.; Abadias, G.; Dub, S. Structural and mechanical stability upon annealing of arc-deposited Ti–Zr–N coatings. Surf. Coat. Technol. 2008, 202, 2394–2398. [Google Scholar] [CrossRef]
- Kuo, Y.-L.; Lee, C.; Lin, J.-C.; Yen, Y.-W.; Lee, W.-H. Evaluation of the thermal stability of reactively sputtered (Ti,Zr)Nx nano-thin films as diffusion barriers between Cu and Silicon. Thin Solid Films 2005, 484, 265–271. [Google Scholar] [CrossRef]
- Tonghe, Z.; Yuguang, W.; Zhiyong, Z.; Zhiwei, D. The ternary Ti (Zr, N) phases formation and modification of TiN coatings by Zr+ MEVVA ion implantation. Surf. Coat. Technol. 2000, 131, 326–329. [Google Scholar] [CrossRef]
- Niu, E.; Li, L.; Lv, G.; Chen, H.; Li, X.; Yang, X.; Yang, S. Characterization of Ti–Zr–N films deposited by cathodic vacuum arc with different substrate bias. Appl. Surf. Sci. 2008, 254, 3909–3914. [Google Scholar] [CrossRef]
- Uglov, V.V.; Anishchik, V.M.; Khodasevich, V.V.; Prikhodko, Z.L.; Zlotski, S.V.; Abadias, G.; Dub, S.N. Structural characterization and mechanical properties of Ti–Zr–N coatings, deposited by vacuum arc. Surf. Coat. Technol. 2004, 180–181, 519–525. [Google Scholar] [CrossRef]
- Regent, F.; Musil, J. Magnetron sputtered Cr–Ni–N and Ti–Mo–N films: Comparison of mechanical properties. Surf. Coat. Technol. 2001, 142–144, 146–151. [Google Scholar] [CrossRef]
- Kuleshov, A.; Uglov, V.V.; Chayevski, V.V.; Anishchik, V.M. Properties of coatings based on Cr, Ti, and Mo nitrides with embedded metals deposited on cutting tools. J. Frict. Wear 2011, 32, 192–198. [Google Scholar] [CrossRef]
- Hangwei, C.; Yuan, G.; Lin, Y.; Zhikang, M.; Chenglei, W. High-temperature oxidation behavior of (Ti,Cr)N coating deposited on 4Cr13 stainless steel by multi-arc ion plating. Rare Met. Mater. Eng. 2014, 43, 1084–1087. [Google Scholar] [CrossRef]
- Panjan, P.; Navinsek, B.; Cvelbar, A.; Zalar, A.; Milosev, I. Oxidation of TiN, ZrN, TiZrN, CrN, TiCrN and TiN/CrN multilayer hard coatings reactively sputtered at low temperature. Thin Solid Films 1996, 281, 298–301. [Google Scholar] [CrossRef]
- Caplan, D.; Cohen, M. The volatilization of chromium oxide. J. Electrochem. Soc. 1961, 108, 438. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, E.Y.; Hong, S.G.; Park, B.G.; Yoon, J.H.; Yong, J.H. Syntheses and mechanical properties of Cr–Mo–N coatings by a hybrid coating system. Surf. Coat. Technol. 2006, 207, 4068–4072. [Google Scholar] [CrossRef]
- Qi, D.; Lei, H.; Wang, T.; Pei, Z.; Gong, J.; Sun, C. Mechanical, microstructural and tribological properties of reactive magnetron sputtered Cr–Mo–N films. J. Mater. Sci. Technol. 2015, 31, 55–64. [Google Scholar] [CrossRef]
- Koshy, R.A.; Graham, M.E.; Marks, L.D. Temperature activated self-lubrication in CrN/Mo2N nanolayer coatings. Surf. Coat. Technol. 2010, 204, 1359–1365. [Google Scholar] [CrossRef]
- Liu, W.; Li, A.; Wu, H.; He, R.; Huang, J.; Long, Y.; Deng, X.; Wang, Q.; Wang, C.; Wu, S. Effects of bias voltage on microstructure, mechanical properties, and wear mechanism of novel quaternary (Ti, Al, Zr)N coating on the surface of silicon nitride ceramic cutting tool. Ceram. Int. 2016, 42, 17693–17697. [Google Scholar] [CrossRef]
- Liu, W.; Li, A.; Wu, H.; Long, Y.; Huang, J.; Deng, X.; Wang, C.; Wang, Q.; Wu, S. Effects of gas pressure on microstructure and performance of (Ti, Al, Zr)N coatings produced by physical vapor deposition. Ceram. Int. 2016, 42, 17436–17441. [Google Scholar] [CrossRef]
- Sergevnin, V.S.; Blinkov, I.V.; Belov, D.; Volkhonskii, A.O.; Skryleva, E.A.; Chernogor, A.V. Phase formation in the Ti–Al–Mo–N system during the growth of adaptive wear-resistant coatings by arc PVD. Inorg. Mater. 2016, 52, 735–742. [Google Scholar] [CrossRef]
- Tomaszewski, Ł.; Gulbiński, W.; Urbanowicz, A.; Suszko, T.; Lewandowski, A.; Gulbiński, W. TiAlN based wear resistant coatings modified by molybdenum addition. Vacuum 2015, 121, 223–229. [Google Scholar] [CrossRef]
- Yang, K.; Xian, G.; Zhao, H.; Fan, H.; Wang, J.; Wang, H.; Du, H. Effect of Mo content on the structure and mechanical properties of TiAlMoN films deposited on WC–Co cemented carbide substrate by magnetron sputtering. Int. J. Refract. Met. Hard Mater. 2015, 52, 29–35. [Google Scholar] [CrossRef]
- Sergevnin, V.S.; Anikin, V.N.; Volkhonskii, A.; Belov, D.; Kuznetsov, D.; Gorshenkov, M.; Skryleva, E. Wear behaviour of wear-resistant adaptive nano-multilayered Ti–Al–Mo–N coatings. Appl. Surf. Sci. 2016, 388, 13–23. [Google Scholar] [CrossRef]
- Grigoriev, S.; Vereschaka, A.; Milovich, F.; Tabakov, V.; Sitnikov, N.; Andreev, N.; Sviridova, T.; Bublikov, J. Investigation of multicomponent nanolayer coatings based on nitrides of Cr, Mo, Zr, Nb, and Al. Surf. Coat. Technol. 2020, 401, 126258. [Google Scholar] [CrossRef]
- Vereschaka, A.A. Development of assisted filtered cathodic vacuum arc deposition of nano-dispersed multi-layered composite coatings on cutting tools. Key Eng. Mater. 2013, 581, 62–67. [Google Scholar] [CrossRef]
- Vereschaka, A.A.; Grigoriev, S.N.; Sitnikov, N.N.; Oganyan, G.V.; Batako, A. Working efficiency of cutting tools with multilayer nano-structured Ti–TiCN–(Ti,Al)CN and Ti–TiCN–(Ti,Al,Cr)CN coatings: Analysis of cutting properties, wear mechanism and diffusion processes. Surf. Coat. Technol. 2017, 332, 198–213. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Sobol, O.V.; Beresnev, V.M.; Serdyuk, I.V.; Pogrebnyak, A.D.; Kolesnikov, D.A.; Nemchenko, U.S. Tribological characteristics of (TiZrHfVNbTa)N coatings applied using the vacuum arc deposition method. J. Frict. Wear 2014, 35, 359–364. [Google Scholar] [CrossRef]
- Vereschaka, A.; Tabakov, V.; Grigoriev, S.; Aksenenko, A.; Sitnikov, N.; Oganyan, G.; Seleznev, A.; Shevchenko, S. Effect of adhesion and the wear-resistant layer thickness ratio on mechanical and performance properties of ZrN–(Zr,Al,Si)N coatings. Surf. Coat. Technol. 2019, 357, 218–234. [Google Scholar] [CrossRef]
- Vereschaka, A.; Tabakov, V.; Grigoriev, S.; Sitnikov, N.; Milovich, F.; Andreev, N.; Sotova, C.; Kutina, N. Investigation of the influence of the thickness of nanolayers in wear-resistant layers of Ti–TiN–(Ti,Cr,Al)N coating on destruction in the cutting and wear of carbide cutting tools. Surf. Coat. Technol. 2020, 385, 125402. [Google Scholar] [CrossRef]
- Vereschaka, A.; Aksenenko, A.; Sitnikov, N.; Migranov, M.; Shevchenko, S.; Sotova, C.; Batako, A.D.L.; Andreev, N. Effect of adhesion and tribological properties of modified composite nano-structured multi-layer nitride coatings on WC–Co tools life. Tribol. Int. 2018, 128, 313–327. [Google Scholar] [CrossRef]
- Vereschaka, A. Improvement of working efficiency of cutting tools by modifying its surface properties by application of wear-resistant complexes. Adv. Mater. Res. 2013, 712, 347–351. [Google Scholar] [CrossRef]
- Volosova, M.A.; Grigor’ev, S.N.; Kuzin, V.V. Effect of titanium nitride coating on stress structural inhomogeneity in oxide-carbide ceramic. Part 4. Action of heat flow. Refract. Ind. Ceram. 2015, 56, 91–96. [Google Scholar] [CrossRef]
- Kuzin, V.V.; Grigor’ev, S.N.; Volosova, M.A. Effect of a TiC coating on the stress-strain state of a plate of a high-density nitride ceramic under nonsteady thermoelastic conditions. Refract. Ind. Ceram. 2014, 54, 376–380. [Google Scholar] [CrossRef]
- Kuzin, V.; Grigoriev, S.N.; Fedorov, M.Y. Role of the thermal factor in the wear mechanism of ceramic tools. Part 2: Microlevel. J. Frict. Wear 2015, 36, 40–44. [Google Scholar] [CrossRef]
- Vereschaka, A.A.; Grigoriev, S.N.; Volosova, M.A.; Batako, A.; Vereschaka, A.S.; Sitnikov, N.; Seleznev, A. Nano-scale multi-layered coatings for improved efficiency of ceramic cutting tools. Int. J. Adv. Manuf. Technol. 2016, 90, 27–43. [Google Scholar] [CrossRef]
- Vereschaka, A.; Tabakov, V.; Grigoriev, S.; Sitnikov, N.; Oganyan, G.; Andreev, N.; Milovich, F. Investigation of wear dynamics for cutting tools with multilayer composite nanostructured coatings in turning constructional steel. Wear 2019, 420–421, 17–37. [Google Scholar] [CrossRef]
- Vereschaka, A.; Grigoriev, S.; Sitnikov, N.; Milovich, F.; Aksenenko, A.; Andreev, N. Investigation of performance and cutting properties of carbide tool with nanostructured multilayer Zr–ZrN–(Zr0.5,Cr0.3,Al0.2)N coating. Int. J. Adv. Manuf. Technol. 2019, 102, 2953–2965. [Google Scholar] [CrossRef]
- Vereschaka, A.S.; Grigoriev, S.N.; Tabakov, V.P.; Sotova, E.S.; Vereschaka, A.A.; Kulikov, M.Y. Improving the efficiency of the cutting tool made of ceramic when machining hardened steel by applying nano-dispersed multi-layered coatings. Key Eng. Mater. 2014, 581, 68–73. [Google Scholar] [CrossRef]
- Engineering ToolBox. Thermal Conductivity of Metals, Metallic Elements and Alloys. 2005. Available online: https://www.engineeringtoolbox.com/thermal-conductivity-metals-d_858.html (accessed on 1 November 2020).
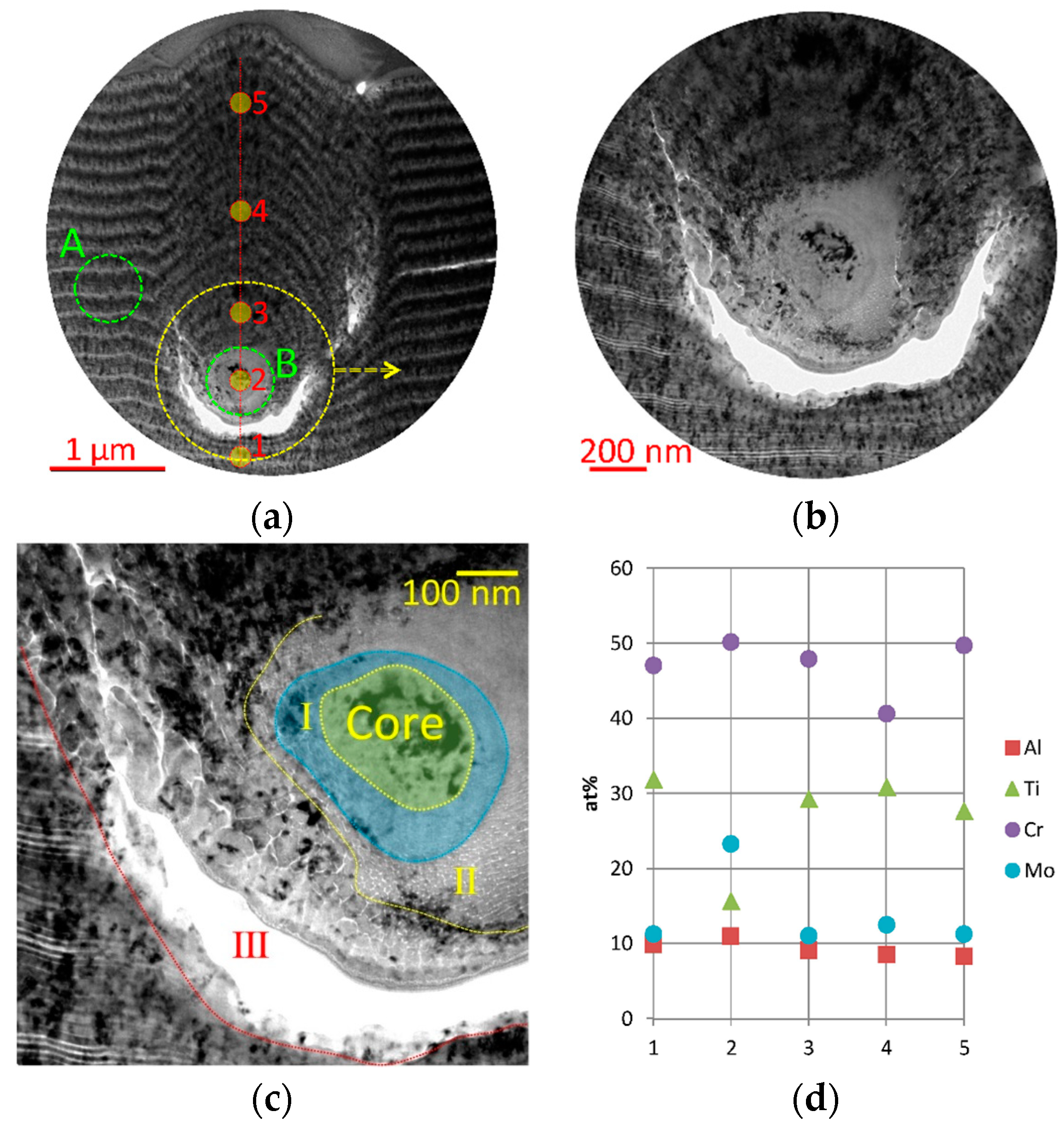
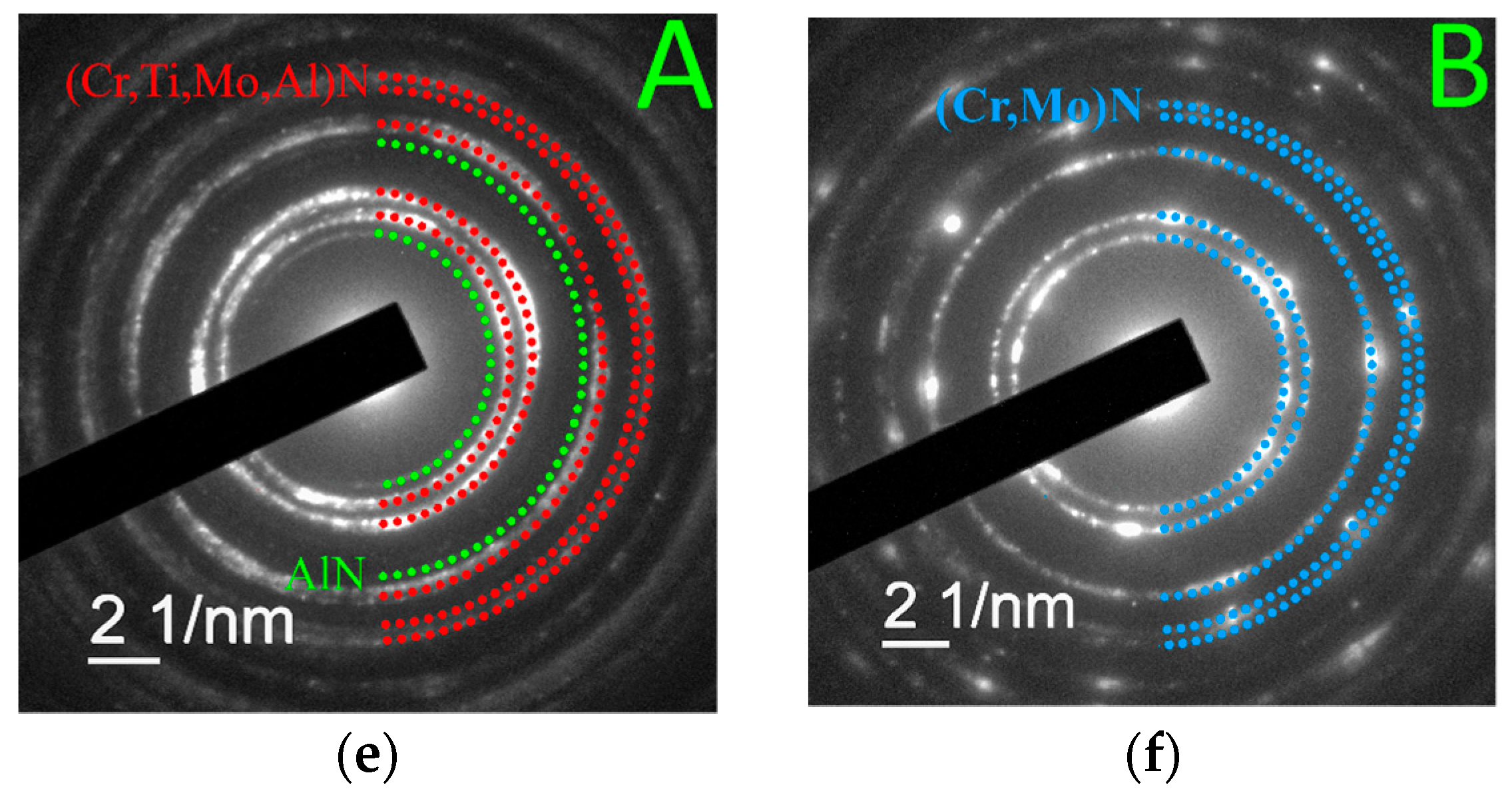

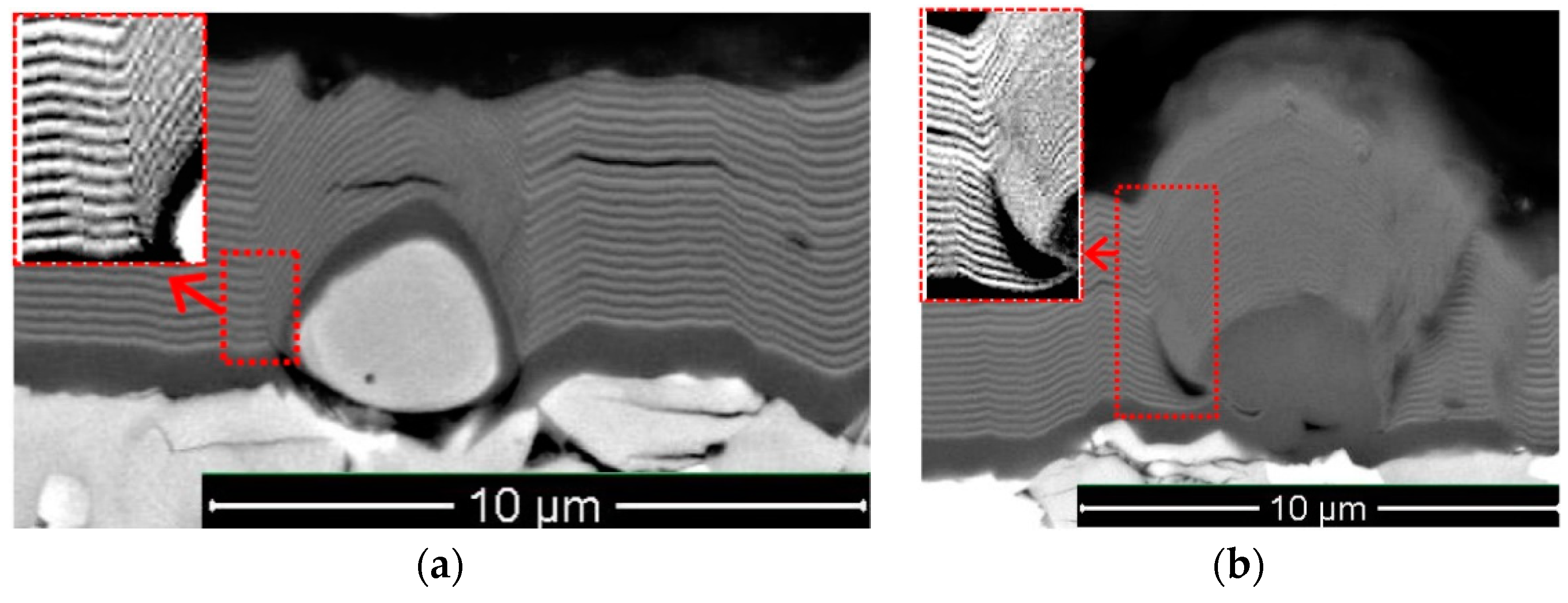
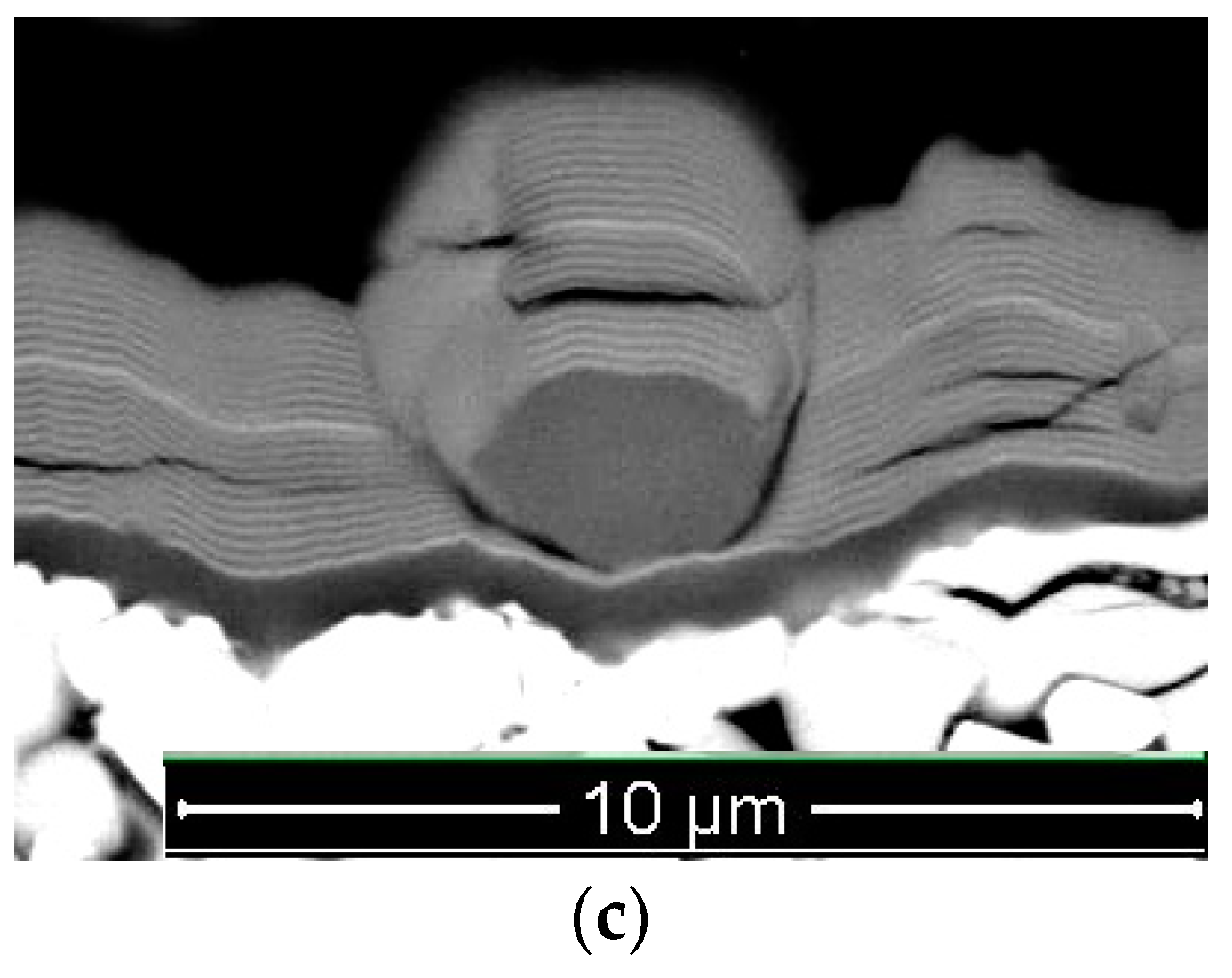
| Process | pN (Pa) | U (V) | ITi (A) | IAl (A) | IZr–Nb (A) | ICr–Mo (A) |
|---|---|---|---|---|---|---|
| Pumping and heating of vacuum chamber | 0.06 | +20 | 75 | 120 | 65 | – |
| Heating and cleaning of products with gaseous plasma | 2.0 | 100 DC/900 AC f = 10 kHz, 2:1 | 85 | 80 | – | – |
| Deposition of coating | 0.42 | −800 DC | 75 | 160 | 55 | 73 |
| Cooling of products | 0.06 | – | – | – | – | – |
| Chemical Element | 127 °C | 527 °C | 727 °C |
|---|---|---|---|
| Al | 240 | 220 | 90 |
| Ti | 20.4 | 19.7 | 20.7 |
| Cr | 87.3 | 71.3 | 65.3 |
| Mo | 134 | 118 | 112 |
| Nb | 55.2 | 61.3 | 64.4 |
| Zr | 21.6 | 21.6 | 23.7 |
| Phase | CrN | Mo2N | (Cr,Mo)N | TiN | HKL |
|---|---|---|---|---|---|
| Space Group | m | m | m | m | |
| 1 | 2.390 Å | 2.408 Å | 2.42 Å | 2.448 Å | (111) |
| 2 | 2.070 Å | 2.085 Å | 2.09 Å | 2.120 Å | (002) |
| 3 | 1.464 Å | 1.475 Å | 1.48 Å | 1.499 Å | (202) |
| 4 | 1.248 Å | 1.257 Å | 1.26 Å | 1.278 Å | (113) |
| 5 | 1.195 Å | 1.204 Å | 1.20 Å | 1.224 Å | (222) |
| 6 | 1.035 Å | 1.043 Å | 1.04 Å | 1.060 Å | (004) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoriev, S.; Vereschaka, A.; Milovich, F.; Sitnikov, N.; Andreev, N.; Bublikov, J.; Sotova, C.; Sadov, I. Investigation of the Influence of Microdroplets on the Coatings Nanolayer Structure. Coatings 2020, 10, 1204. https://doi.org/10.3390/coatings10121204
Grigoriev S, Vereschaka A, Milovich F, Sitnikov N, Andreev N, Bublikov J, Sotova C, Sadov I. Investigation of the Influence of Microdroplets on the Coatings Nanolayer Structure. Coatings. 2020; 10(12):1204. https://doi.org/10.3390/coatings10121204
Chicago/Turabian StyleGrigoriev, Sergey, Alexey Vereschaka, Filipp Milovich, Nikolay Sitnikov, Nikolay Andreev, Jury Bublikov, Catherine Sotova, and Ilya Sadov. 2020. "Investigation of the Influence of Microdroplets on the Coatings Nanolayer Structure" Coatings 10, no. 12: 1204. https://doi.org/10.3390/coatings10121204
APA StyleGrigoriev, S., Vereschaka, A., Milovich, F., Sitnikov, N., Andreev, N., Bublikov, J., Sotova, C., & Sadov, I. (2020). Investigation of the Influence of Microdroplets on the Coatings Nanolayer Structure. Coatings, 10(12), 1204. https://doi.org/10.3390/coatings10121204







