Significant Improvement of Anticorrosion Properties of Zinc-Containing Coating Using Sodium Polystyrene Sulfonate Noncovalent Modified Graphene Dispersions
Abstract
1. Introduction
2. Experimental
2.1. Materials
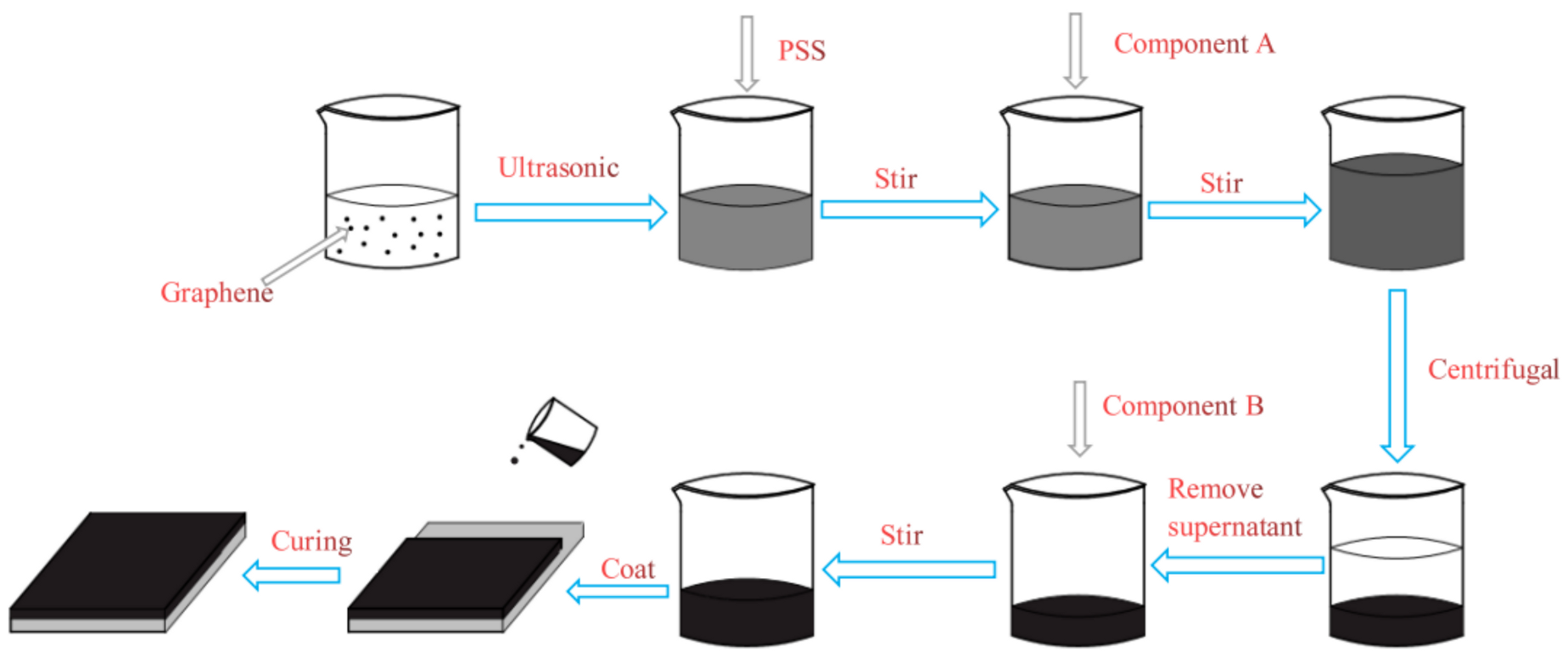
2.2. Preparation of Graphene Dispersion Modified by PSS
2.3. Preparation of Composite Coatings
2.4. Characterization
3. Results and Discussion
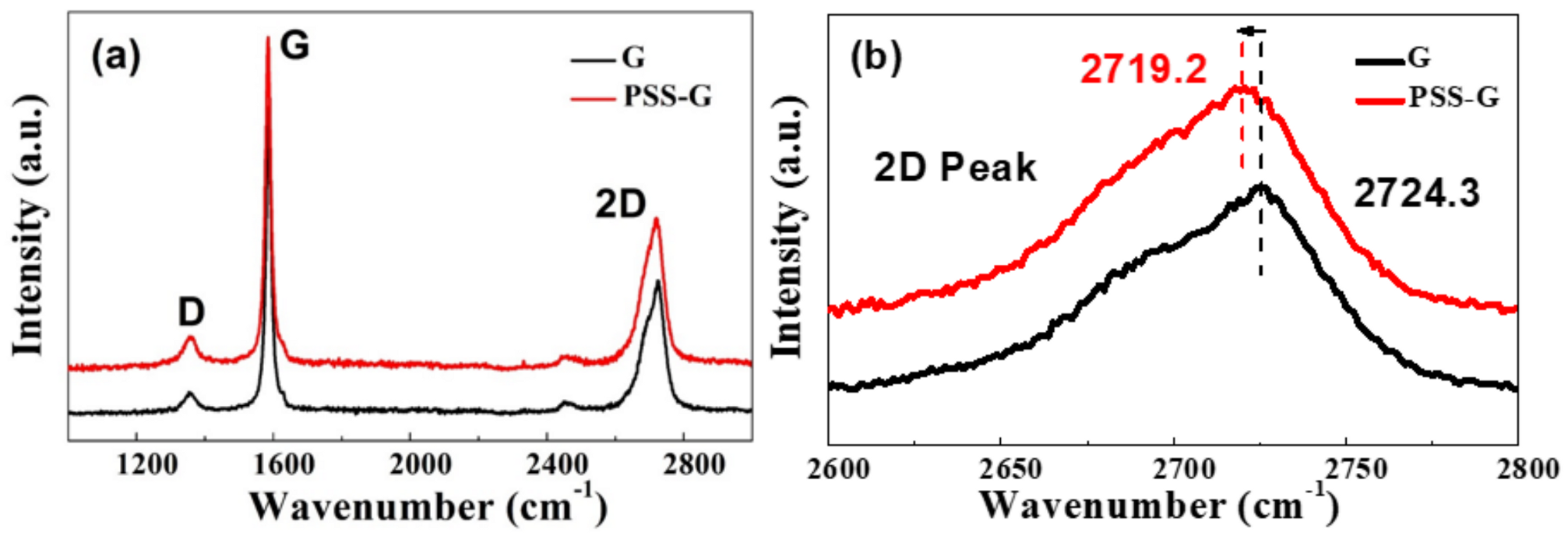
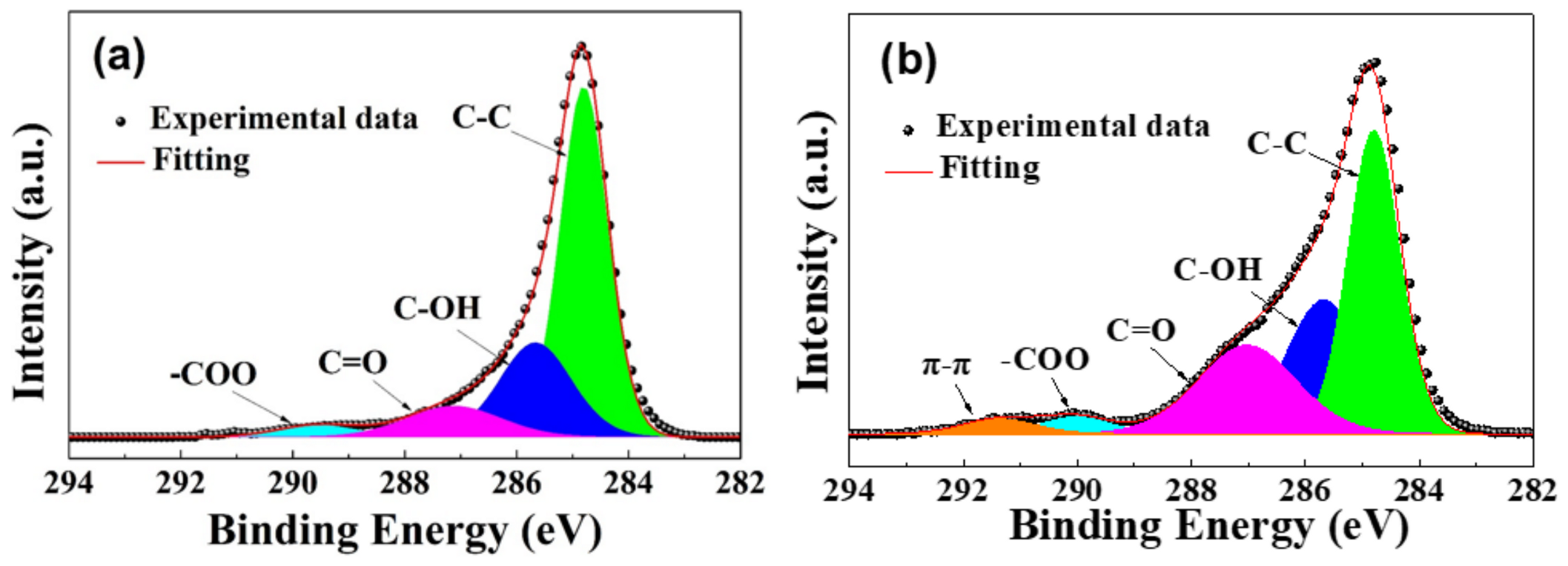
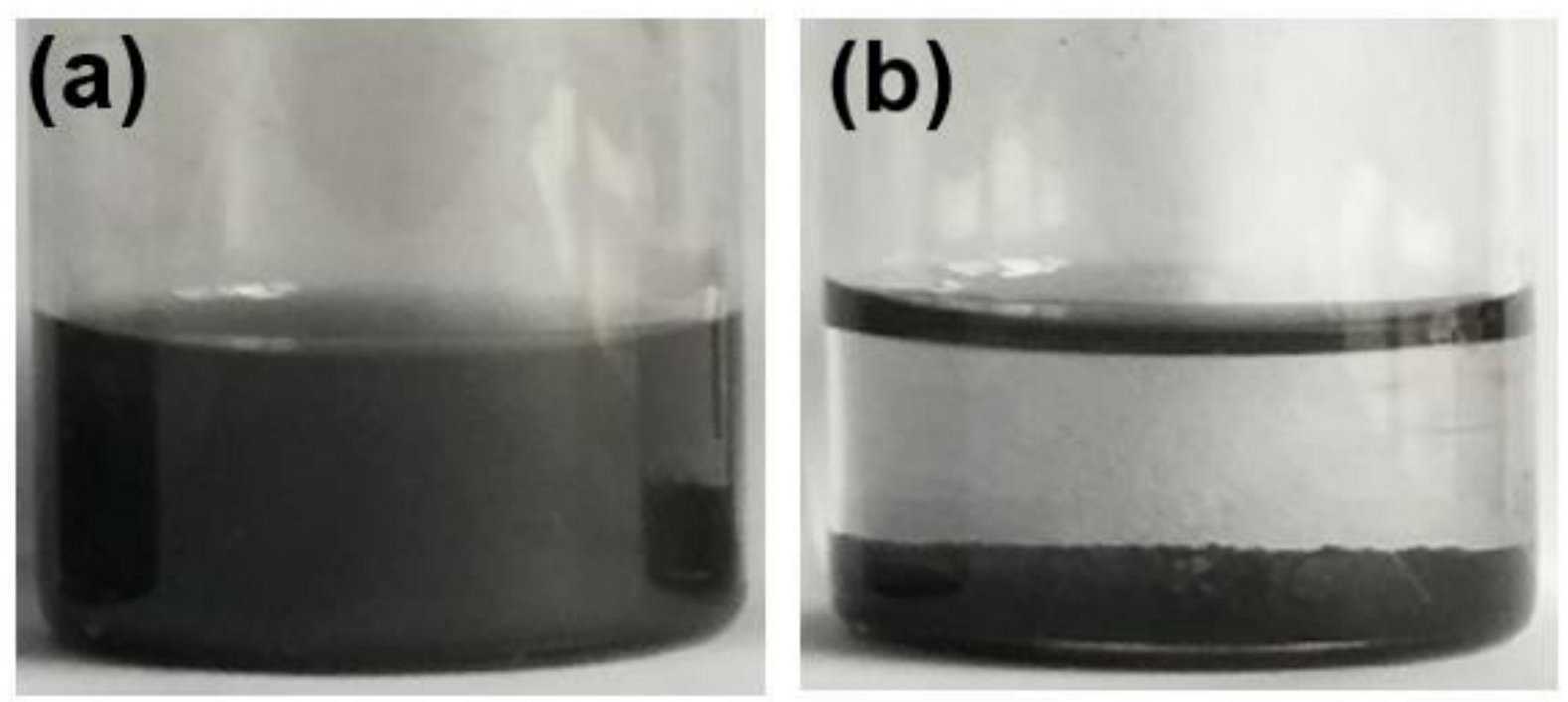
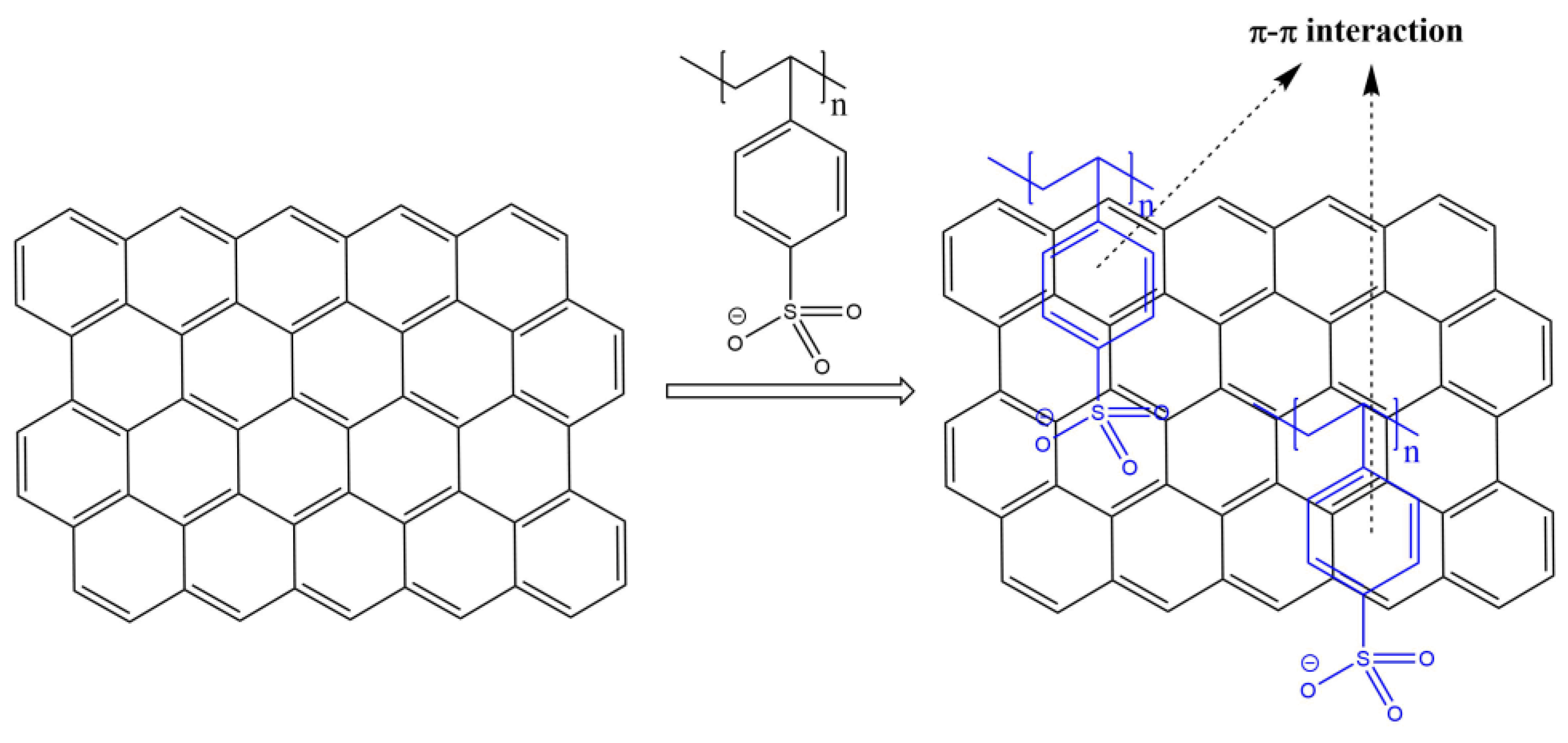
3.1. Characterization of PSS-G
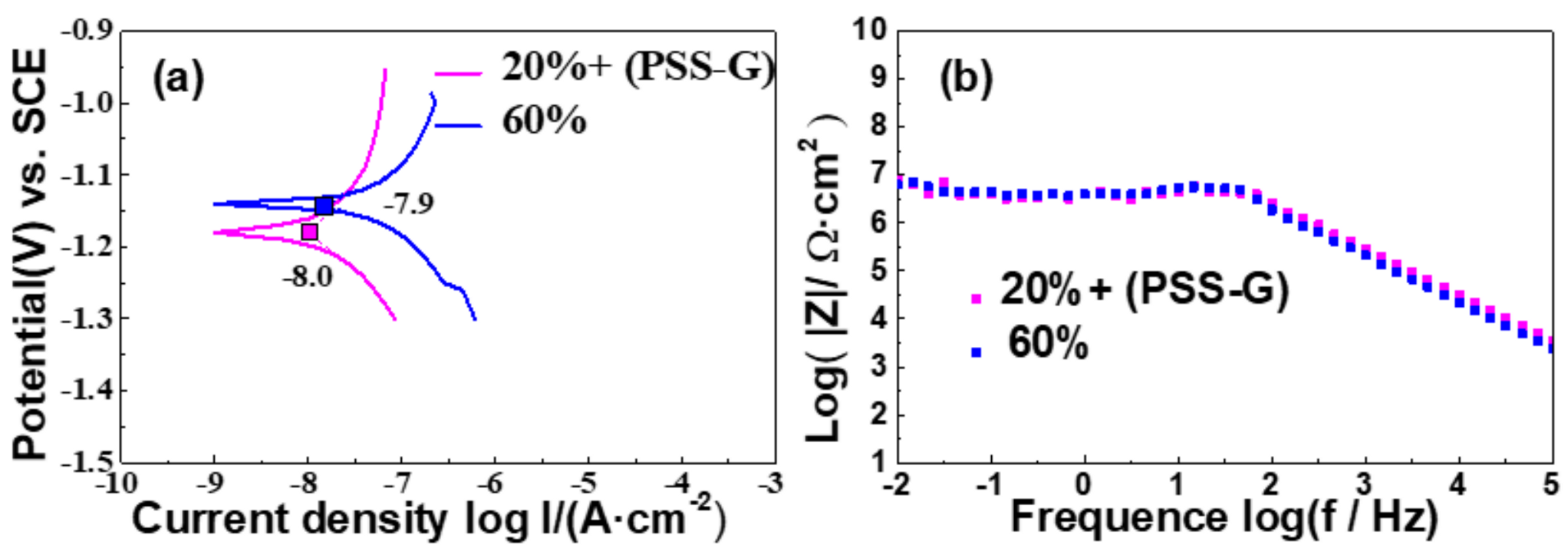
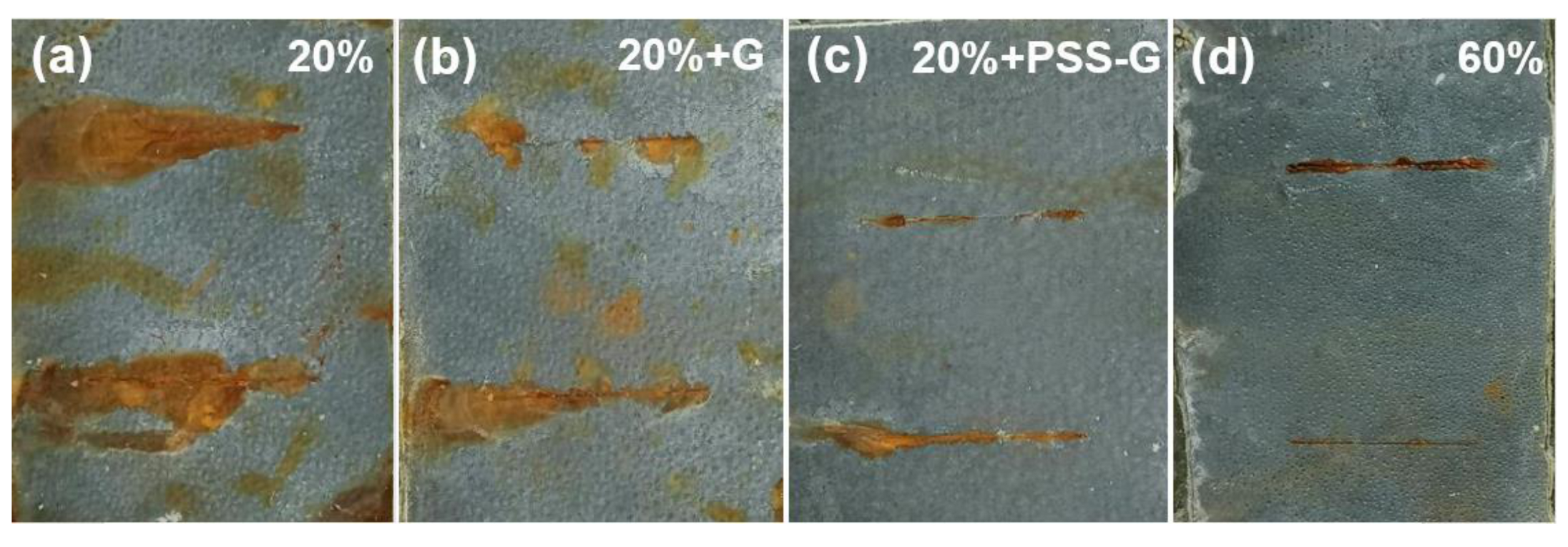
3.2. Anticorrosion Properties of PSS-G Coatings
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hayatdavoudi, H.; Rahsepar, M. A mechanistic study of the enhanced cathodic protection performance of graphene-reinforced zinc rich nanocomposite coating for corrosion protection of carbon steel substrate. J. Alloys Compd. 2017, 727, 1148–1156. [Google Scholar] [CrossRef]
- Marchebois, H.; Touzain, S.; Joiret, S.; Bernard, J.; Savall, C. Zinc-rich powder coatings corrosion in sea water: Influence of conductive pigments. Prog. Org. Coat. 2002, 45, 415–421. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Schiller, T.; Medhekar, N.; Birbilis, N. Exploring graphene as a corrosion protection barrier. Corros. Sci. 2012, 56, 1–4. [Google Scholar] [CrossRef]
- Chang, C.-H.; Huang, T.-C.; Peng, C.-W.; Yeh, T.-C.; Lu, H.-I.; Hung, W.-I.; Weng, C.-J.; Yang, T.-I.; Yeh, J.-M. Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 2012, 50, 5044–5051. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z. A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Yu, Y.-H.; Lin, Y.-Y.; Lin, C.-H.; Chan, C.-C.; Huang, Y.-C. High-performance polystyrene/graphene-based nanocomposites with excellent anti-corrosion properties. Polym. Chem. 2014, 5, 535–550. [Google Scholar] [CrossRef]
- Sahu, S.C.; Samantara, A.K.; Seth, M.; Parwaiz, S.; Singh, B.P.; Rath, P.C.; Jena, B.K. A facile electrochemical approach for development of highly corrosion protective coatings using graphene nanosheets. Electrochem. Commun. 2013, 32, 22–26. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Randviir, E.P.; Brownson, D.A.C.; Banks, C.E. A decade of graphene research: Production, applications and outlook. Mater. Today 2014, 17, 426–432. [Google Scholar] [CrossRef]
- Texter, J. Graphene dispersions. Curr. Opin. Colloid Interface Sci. 2014, 19, 163–174. [Google Scholar] [CrossRef]
- Chen, C.; Qiu, S.; Cui, M.; Qin, S.; Yan, G.; Zhao, H.; Wang, L.; Xue, Q. Achieving high performance corrosion and wear resistant epoxy coatings via incorporation of noncovalent functionalized graphene. Carbon 2017, 114, 356–366. [Google Scholar] [CrossRef]
- Shih, C.-J.; Lin, S.; Strano, M.S.; Blankschtein, D. Understanding the stabilization of liquid-phase-exfoliated graphene in polar solvents: Molecular dynamics simulations and kinetic theory of colloid aggregation. J. Am. Chem. Soc. 2010, 132, 14638–14648. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Xu, Y.; Zhao, L.; Li, C.; Shi, G. Non-covalent functionalization of graphene sheets by sulfonated polyaniline. Chem. Commun. 2009, 13, 1667–1669. [Google Scholar] [CrossRef]
- Chang, H.; Wang, G.; Yang, A.; Tao, X.; Liu, X.; Shen, Y.; Zheng, Z. A transparent, flexible, low-temperature, and solution-processible graphene composite electrode. Adv. Funct. Mater. 2010, 20, 2893–2902. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Chen, X.Q.; Wu, N.Q.; Nguyen, S.T.; Ruoff, R.S. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Kratochvílová, I.; Ashcheulov, P.; ÅkarohlÃd, J.; Škoda, R.; Kopeček, J.; Sajdl, P.; Macák, J.; Lajčinová, M.; Nováková, A.; Neethling, J.; et al. Zr alloy protection against high-temperature oxidation: Coating by a double layered structure with active and passive functional properties. Corros. Sci. 2020, 163, 108270. [Google Scholar] [CrossRef]
- GB/T 1771-2007. Paints and Varnishes-Determination of Resistance to Neutral Salt Spray(fog); Standardization Administration of China: Beijing, China, 2007. [Google Scholar]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B. 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Trung Dung, D.; Lee H-i Jeong, H.M.; Kim, B.K. Direct covalent modification of thermally exfoliated graphene forming functionalized graphene stably dispersible in water and poly(vinyl alcohol). Colloid Polym. Sci. 2013, 291, 2365–2374. [Google Scholar]
- Gao, J.; Liu, F.; Liu, Y.; Ma, N.; Wang, Z.; Zhang, X. Environment-friendly method to produce graphene that employs vitamin C and amino acid. Chem. Mater. 2010, 22, 2213–2218. [Google Scholar] [CrossRef]
- Kear, G.; Barker, B.D.; Walsh, F.C. Electrochemical corrosion of unalloyed copper in chloride media—A critical review. Corros. Sci. 2004, 46, 109–135. [Google Scholar] [CrossRef]
- Li, P.; He, X.; Huang, T.-C.; White, K.L.; Zhang, X.; Liang, H.; Nishimura, R.; Sue, H.J. Highly effective anti-corrosion epoxy spray coatings containing self-assembled clay in smectic order. J. Mater. Chem. A 2015, 3, 2669–2676. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Niroumandrad, S.; Ahmadi, A.; Mahdavian, M.; Moghadam, M.H.M. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide. Corros. Sci. 2016, 103, 283–304. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Niu, G.; Bai, W.; Ma, Y.; Xiong, Q.; Qin, C.; Zhang, J.; An, R.; Ren, W. Significant Improvement of Anticorrosion Properties of Zinc-Containing Coating Using Sodium Polystyrene Sulfonate Noncovalent Modified Graphene Dispersions. Coatings 2020, 10, 1150. https://doi.org/10.3390/coatings10121150
Li J, Niu G, Bai W, Ma Y, Xiong Q, Qin C, Zhang J, An R, Ren W. Significant Improvement of Anticorrosion Properties of Zinc-Containing Coating Using Sodium Polystyrene Sulfonate Noncovalent Modified Graphene Dispersions. Coatings. 2020; 10(12):1150. https://doi.org/10.3390/coatings10121150
Chicago/Turabian StyleLi, Jiehui, Gang Niu, Wei Bai, Yanjie Ma, Qingren Xiong, Changyi Qin, Junjie Zhang, Ruihua An, and Wei Ren. 2020. "Significant Improvement of Anticorrosion Properties of Zinc-Containing Coating Using Sodium Polystyrene Sulfonate Noncovalent Modified Graphene Dispersions" Coatings 10, no. 12: 1150. https://doi.org/10.3390/coatings10121150
APA StyleLi, J., Niu, G., Bai, W., Ma, Y., Xiong, Q., Qin, C., Zhang, J., An, R., & Ren, W. (2020). Significant Improvement of Anticorrosion Properties of Zinc-Containing Coating Using Sodium Polystyrene Sulfonate Noncovalent Modified Graphene Dispersions. Coatings, 10(12), 1150. https://doi.org/10.3390/coatings10121150



