RETRACTED: New Imidazolium Ionic Liquids from Recycled Polyethylene Terephthalate Waste for Curing Epoxy Resins as Organic Coatings of Steel
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Imidazolium Ionic Liquid from PET Waste
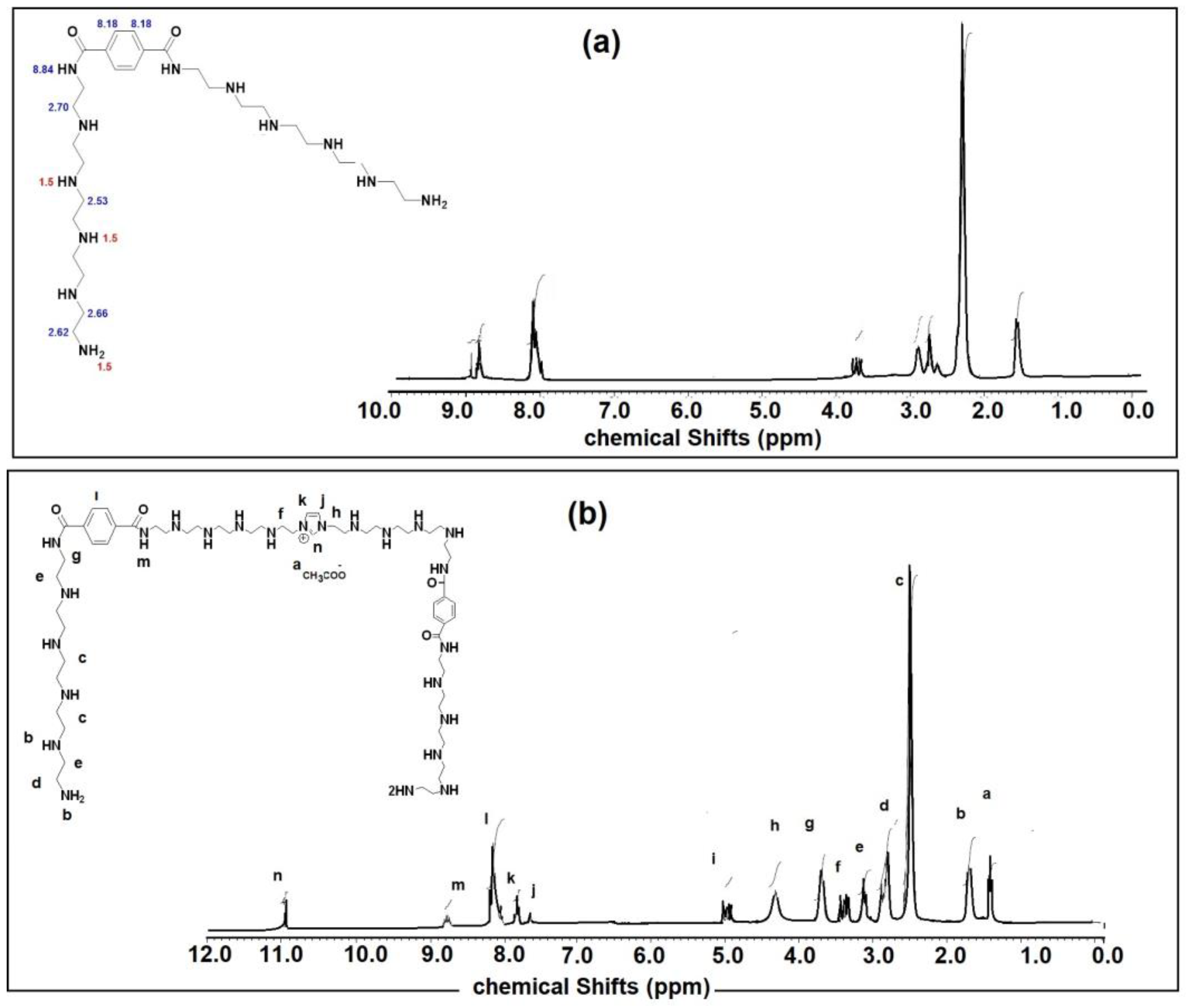
2.3. Characterization of APA and PAIIL
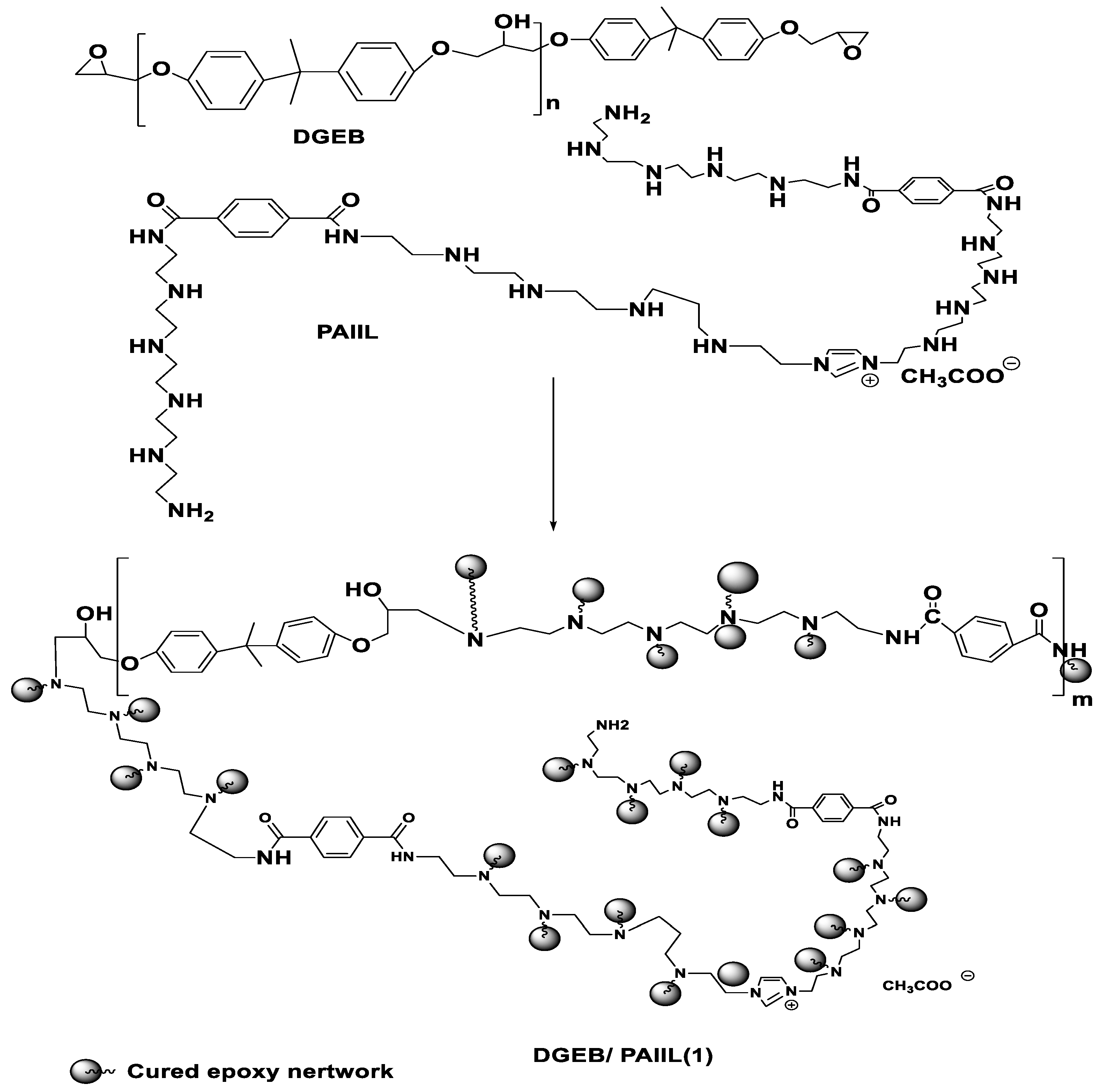
2.4. Curing of DGEB/PAIIL System
2.5. Application of DGEB/PAIIL as Coatings for Steel
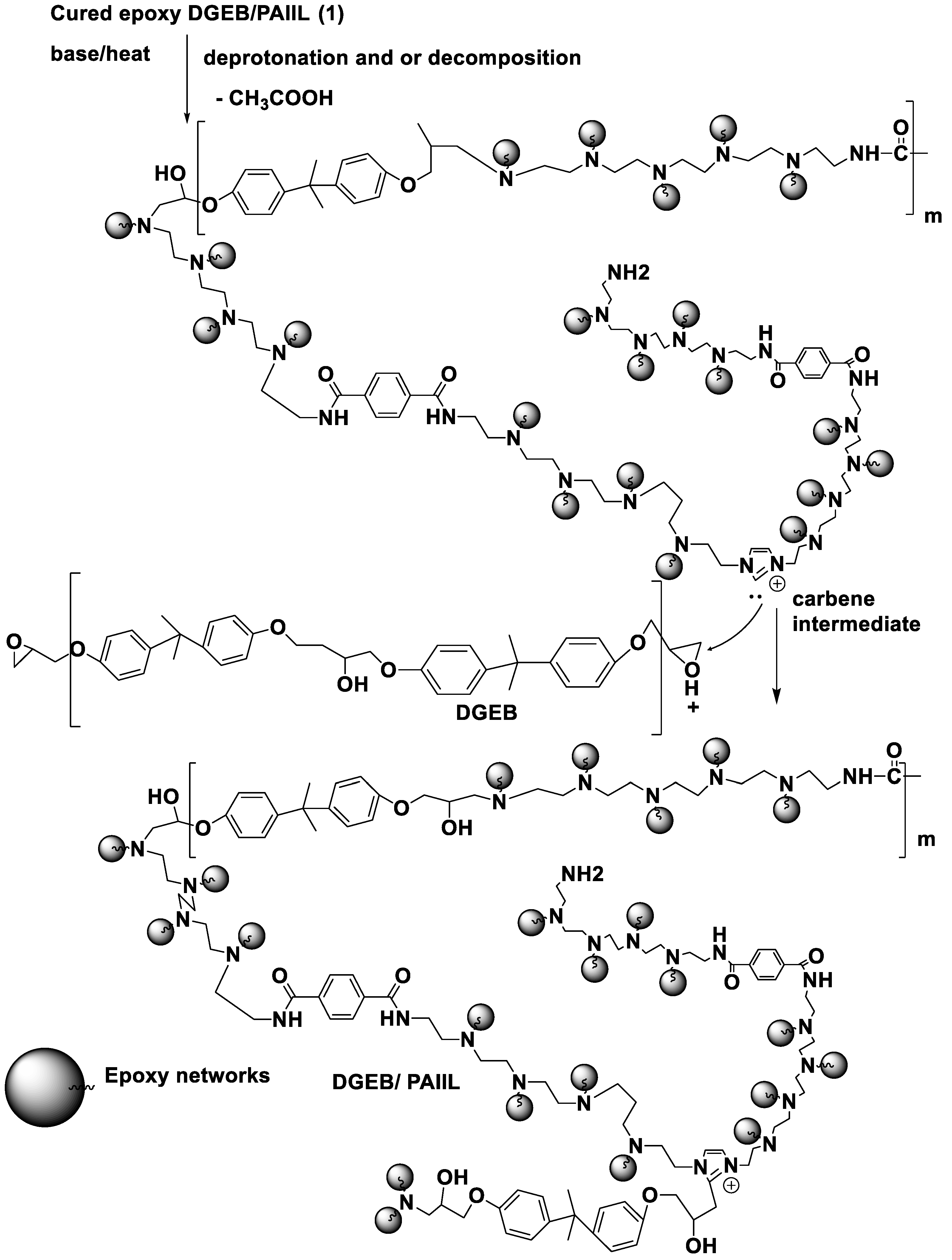
3. Results and Discussion
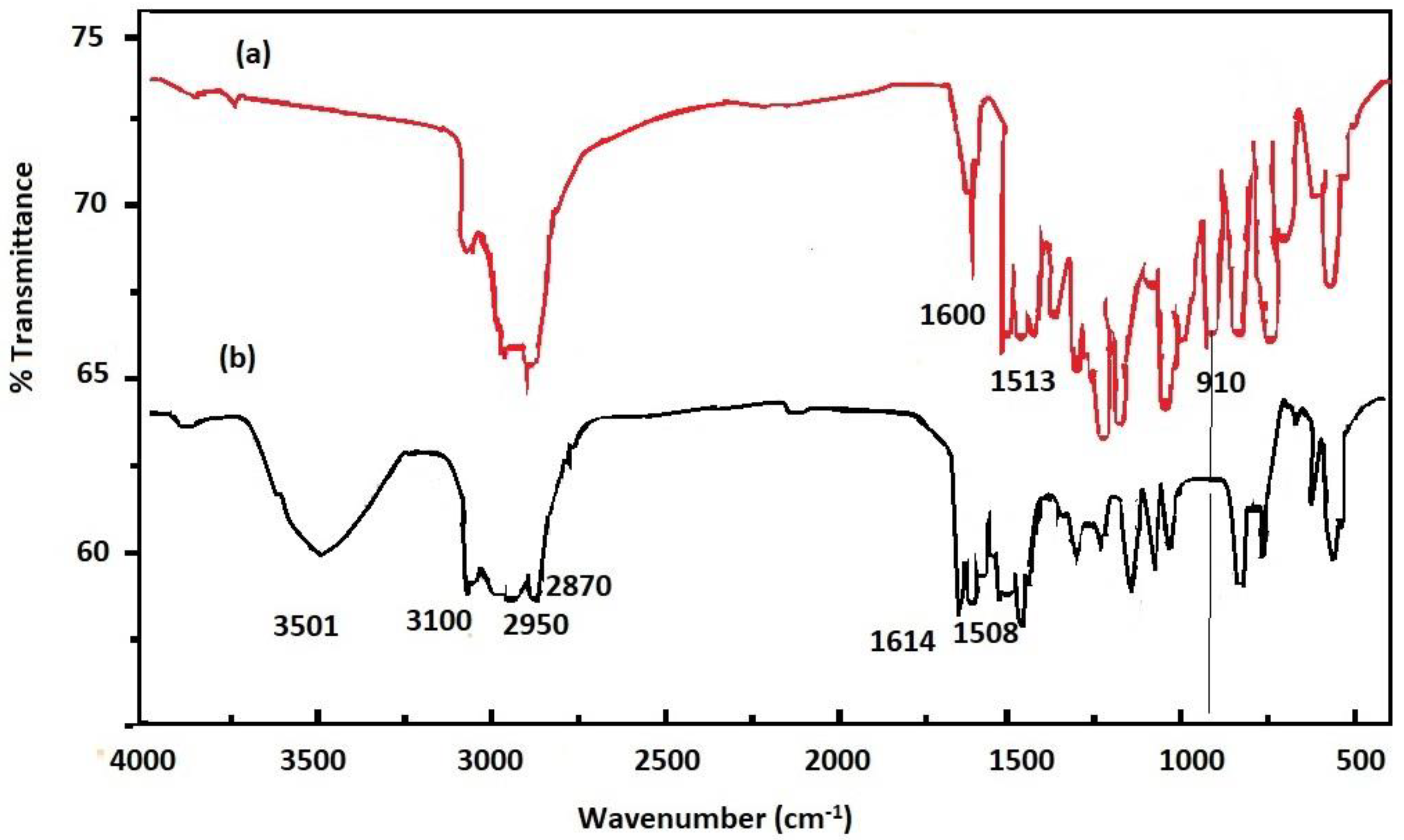
3.1. Characterization of APA and PAIIL
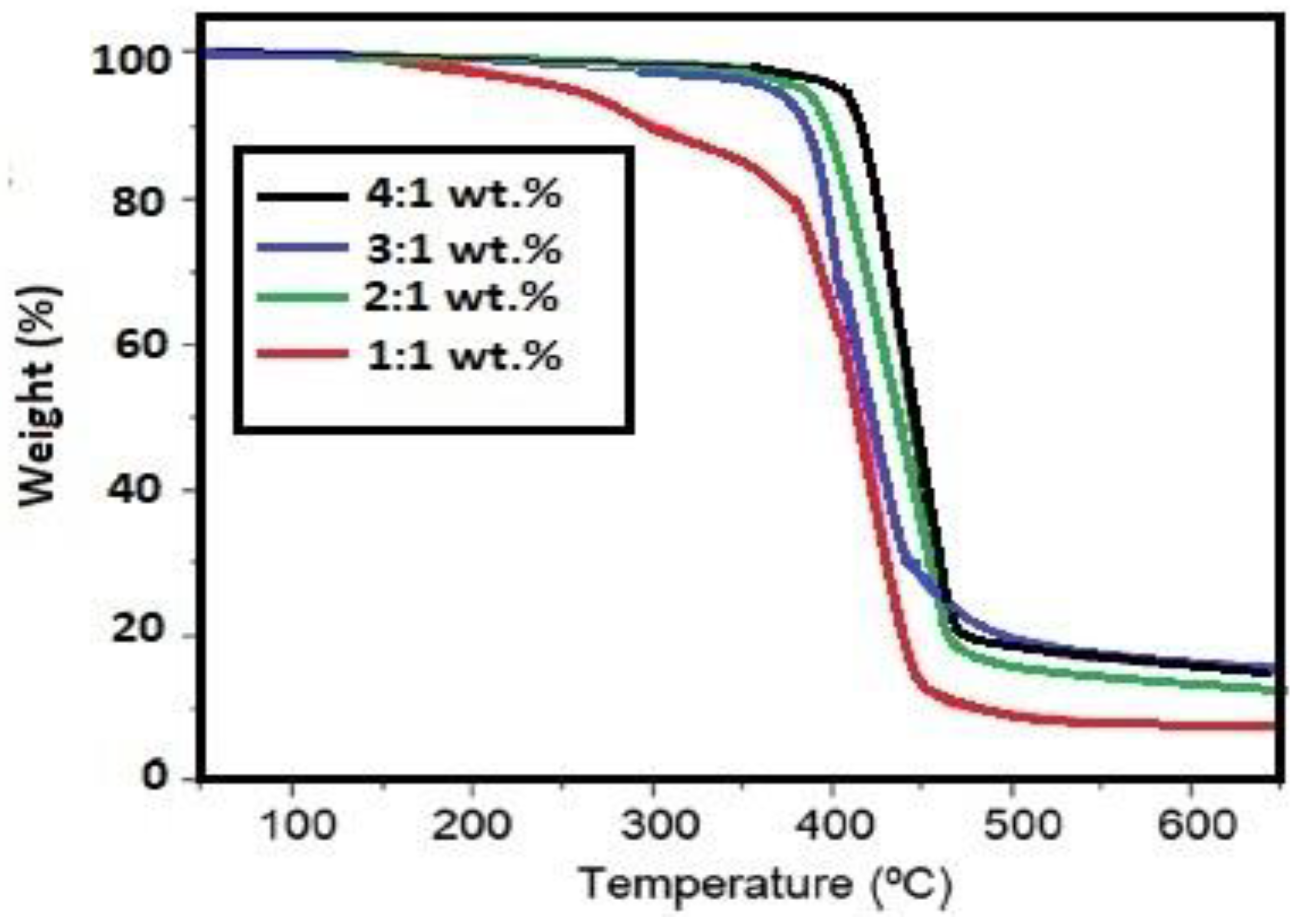
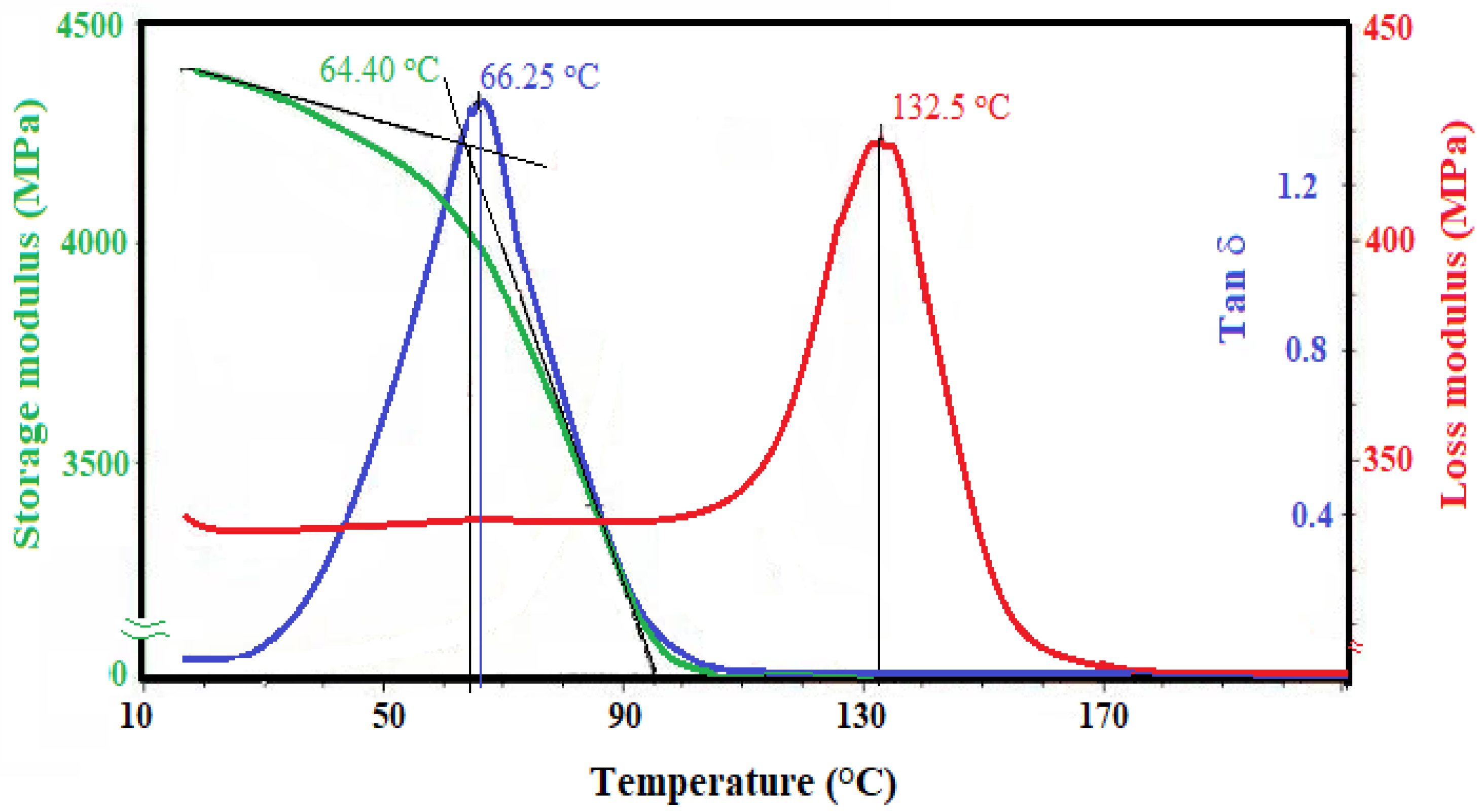
3.2. Curing of DGEB Epoxy with PAIIL
3.3. Adhesion and Salt Spray Resistance of DGEB/PAIIL
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prolongo, S.G.; del Rosario, G.; Ureña, A. Comparative study on the adhesive properties of different epoxy resins. Int. J. Adhes. Adhes. 2006, 26, 125–132. [Google Scholar] [CrossRef]
- Wan, J.; Li, C.; Bu, Z.-Y.; Fan, H.; Li, B.-G. Evaluating a four-directional benzene-centered aliphatic polyamine curing agent for epoxy resins. J. Therm. Anal. Calorim. 2013, 114, 365–375. [Google Scholar] [CrossRef]
- Benedetti, A.; Fernandes, P.; Granja, J.L.; Sena-Cruz, J.; Azenha, M. Influence of temperature on the curing of an epoxy adhesive and its influence on bond behaviour of NSM-CFRP systems. Compos. Part B Eng. 2016, 89, 219–229. [Google Scholar] [CrossRef]
- Unnikrishnan, K.; Thachil, E.T. Toughening of epoxy resins. Des. Monomers Polym. 2006, 9, 129–152. [Google Scholar] [CrossRef]
- Silva, P.; Fernandes, P.; Sena-Cruz, J.; Xavier, J.; Castro, F.; Soares, D.; Carneiro, V. Effects of different environmental conditions on the mechanical characteristics of a structural epoxy. Compos. Part B Eng. 2016, 88, 55–63. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, X.; Yu, X. Reaction mechanism, cure behavior and properties of a multifunctional epoxy resin, TGDDM, with latent curing agent dicyandiamide. RSC Adv. 2018, 8, 8248–8258. [Google Scholar] [CrossRef]
- Wang, L.; Shui, X.; Zheng, X.; You, J.; Li, Y. Investigations on the morphologies and properties of epoxy/acrylic rubber/nanoclay nanocomposites for adhesive films. Compos. Sci. Technol. 2014, 93, 46–53. [Google Scholar] [CrossRef]
- Liu, X.D.; Zhao, C.H.; Sudo, A.; Endo, T. Storage stability and curing behavior of epoxy-dicyandiamide systems with carbonyldiimidazole-Cu (II) complexes as the accelerator. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3470–3476. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.Z.; Fu, Y.F.; Liu, X.D. Latent curing systems stabilized by reaction equilibrium in homogeneous mixtures of benzoxazine and amine. Sci. Rep. 2016, 6, 38584. [Google Scholar] [CrossRef]
- Vietri, U.; Guadagno, L.; Raimondo, M.; Vertuccio, L.; Lafdi, K. Nanofilled epoxy adhesive for structural aeronautic materials. Compos. Part B Eng. 2014, 61, 73–83. [Google Scholar] [CrossRef]
- Kong, X.; Xu, Z.; Guan, L.; Di, M. Study on polyblending epoxy resin adhesive with lignin I-curing temperature. Int. J. Adhes. Adhes. 2014, 48, 75–79. [Google Scholar] [CrossRef]
- Zhao, C.H.; Wan, S.J.; Wang, L.; Liu, X.D.; Endo, T. Carbonyldiimidazole-accelerated efficient cure of epoxidized soybean oil with dicyandiamide. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 375–382. [Google Scholar] [CrossRef]
- Acebo, C.; Fernández-Francos, X.; Ferrando, F.; Serra, À.; Salla, J.M.; Ramis, X. Multiarm star with poly (ethyleneimine) core and poly (ε-caprolactone) arms as modifiers of diglycidylether of bisphenol A thermosets cured by 1-methylimidazole. React. Funct. Polym. 2013, 73, 431–441. [Google Scholar] [CrossRef]
- Lee, D.H.; Yang, M.; Kim, S.H.; Shin, M.J.; Shin, J.S. Microencapsulation of imidazole curing agents by spray-drying method. J. Appl. Polym. Sci. 2011, 122, 782–788. [Google Scholar] [CrossRef]
- Shin, M.J.; Kim, J.G.; Shin, J.S. Microencapsulation of imidazole curing agents by spray-drying method using W/O emulsion. J. Appl. Polym. Sci. 2012, 126, E108–E115. [Google Scholar] [CrossRef]
- Soares, B.G.; Livi, S.; Duchet-Rumeau, J.; Gerard, J.F. Synthesis and characterization of epoxy/MCDEA networks modified with imidazolium-based ionic liquids. Macromol. Mater. Eng. 2011, 296, 826–834. [Google Scholar] [CrossRef]
- Pire, M.; Norvez, S.; Iliopoulos, I.; Le Rossignol, B.; Leibler, L. Imidazole-promoted acceleration of crosslinking in epoxidized natural rubber/dicarboxylic acid blends. Polymer 2011, 52, 5243–5249. [Google Scholar] [CrossRef]
- Atta, A.M.; Ezzat, A.O.; El-Saeed, A.M.; Tawfeek, A.M.; Sabeela, N.I. Self-healing of chemically bonded hybrid silica/epoxy for steel Coating. Prog. Org. Coat. 2020, 141, 105549. [Google Scholar] [CrossRef]
- Atta, A.M.; El-Faham, A.; Al-Lohedan, H.A.; Othman, Z.A.A.; Abdullah, M.M.; Ezzat, A.O. Modified triazine decorated with Fe3O4 and Ag/Ag2O nanoparticles for self-healing of steel epoxy coatings in seawater. Prog. Org. Coat. 2018, 121, 247–262. [Google Scholar] [CrossRef]
- Atta, A.M.; Al-Hodan, H.A.; Hameed, R.S.A.; Ezzat, A.O. Preparation of green cardanol-based epoxy and hardener as primer coatings for petroleum and gas steel in marine environment. Prog. Org. Coat. 2017, 111, 283–293. [Google Scholar] [CrossRef]
- Achilias, D.; Karayannidis, G. The chemical recycling of PET in the framework of sustainable development. Water Air Soil Pollut. Focus 2004, 4, 385–396. [Google Scholar] [CrossRef]
- George, N.; Kurian, T. Recent developments in the chemical recycling of postconsumer poly (ethylene terephthalate) waste. Ind. Eng. Chem. Res. 2014, 53, 14185–14198. [Google Scholar] [CrossRef]
- Atta, A.M.; El-Kafrawy, A.F.; Aly, M.H.; Abdel-Azim, A.-A.A. New epoxy resins based on recycled poly (ethylene terephthalate) as organic coatings. Prog. Org. Coat. 2007, 58, 13–22. [Google Scholar] [CrossRef]
- Atta, A. ElsaeedS. M. and Abdel-Azim AA. Prog. Org. Coat. 2006, 12, 50–55. [Google Scholar] [CrossRef]
- Atta, A.M.; Elnagdy, S.I.; Abdel-Raouf, M.E.; Elsaeed, S.M.; Abdel-Azim, A.-A.A. Compressive properties and curing behaviour of unsaturated polyester resins in the presence of vinyl ester resins derived from recycled poly (ethylene terephthalate). J. Polym. Res. 2005, 12, 373–383. [Google Scholar] [CrossRef]
- Spychaj, T.; Fabrycy, E.; Spychaj, S.; Kacperski, M. Aminolysis and aminoglycolysis of waste poly (ethylene terephthalate). J. Mater. Cycles Waste Manag. 2001, 3, 24–31. [Google Scholar]
- Kárpáti, L.; Fejér, M.; Kalocsai, D.; Molnár, J.; Vargha, V. Synthesis and characterization of isophorondiamine based epoxy hardeners from aminolysis of PET. eXPRESS Polym. Lett. 2019, 7, 618–631. [Google Scholar] [CrossRef]
- Dutt, K.; Soni, R. Synthesis and characterization of bis-amino ethyl terephthalamide from PET waste and its applications as hardener in DGEBA. Int. J. Plast. Technol. 2014, 18, 16–26. [Google Scholar] [CrossRef]
- Ehlers, J.-E.; Rondan, N.G.; Huynh, L.K.; Pham, H.; Marks, M.; Truong, T.N. Theoretical study on mechanisms of the epoxy− amine curing reaction. Macromolecules 2007, 40, 4370–4377. [Google Scholar] [CrossRef]
- Yamada, T.; Tominari, Y.; Tanaka, S.; Mizuno, M. Infrared spectroscopy of ionic liquids consisting of imidazolium cations with different alkyl chain lengths and various halogen or molecular anions with and without a small amount of water. J. Phys. Chem. B 2017, 121, 3121–3129. [Google Scholar] [CrossRef] [PubMed]
- Тютюкин, К.В.; Маркелов, Д.А.; Матвеев, В.В.; Иевлев, А.В. Molecular mobility in several imidazolium-based ionic liquids according to data of 1H and 13C NMR relaxation. Magn. Reson. Chem. 2018, 56, 140–143. [Google Scholar]
- Jog, J. Crystallization of polyethyleneterephthalate. J. Macromol. Sci. Part C Polym. Rev. 1995, 35, 531–553. [Google Scholar] [CrossRef]
- Souda, R. Glass-Liquid Transition, Crystallization, and Melting of a Room Temperature Ionic Liquid: Thin Films of 1-Ethyl-3-methylimidazolium Bis [trifluoromethanesulfonyl] imide Studied with TOF-SIMS. J. Phys. Chem. B 2008, 112, 15349–15354. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Tiwari, P. Thermal degradation study of waste polyethylene terephthalate (PET) under inert and oxidative environments. Thermochim. Acta 2019, 679, 178340. [Google Scholar] [CrossRef]
- Maka, H.; Spychaj, T.; Pilawka, R. Epoxy resin/ionic liquid systems: The influence of imidazolium cation size and anion type on reactivity and thermomechanical properties. Ind. Eng. Chem. Res. 2012, 51, 5197–5206. [Google Scholar] [CrossRef]
- Carvalho, A.P.A.; Santos, D.F.; Soares, B.G. Epoxy/imidazolium-based ionic liquid systems: The effect of the hardener on the curing behavior, thermal stability, and microwave absorbing properties. J. Appl. Polym. Sci. 2020, 137, 48326. [Google Scholar] [CrossRef]
- Binks, F.C.; Cavalli, G.; Henningsen, M.; Howlin, B.J.; Hamerton, I. Investigating the mechanism through which ionic liquids initiate the polymerisation of epoxy resins. Polymer 2018, 139, 163–176. [Google Scholar] [CrossRef]
- Clough, M.T.; Geyer, K.; Hunt, P.A.; Mertes, J.; Welton, T. Thermal decomposition of carboxylate ionic liquids: Trends and mechanisms. Phys. Chem. Chem. Phys. 2013, 15, 20480–20495. [Google Scholar] [CrossRef]
- Jaques, N.G.; Barros, J.J.P.; dos Santos Silva, I.D.; Popp, M.; Kolbe, J.; Wellen, R.M.R. New approaches of curing and degradation on epoxy/eggshell composites. Compos. Part B Eng. 2020, 196, 108125. [Google Scholar] [CrossRef]
- Chena, J.-S.; Obera, C.K.; Poliksb, M.D.; Zhang, Y.; Wiesner, U.; Cohen, C. Controlled degradation of epoxy networks: Analysis of crosslink density and glass transition temperature changes in thermally reworkable thermosets. Polymer 2004, 45, 1939–1950. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, Q.; Liu, L.; Zhang, Y.; Du, F.; Pang, A. Dual-Functionalized Imidazolium Ionic Liquids as Curing Agents for Epoxy Resins. Ind. Eng. Chem. Res. 2020, 59, 3024–3034. [Google Scholar] [CrossRef]
- Wei, H.; Xia, J.; Zhou, W.; Zhou, L.; Hussain, G.; Li, Q.; Ostrikov, K.K. Adhesion and cohesion of epoxy-based industrial composite coatings. Compos. Part B Eng. 2020, 193, 108035. [Google Scholar] [CrossRef]
- Dwivedi, D.; Lepková, K.; Becker, T. Carbon steel corrosion: A review of key surface properties and characterization methods. RSC Adv. 2017, 7, 4580–4610. [Google Scholar] [CrossRef]




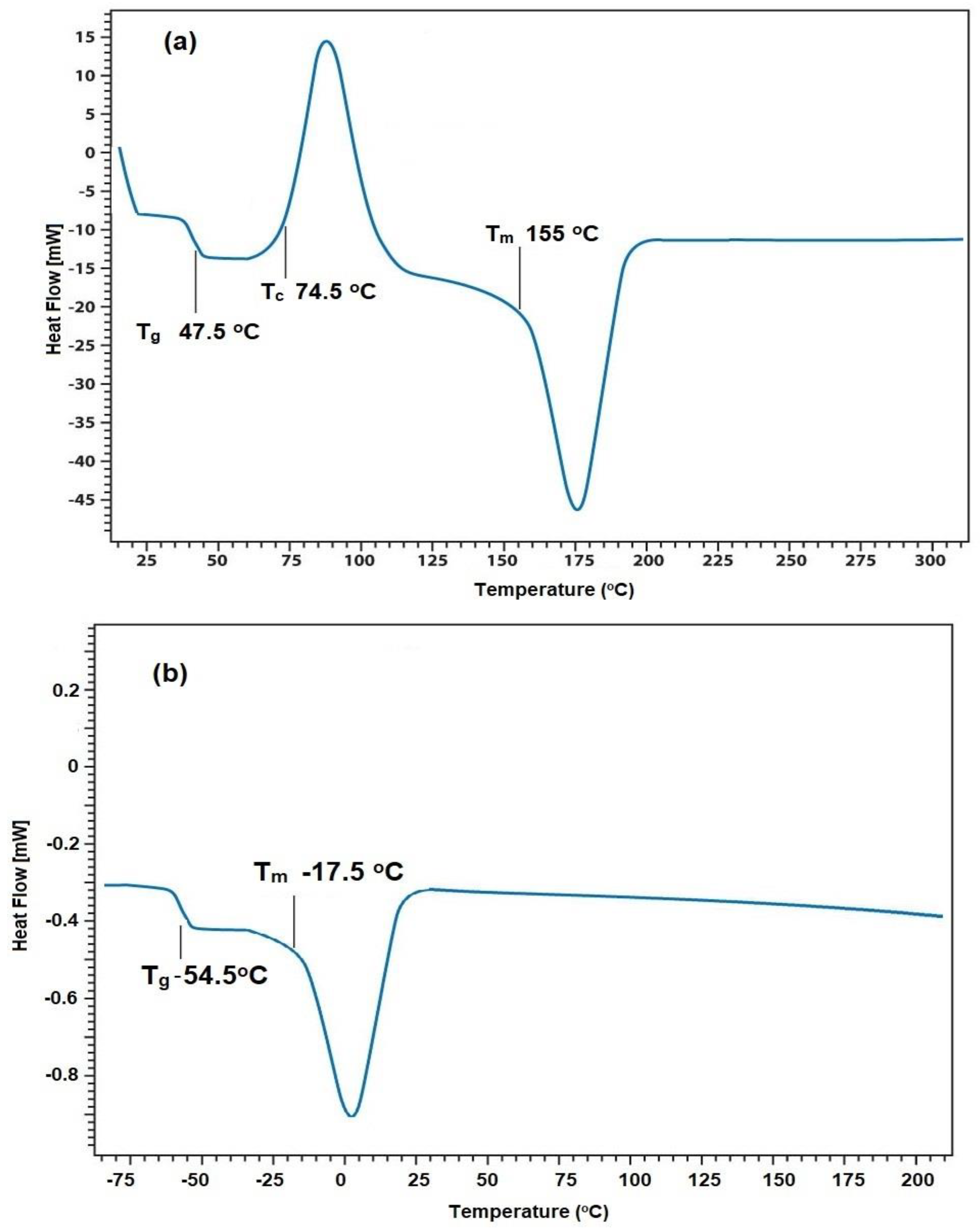
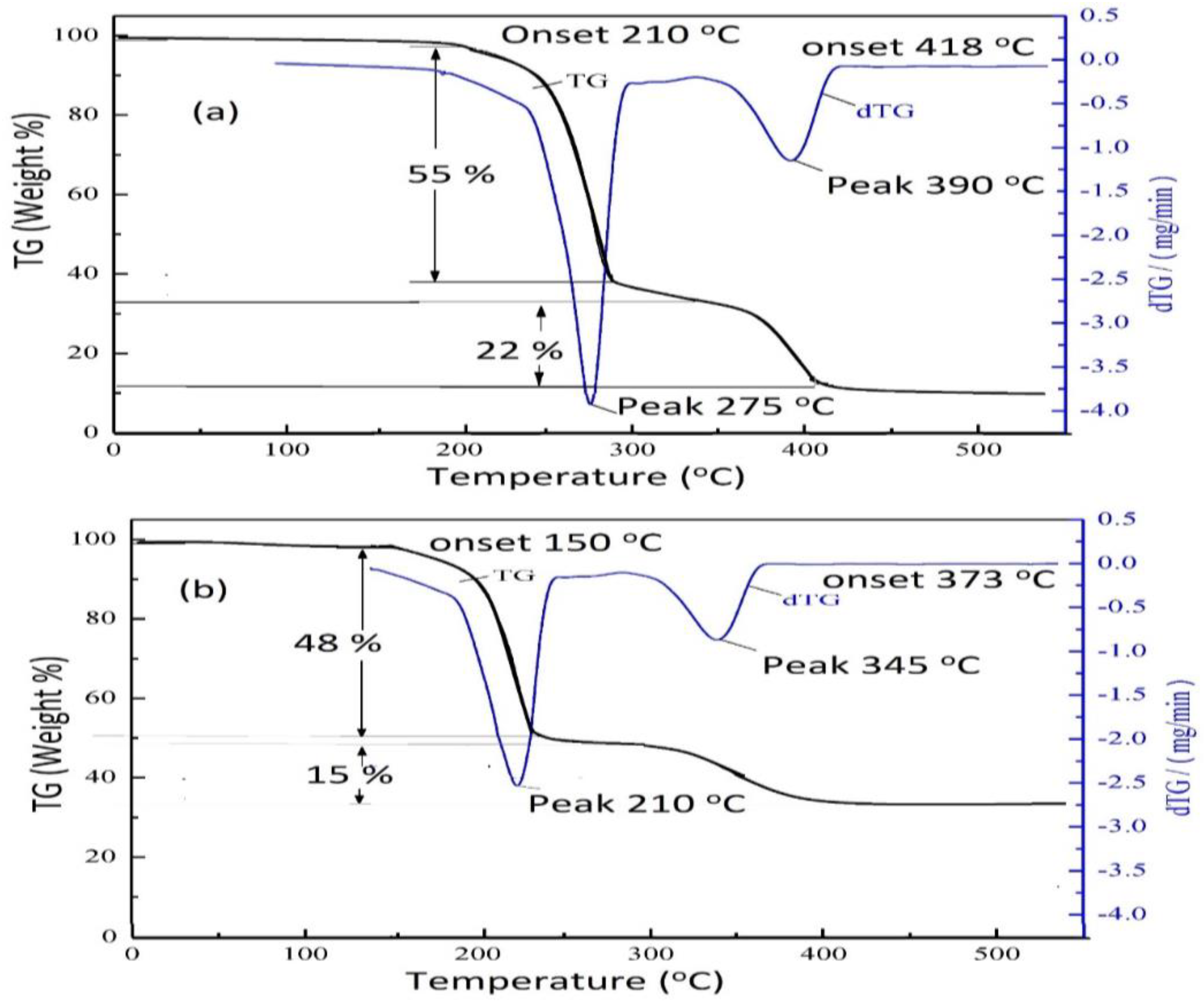
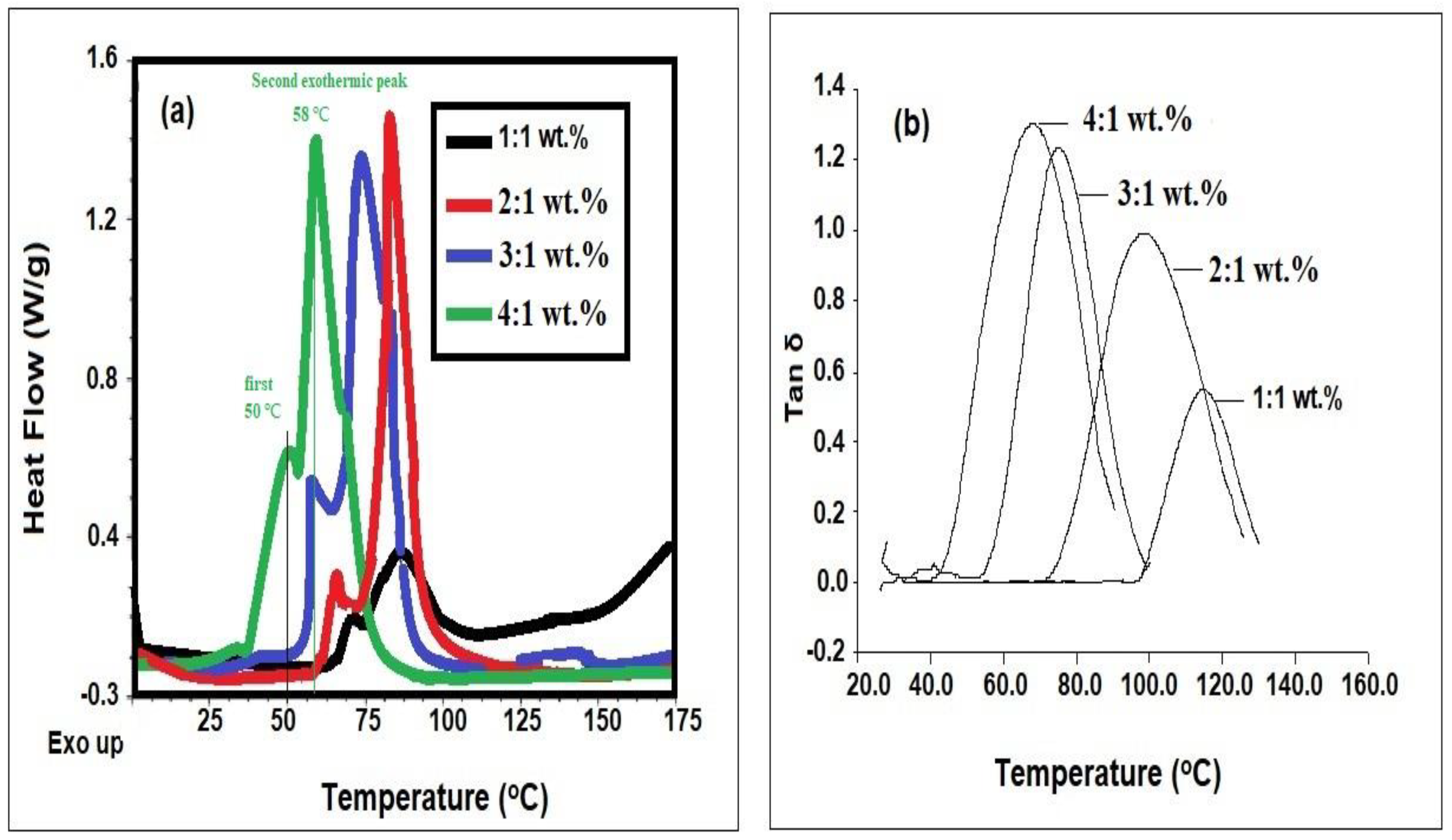

| DGEB/PAIIL (wt.%) | Tg DSC (°C) | ΔH (J g−1) | Tg DMA (°C) | ||
|---|---|---|---|---|---|
| First | Second | First | Second | ||
| 1:1 | 72 | 90 | 20 | 462 | 115 |
| 2:1 | 64 | 84 | 30 | 510 | 98 |
| 3:1 | 57 | 72 | 35 | 515 | 75 |
| 4:1 | 50 | 58 | 45 | 522 | 67 |
| DGEB/PAIIL (wt.%) | Tg (°C) | Ge (MPa) | Te (K) | ρ × 103 (mol dm−3) |
|---|---|---|---|---|
| 1:1 | 115 | 2200 | 453 | 0.5841 |
| 2:1 | 98 | 2800 | 436 | 0.7724 |
| 3:1 | 75 | 2600 | 413 | 0.757 |
| 4:1 | 67 | 4200 | 405 | 1.247 |
| DGEB/PAIIL (wt.%) | Adhesion Strength (MPa) | Exposure Time | Disbanded Area % | Rating Number (ASTM D-1654) |
|---|---|---|---|---|
| 1:1 | 4.5 | 500 | 5 ± 0.05 | 7 |
| 2:1 | 15 | 1500 | 2 ± 0.08 | 8 |
| 3:1 | 9.0 | 1000 | 10 ± 0.08 | 5 |
| 4:1 | 12.5 | 1500 | 2 ± 0.04 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atta, A.M.; Al-Lohedan, H.A.; Ezzat, A.O.; Sabeela, N.I. RETRACTED: New Imidazolium Ionic Liquids from Recycled Polyethylene Terephthalate Waste for Curing Epoxy Resins as Organic Coatings of Steel. Coatings 2020, 10, 1139. https://doi.org/10.3390/coatings10111139
Atta AM, Al-Lohedan HA, Ezzat AO, Sabeela NI. RETRACTED: New Imidazolium Ionic Liquids from Recycled Polyethylene Terephthalate Waste for Curing Epoxy Resins as Organic Coatings of Steel. Coatings. 2020; 10(11):1139. https://doi.org/10.3390/coatings10111139
Chicago/Turabian StyleAtta, Ayman M., Hamad A. Al-Lohedan, Abdelrahman O. Ezzat, and Nourah I. Sabeela. 2020. "RETRACTED: New Imidazolium Ionic Liquids from Recycled Polyethylene Terephthalate Waste for Curing Epoxy Resins as Organic Coatings of Steel" Coatings 10, no. 11: 1139. https://doi.org/10.3390/coatings10111139
APA StyleAtta, A. M., Al-Lohedan, H. A., Ezzat, A. O., & Sabeela, N. I. (2020). RETRACTED: New Imidazolium Ionic Liquids from Recycled Polyethylene Terephthalate Waste for Curing Epoxy Resins as Organic Coatings of Steel. Coatings, 10(11), 1139. https://doi.org/10.3390/coatings10111139





