Production of Microbial Cellulose Films from Green Tea (Camellia Sinensis) Kombucha with Various Carbon Sources
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Kombucha Cultures and Fermentation
2.3. Physicochemical Analysis of Fermentations
2.3.1. Determination of pH, Total Acidity, and a Total of Soluble Solids
2.3.2. Determination of Total Sugars and Proteins
2.3.3. Color Analysis
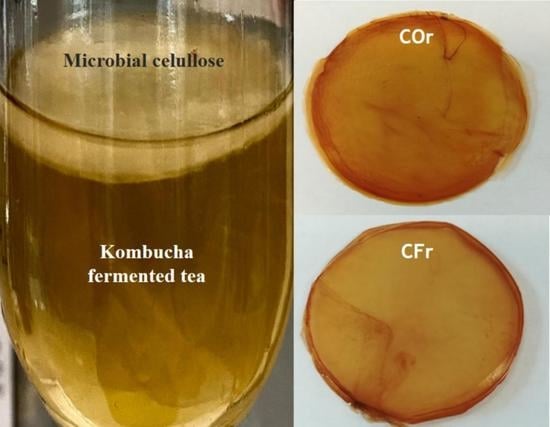
2.3.4. Production of MCFs
2.3.5. Determination of Wet Weight, Dry Weight, and Yields of the MCFs
2.4. Statistical Analysis
3. Results and Discussion
3.1. pH, TSS and TA
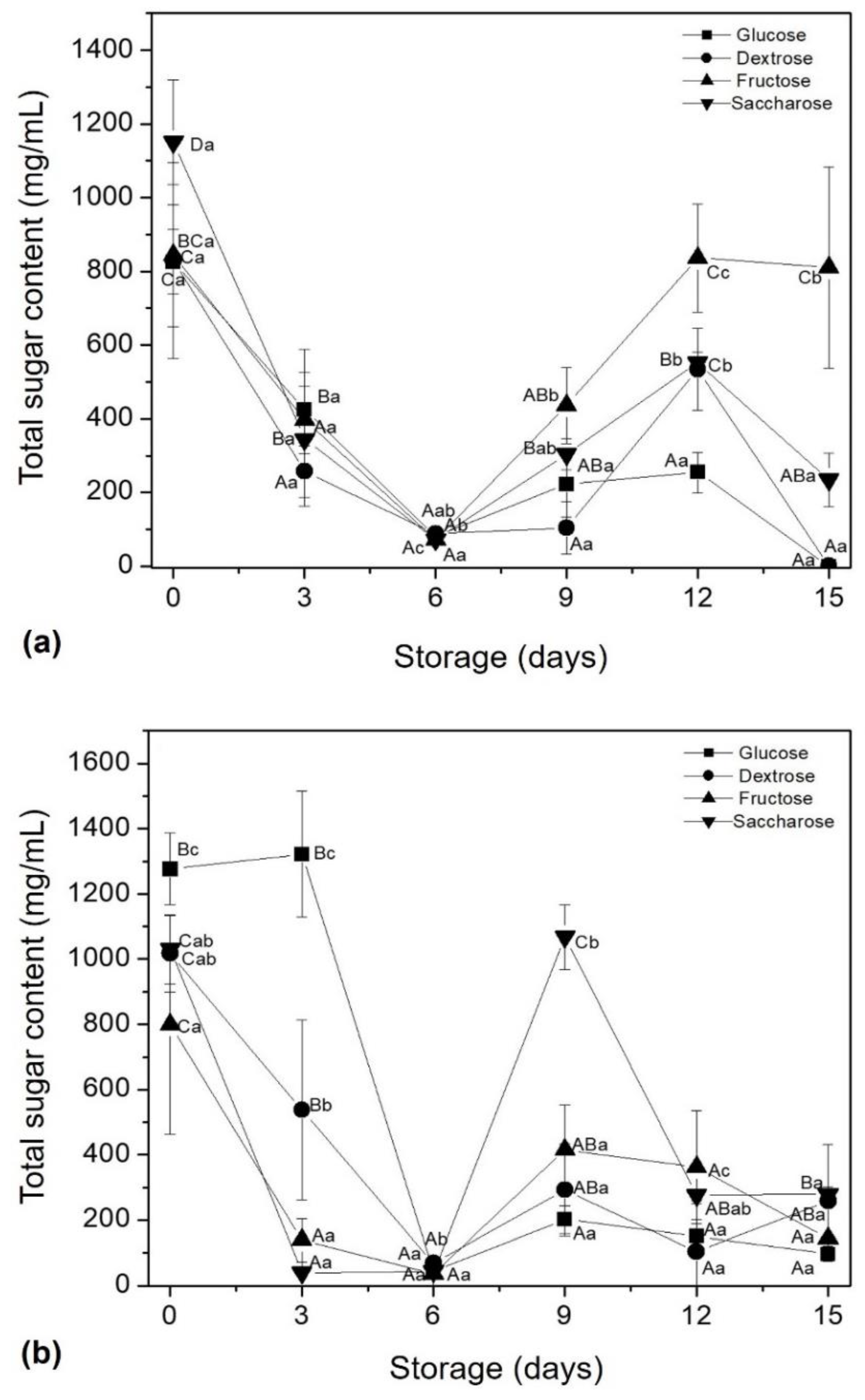
3.2. Total of Sugars and Protein Content
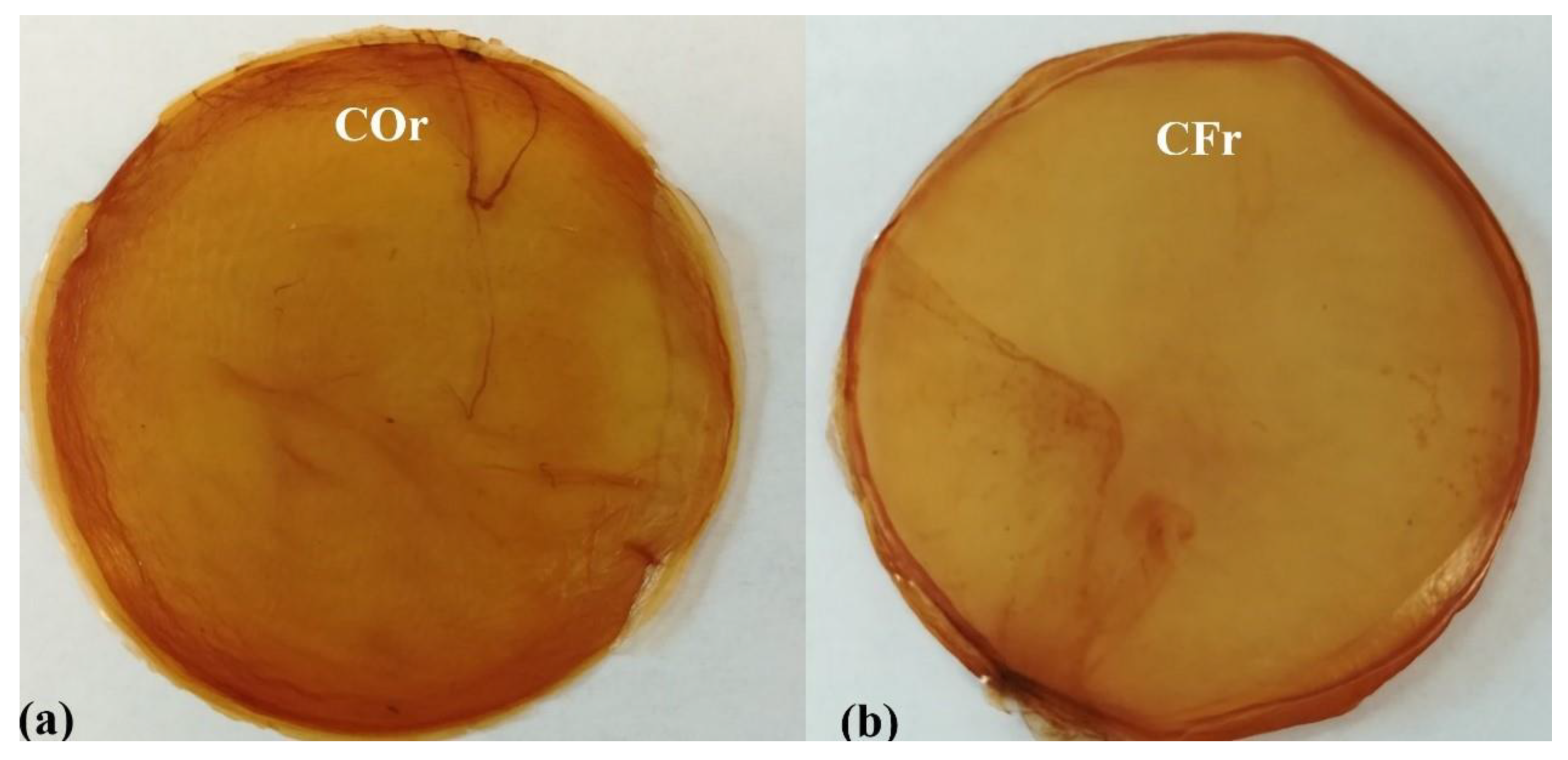
3.3. Color Analysis and Formation of MCFs
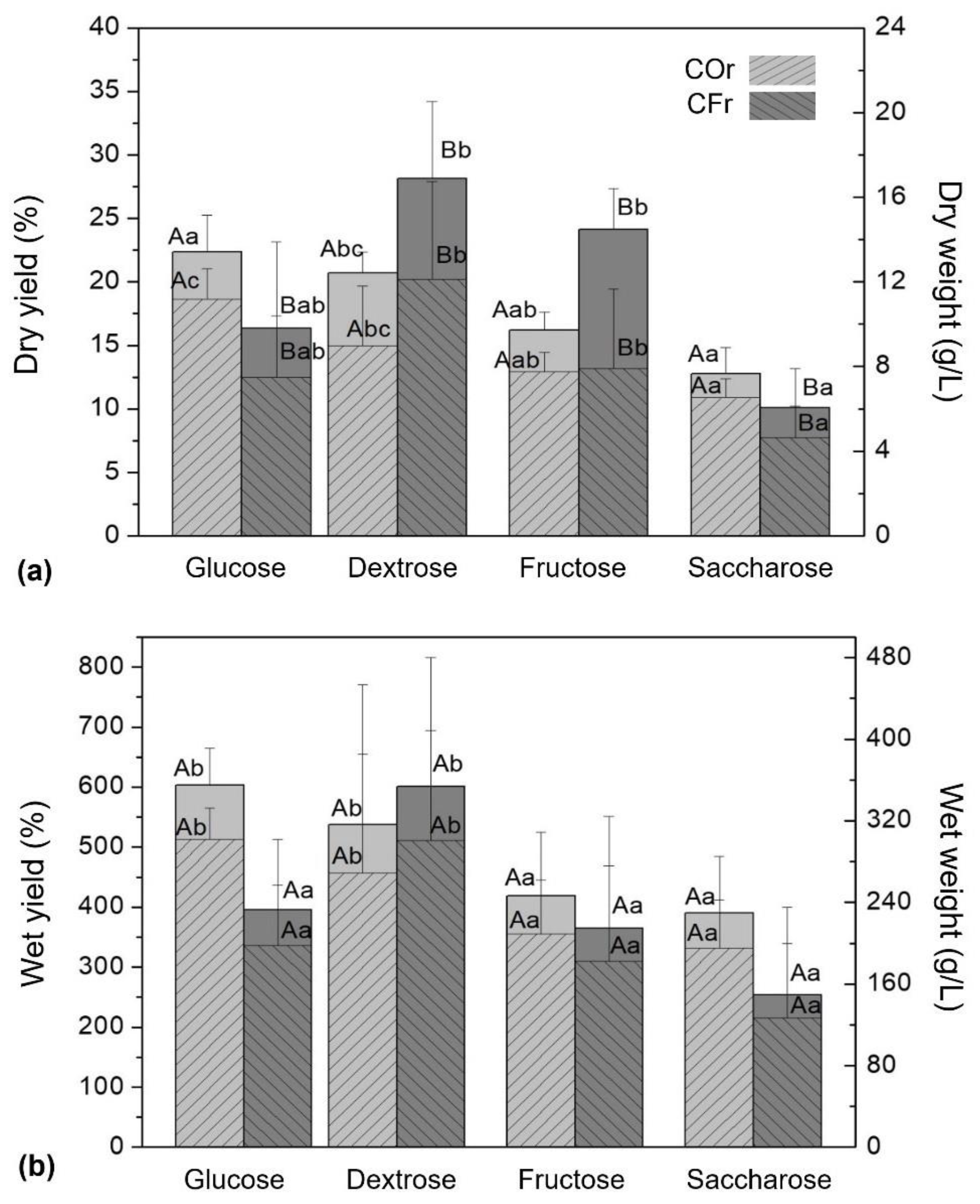
3.4. MCFs Yields
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sederavičiūtė, F.; Bekampienė, P.; Domskienė, J. Effect of pretreatment procedure on properties of kombucha fermented bacterial cellulose membrane. Polym. Test. 2019, 78, 10594. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Renard, T.; Rollan, S.; Taillandier, P. Impact of fermentation conditions on the production of bioactive compounds with anticancer, anti-inflammatory and antioxidant properties in kombucha tea extracts. Process Biochem. 2019, 83, 44–54. [Google Scholar] [CrossRef]
- Amarasinghe, H.; Weerakkody, N.S.; Waisundara, V.Y. Evaluation of physicochemical properties and antioxidant activities of kombucha “Tea Fungus” during extended periods of fermentation. Food Sci. Nutr. 2018, 6, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.K.; Kumar, V. Influence of different sugar sources, nitrogen sources and inocula on the quality characteristics pf apple tea wine. J. Inst. Brew. 2017, 123, 268–276. [Google Scholar] [CrossRef]
- Yıkmış, S.; Tuğgüm, S. Evaluation of Microbiological, Physicochemical and sensorial properties of purple basil kombucha beverage. Turk. J. Agric. Food Sci. Technol. 2019, 7, 1321–1327. [Google Scholar] [CrossRef]
- Ashrafi, A.; Jokar, M.; Nafchia, A.M. Preparation and characterization of biocomposite film based on chitosan and kombucha tea as active food packaging. Int. J. Biol. Macromol. 2018, 108, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, A.S.; Wang, D.; Grady, T.L. A comparison of kombucha SCOBY bacterial cellulose purification methods. SN Appl. Sci. 2020, 2, 240. [Google Scholar] [CrossRef]
- Greenwalt, C.J.; Steinkraus, K.H.; Ledford, R.A. Kombucha, the fermented tea: Microbiology, composition, and claimed health effects. J. Food Prot. 2000, 63, 976–981. [Google Scholar] [CrossRef]
- Carreño, L.D.; Caicedo, L.A.; Martínez, C.A. Técnicas de fermentación y aplicaciones de la celulosa bacteriana: Una revisión. Ing. Cienc 2012, 8, 307–335. [Google Scholar] [CrossRef]
- Spedding, G. So What Is Kombucha? An Alcoholic or a Non-Alcoholic Beverage? A Brief Selected Literature Review and Personal Reflection; Abstract-Overview; Bdas, Llc Wpsp#2; Brewing and Distilling Analytical Services, LLC: Lexington, KY, USA, 2015. [Google Scholar]
- Gomes, R.J.; de Borges, M.F.; de Rosa, M.F.; Castro-Gómez, R.J.H.; Spinosa, W.A. Acetic acid bacteria in the food industry: Systematics, characteristics and applications. Food Technol. Biotechnol. 2018, 56, 139–151. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.; Taillandier, P. Understanding kombucha tea fermentation: A review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Römling, U.; Galperin, M.Y. Bacterial cellulose biosynthesis: Diversity of operons, subunits, products, and functions. Trends Microbiol. 2015, 23, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Lopez-Sanchez, P.; Gidley, M.J. Binding of arabinan or galactan during cellulose synthesis is extensive and reversible. Carbohydr. Polym. 2015, 126, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Lopez-Sanchez, P.; Gidley, M.J. Interactions of pectins with cellulose during its synthesis in the absence of calcium. Food Hydrocoll. 2016, 52, 57–68. [Google Scholar] [CrossRef]
- Chawla, P.R.; Bajaj, I.B.; Survase, S.A.; Singhal, R.S. Microbial Cellulose: Fermentative production and applications. Food Technol. Biotechnol. 2009, 47, 107–124. [Google Scholar]
- Pereda, M.; Dufresne, A.; Aranguren, M.I.; Marcovich, N.E. Polyelectrolyte films based on chitosan/olive oil and reinforced with cellulose nanocrystals. Carbohydr. Polym. 2014, 101, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.C.F.; Wang, W. A Study of the Receptivity to Bacterial Cellulosic Pellicle for Fashion. Res. J. Text. Apparel. 2015, 19, 65–69. [Google Scholar] [CrossRef]
- Frone, A.N.; Panaitescu, D.M.; Chiulan, I.; Nicolae, C.A.; Casarica, A.; Gabor, A.R.; Tusca, R.; Damian, C.M.; Purcar, V.; Alexandrescu, E.; et al. Surface Treatment of Bacterial Cellulose in Mild, Eco-Friendly Conditions. Coatings 2018, 8, 221. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K. Biotransformation of fermented black tea into bacterial nanocellulose via symbiotic interplay of microorganisms. Int. J. Biol. Macromol. 2019, 132, 166–177. [Google Scholar] [CrossRef]
- Schramm, B.M.; Hestrin, S. Factors affecting production of cellulose at the air/liquid interface of a culture of acetobacter xylinum. Microbiology 1954, 11, 123–129. [Google Scholar] [CrossRef]
- Gorgieva, S.; Trcek, J. Bacterial cellulose: Production, modification and perspectives in biomedical applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Flanagan, B.; Gidley, M.J.; Dykes, G.A. Characterization of cellulose production by a Gluconacetobacter xylinus strain from kombucha. Curr. Microbiol. 2008, 57, 449. [Google Scholar] [CrossRef] [PubMed]
- Neera, K.; Ramana, V.; Batra, V.H. Occurrence of cellulose-producing gluconacetobacter spp. in Fruit samples and kombucha tea, and production of the biopolymer. Appl. Biochem. Biotechnol. 2015, 176, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Goh, W.N.; Rosma, A.; Kaur, B.; Fazilah, A.; Karim, A.A.; Bhat, R. Fermentation of black tea broth (Kombucha): I. effects of sucrose concentration and fermentation time on the yield of microbial cellulose. Int. Food Res. J. 2012, 19, 109–117. [Google Scholar]
- Kallel, L.; Desseaux, V.; Hamdi, M.; Stocker, P.; Ajandouz, E.H. Insights into the fermentation biochemistry of kombucha teas and potential impacts of kombucha drinking on starch digestion. Food Res. Int. 2012, 49, 226–232. [Google Scholar] [CrossRef]
- Khosravi, S.; Safari, M.; Emam-Djomeh, Z.; Golmakani, M.T. Development of fermented date syrup using kombucha starter culture. J. Food Process. Preserv. 2018, 43, e13872. [Google Scholar] [CrossRef]
- Ahmed, R.F.; Hikal, M.S.; Abou-Taleb, K.A. Biological, chemical and antioxidant activities of different types Kombucha. Ann. Agric. Sci. 2020, 65, 35–41. [Google Scholar] [CrossRef]
- Matsuoka, M.; Tsuchida, T.; Matsushita, K.; Adachi, O.; Yoshinaga, F. A Synthetic medium for bacterial cellulose production by acetobacter xylinum subsp. sucrofermentans. Biosci. Biotechnol. Biochem. 2011, 60, 575–579. [Google Scholar] [CrossRef]
- Lin, D.; Lopez-Sanchez, P.; Li, R.; Li, Z. Production of bacterial cellulose by gluconacetobacter hanseniiCGMCC3917 using only waste beer yeast as nutrient source. Bioresour. Technol. 2014, 151, 113–119. [Google Scholar] [CrossRef]
- Huang, C.; Yang, X.-Y.; Xiong, L.; Guo, H.J.; Luo, J.; Wang, B.; Zhang, H.R.; Lin, X.Q.; Chen, X.D. Evaluating the possibility of using acetone-butanol-ethanol (ABE) fermentation wastewater for bacterial cellulose production by Gluconacetobacter xylinus. Lett. Appl. Microbiol. 2015, 60, 491–496. [Google Scholar] [CrossRef]
- Bayrak, E.; Büyükkileci, A.O. Utilization of white grape pomace for lactic acid production. Gıda J. Food. 2018, 43, 129–138. [Google Scholar] [CrossRef]
- Toda, K.; Asakura, T.; Fukaya, M.; Entani, E.; Kawamura, Y. Cellulose production by acetic acid-resistant Acetobacter xylinum. J. Ferment. Bioeng. 1997, 84, 228–231. [Google Scholar] [CrossRef]
- Srihari, T.; Satyanarayana, U. Changes in free radical scavenging activity of kombucha during fermentation. J. Pharm. Sci. Res. 2012, 4, 1978. [Google Scholar]
- Martínez, J.; Valenzuela, L.; Jayabalan, R.; Huerta, J.; Escalante-Aburto, A. A review on health benefits of kombucha nutritional compounds and metabolites. CyTA J. Food 2018, 1, 390–399. [Google Scholar] [CrossRef]
- Juyoung, K.; Koushik, A. Current trends in Kombucha: Marketing perspectivesand the need for improved sensory research. Beverages 2020, 6, 15. [Google Scholar]
- Jayabalan, R.; Malbasa, R.V.; Loncar, E.S.; Vitas, J.S.; Stahishkumar, M. A Review on kombucha tea–microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Vitas, J.; Vukmanovic, S.; Cakarevic, J.; Popovic, L.; Malbasa, R. Kombucha fermentation of six medicinal herbs: Chemical profile and biological activity. Chem. Ind. Chem. Eng. Q. 2020, 26, 157–170. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Ladunska, J.; Kochman, J.; Janda, K. Chemical profile and antioxidant activity of the kombucha beverage derived from white, green, black and red tea. Antioxidants 2020, 9, 447. [Google Scholar] [CrossRef]
- Kaewkod, T.; Bovonsombut, S.; Traagoolpua, Y. Efficacy of kombucha obtained from green, oolong, and black teas on inhibition of pathogenic bacteria, antioxidation, and toxicity on colorectal cancer cell line. Microorganisms 2019, 7, 700. [Google Scholar] [CrossRef]
- López-Legarda, X.; Taramuel-Gallardo, A.; Arboleda-Echavarría, C.; Segura-Sánchez, F.; Restrepo-Betancur, L.F. Comparison of methods using sulfuric acid for determination of total sugars. Rev. Cubana Quím. 2017, 29, 180–198. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Suna, S.; Çiftçi1, K.; Tamer, C.E. Determination of physicochemical and sensory properties of kombucha beverage prepared with saffron. GIDA 2020, 45, 20–30. [Google Scholar]
- Shahbazi, H.; Gahruied, H.; Golmakani, M.T.; Eskandari, M.H.; Movahedi, M. Effect of medicinal plant type and concentration on physicochemical, antioxidant, antimicrobial, and sensorial properties of Kombucha. Food Nutr. Res. 2018, 6, 2568–2577. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Tang, S.; Azi, F.; Hu, W.; Dong, M. Use of kombucha consortium to transform soy whey into a novel functional beverage. J. Funct. Foods 2019, 52, 81–89. [Google Scholar] [CrossRef]
- Neffe-Skocinska, K.; Sionek, B.; Scibisz, I.; Kolozyn-Krajewska, D. Acid contents and the effect of fermentation condition of kombucha tea beverages on physicochemical, microbiological, and sensory properties. CyTA J. Food 2017, 15, 601–607. [Google Scholar] [CrossRef]
- Nurikasari, M.; Puspitasari, Y.; Yoni Siwi, R.P. Characterization and analysis kombucha tea antioxidant activity based on long fermentation as a beverage functional. J. Glob. Res. Public Health 2017, 2, 90–96. [Google Scholar]
- AL-Kalifawi, E.J.; Hassan, I.A. Factors Influence on the yield of bacterial cellulose of kombucha (khubdat humza). Baghdad Sci. J. 2014, 11, 1420–1428. [Google Scholar]


| Initial (day 0) | Final (day 15) | |||
|---|---|---|---|---|
| Carbon Source | pH | |||
| COr | CFr | COr | CFr | |
| Glucose | B3.03 ± 0.01a | A2.96 ± 0.02a | B2.28 ± 0.01b | A2.24 ± 0.07b |
| Dextrose | AB3.01 ± 0.04a | A2.96 ± 0.01a | A2.20 ± 0.01b | A2.48 ± 0.19b |
| Fructose | A2.97 ± 0.00a | B2.99 ± 0.01a | C2.62 ± 0.04b | A2.50 ± 0.26b |
| Saccharose | AB2.98 ± 0.04a | A2.94 ± 0.01a | D2.73 ± 0.02b | A2.53 ± 0.06a |
| Carbon Source | TSS (°Brix) | |||
| COr | CFr | COr | CFr | |
| Glucose | C4.95 ± 0.07a | A4.65 ± 0.35a | A3.80 ± 0.17b | A4.07 ± 0.12b |
| Dextrose | B4.70 ± 0.14a | A4.60 ± 0.57a | A3.43 ± 0.35b | A3.97 ± 0.06a |
| Fructose | A4.30 ± 0.14a | A4.80 ± 0.28a | A3.73 ± 0.06b | A3.83 ± 0.38b |
| Saccharose | B4.60 ± 0.00a | A4.70 ± 0.14a | A3.93 ± 0.12b | A4.07 ± 0.12a |
| Carbon Source | TA (acetic acid %) | |||
| COr | CFr | COr | CFr | |
| Glucose | A0.12 ± 0.03a | A0.09 ± 0.02a | B1.61 ± 0.08b | AB1.25 ± 0.14b |
| Dextrose | A0.10 ± 0.03a | A0.11 ± 0.05a | AB1.20 ± 0.59b | B1.46 ± 0.09b |
| Fructose | A0.12 ± 0.03b | A0.09 ± 0.02a | A0.54 ± 0.11b | AB0.96 ± 0.62b |
| Saccharose | A0.12 ± 0.00a | A0.10 ± 0.02a | A0.42 ± 0.12b | A0.44 ± 0.17b |
| Carbon Source | Initial (day 0) | Final (day 15) | ||
|---|---|---|---|---|
| Carbon Source | L* | |||
| COr | CFr | COr | CFr | |
| Glucose | A23.73 ± 0.87b | A21.60 ± 0.23b | A18.33 ± 0.59a | A18.10 ± 0.35a |
| Dextrose | A24.85 ± 0.98a | A23.10 ± 3.58a | A20.67 ± 4.82 a | A19.00 ± 0.78a |
| Fructose | A23.63 ± 0.06b | A22.50 ± 2.42b | A19.60 ± 0.69a | A18.70 ± 0.56a |
| Saccharose | B28.45 ± 0.17b | A25.15 ± 2.14b | A18.10 ± 2.96a | A19.30 ± 1.04a |
| Carbon Source | a* | |||
| COr | CFr | COr | CFr | |
| Glucose | A2.95 ± 0.64a | A4.75 ± 1.79a | A4.07 ± 0.72a | A4.07 ± 0.81a |
| Dextrose | A1.45 ± 0.40a | A2.30 ± 2.66a | A3.83 ± 0.91b | A4.47 ± 1.89a |
| Fructose | A3.10 ± 1.73a | A2.65 ± 2.02a | A5.13 ± 1.76a | A4.83 ± 0.12a |
| Saccharose | A3.20 ± 0.92a | A2.35 ± 0.29a | A3.70 ± 1.18a | A4.83 ± 0.06b |
| Carbon Source | b* | |||
| COr | CFr | COr | CFr | |
| Glucose | A14.00 ± 0.81a | A10.05 ± 0.52a | A14.90 ±1.40a | A15.60 ± 2.94b |
| Dextrose | B17.55 ± 0.17a | B15.35 ± 3.52a | A16.27 ± 4.45a | A15.47 ± 1.53a |
| Fructose | C15.75 ± 0.98a | AB12.30 ± 3.00a | A14.90 ± 1.65a | A14.73 ± 1.89a |
| Saccharose | D21.90 ± 0.23b | B17.30 ± 1.39a | A14.57 ± 4.35a | A15.10 ± 0.69 |
| Carbon Source | C | |||
| COr | CFr | COr | CFr | |
| Glucose | A14.30 ± 0.92a | A11.25 ± 1.21a | A15.47 ± 1.29a | A16.17 ± 3.06b |
| Dextrose | AB17.65 ± 0.17a | AB15.60 ± 3.81a | A16.77 ± 4.10a | A16.13 ± 1.79a |
| Fructose | AB16.1 ± 1.27a | AB12.65 ± 3.41a | A15.87 ± 1.48a | A15.53 ± 1.79a |
| Saccharose | B22.15 ± 0.40b | B17.45 ± 1.33a | A15.10 ± 4.11a | A15.90 ± 0.69a |
| Carbon Source | H | |||
| COr | CFr | COr | CFr | |
| Glucose | A77.95 ± 1.79a | A65.20 ± 7.39a | A74.60 ± 3.15a | A75.33 ± 0.29a |
| Dextrose | B85.20 ± 1.39b | B82.90 ± 8.08a | A75.37 ± 7.23a | A74.20 ± 5.63a |
| Fructose | AB79.05 ± 5.37a | B78.80 ± 6.41a | A70.77 ± 6.99a | A71.60 ± 1.80a |
| Saccharose | AB81.60 ± 2.19a | B81.90 ± 1.56b | A74.40 ± 6.74a | A72.23 ± 0.98a |
| COr | ||||
| Storage (days) | Glucose | Dextrose | Fructose | Saccharose |
| 0 | Very clear | Very clear | Very clear | Very clear |
| 3 | Turbid | Clear | Clear | Clear |
| 6 | Turbid | Turbid | Turbid | Turbid |
| 9 | Very Turbid | Turbid | Turbid | Turbid |
| 12 | Very turbid | Very turbid | Very turbid | Very turbid |
| 15 | Very turbid | Very turbid | Very turbid | Very turbid |
| CFr | ||||
| Storage (days) | Glucose | Dextrose | Fructose | Saccharose |
| 0 | Very clear | Very clear | Very clear | Very clear |
| 3 | Turbid | Clear | Clear | Clear |
| 6 | Turbid | Turbid | Turbid | Turbid |
| 9 | Turbid | Turbid | Turbid | Turbid |
| 12 | Very turbid | Very turbid | Very turbid | Very turbid |
| 15 | Very turbid | Very turbid | Very turbid | Very turbid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treviño-Garza, M.Z.; Guerrero-Medina, A.S.; González-Sánchez, R.A.; García-Gómez, C.; Guzmán-Velasco, A.; Báez-González, J.G.; Márquez-Reyes, J.M. Production of Microbial Cellulose Films from Green Tea (Camellia Sinensis) Kombucha with Various Carbon Sources. Coatings 2020, 10, 1132. https://doi.org/10.3390/coatings10111132
Treviño-Garza MZ, Guerrero-Medina AS, González-Sánchez RA, García-Gómez C, Guzmán-Velasco A, Báez-González JG, Márquez-Reyes JM. Production of Microbial Cellulose Films from Green Tea (Camellia Sinensis) Kombucha with Various Carbon Sources. Coatings. 2020; 10(11):1132. https://doi.org/10.3390/coatings10111132
Chicago/Turabian StyleTreviño-Garza, Mayra Z., Ana S. Guerrero-Medina, Ricardo A. González-Sánchez, Celestino García-Gómez, Antonio Guzmán-Velasco, Juan G. Báez-González, and Julia M. Márquez-Reyes. 2020. "Production of Microbial Cellulose Films from Green Tea (Camellia Sinensis) Kombucha with Various Carbon Sources" Coatings 10, no. 11: 1132. https://doi.org/10.3390/coatings10111132
APA StyleTreviño-Garza, M. Z., Guerrero-Medina, A. S., González-Sánchez, R. A., García-Gómez, C., Guzmán-Velasco, A., Báez-González, J. G., & Márquez-Reyes, J. M. (2020). Production of Microbial Cellulose Films from Green Tea (Camellia Sinensis) Kombucha with Various Carbon Sources. Coatings, 10(11), 1132. https://doi.org/10.3390/coatings10111132