Radical Species Production and Color Change Behavior of Wood Surfaces Treated with Suppressed Photoactivity and Photoactive TiO2 Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticles
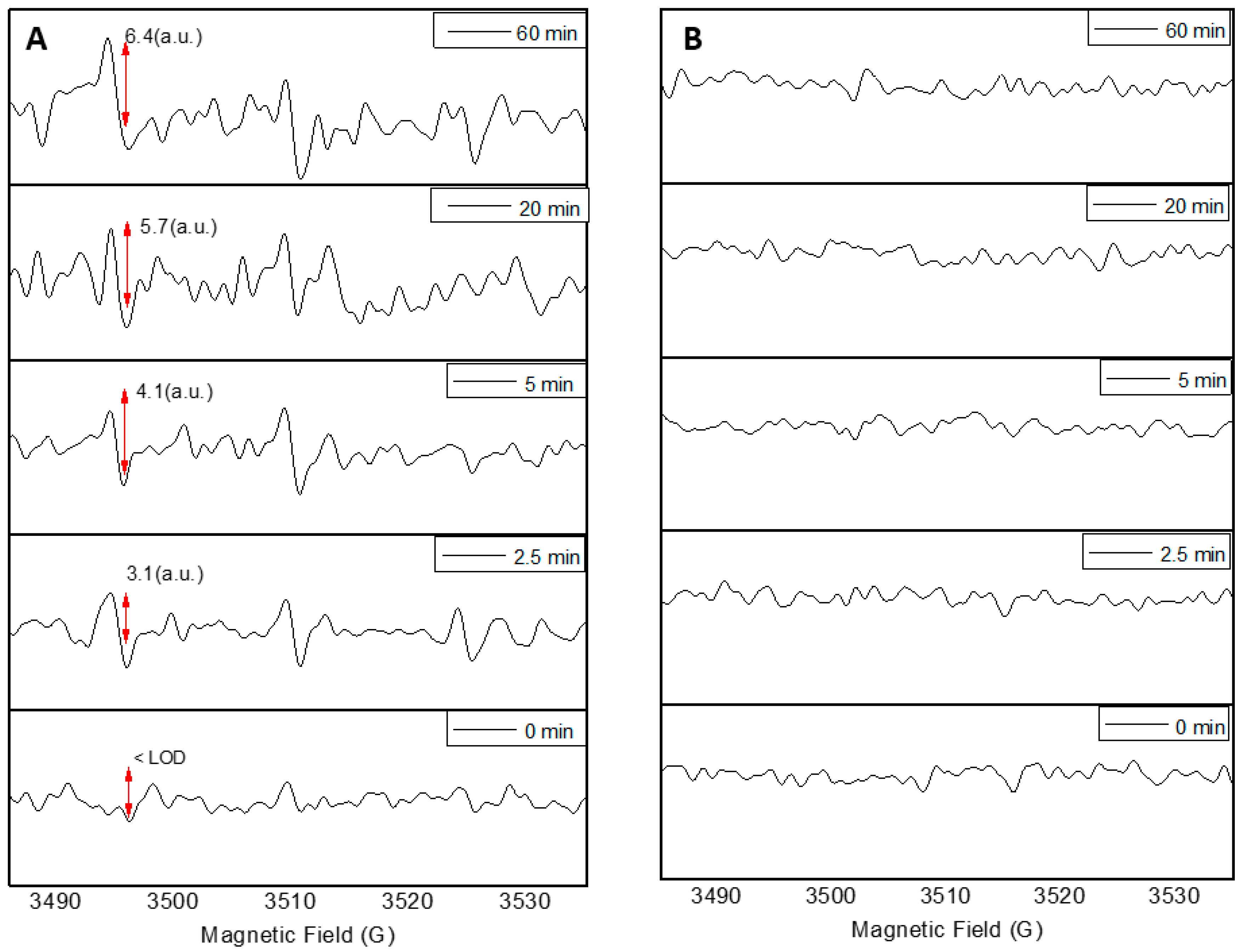
2.2. Photoactivity Tests on Nanoparticles
2.3. Application of Nanoparticles on Wood Surfaces
2.4. UV Exposure
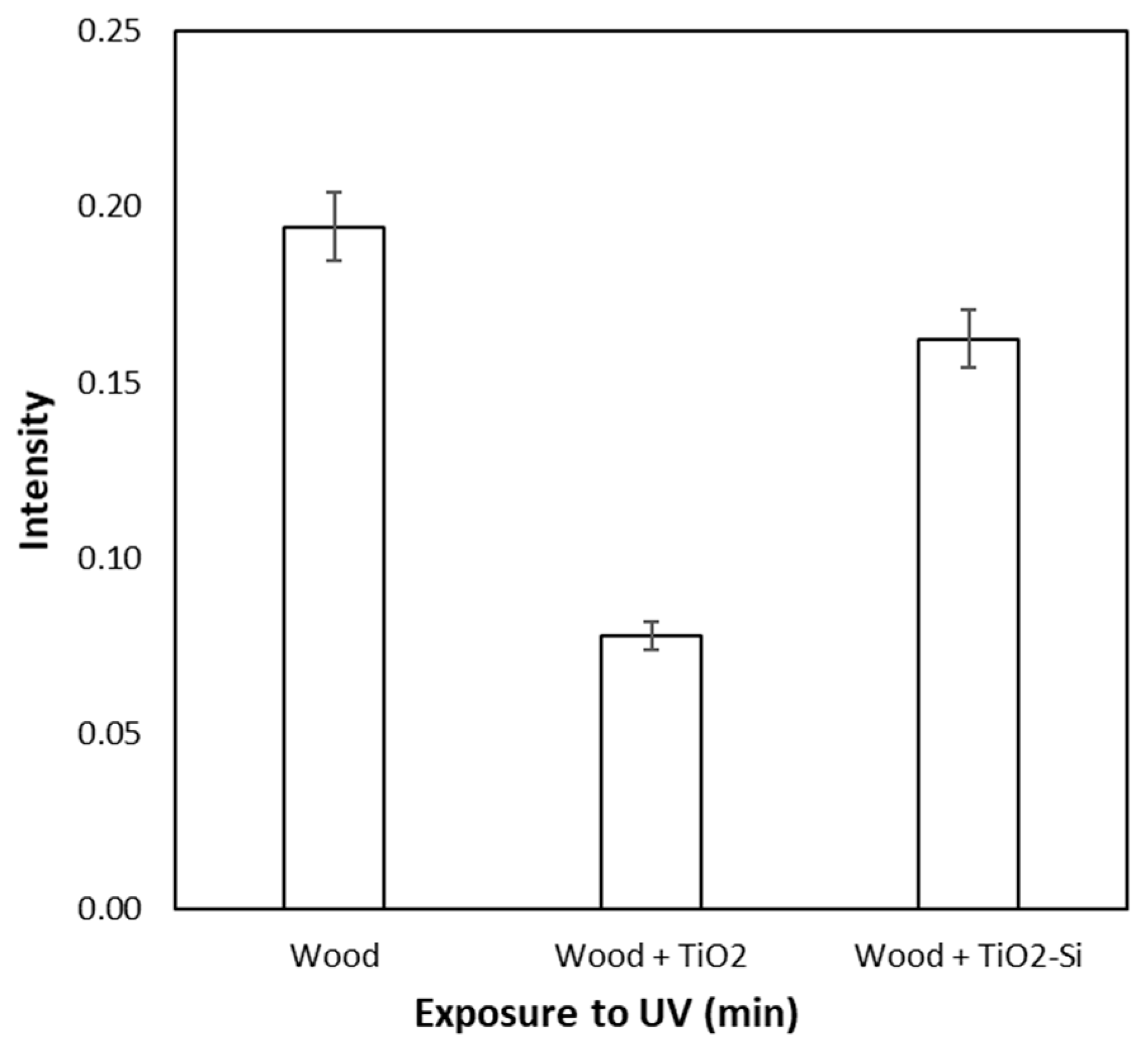
2.5. Wood Surface Color Change Measurements
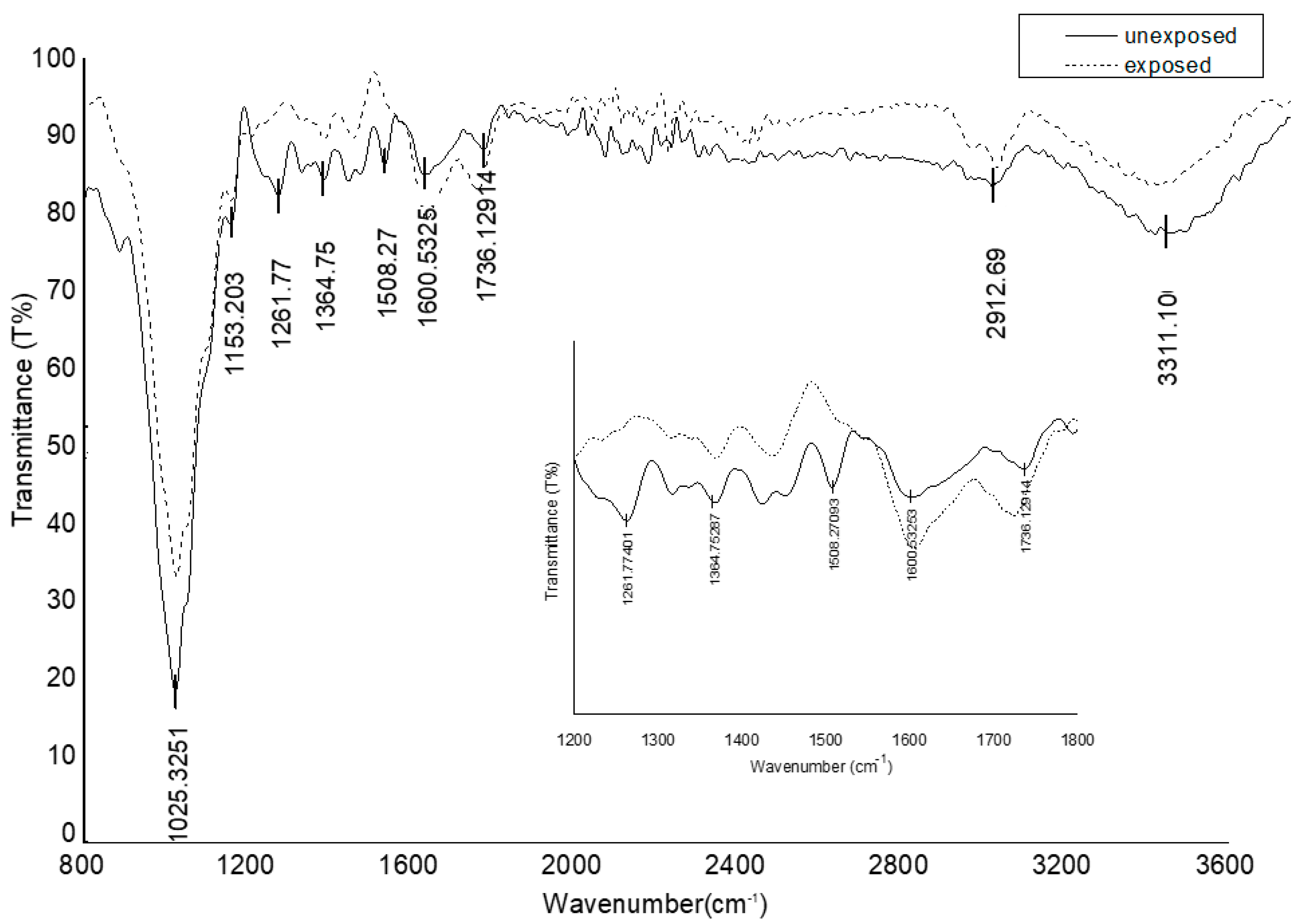
2.6. Chemical Changes of Untreated Wood Surfaces
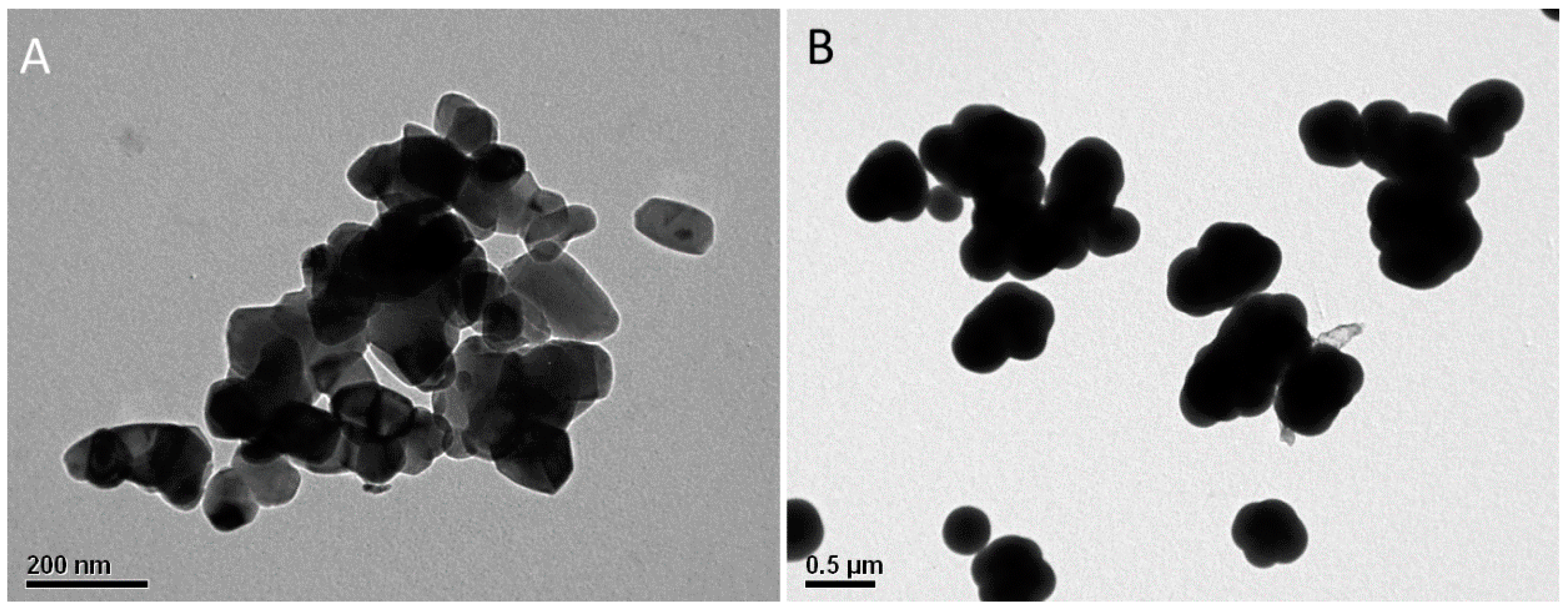
2.7. Transmission Electron Microscopy
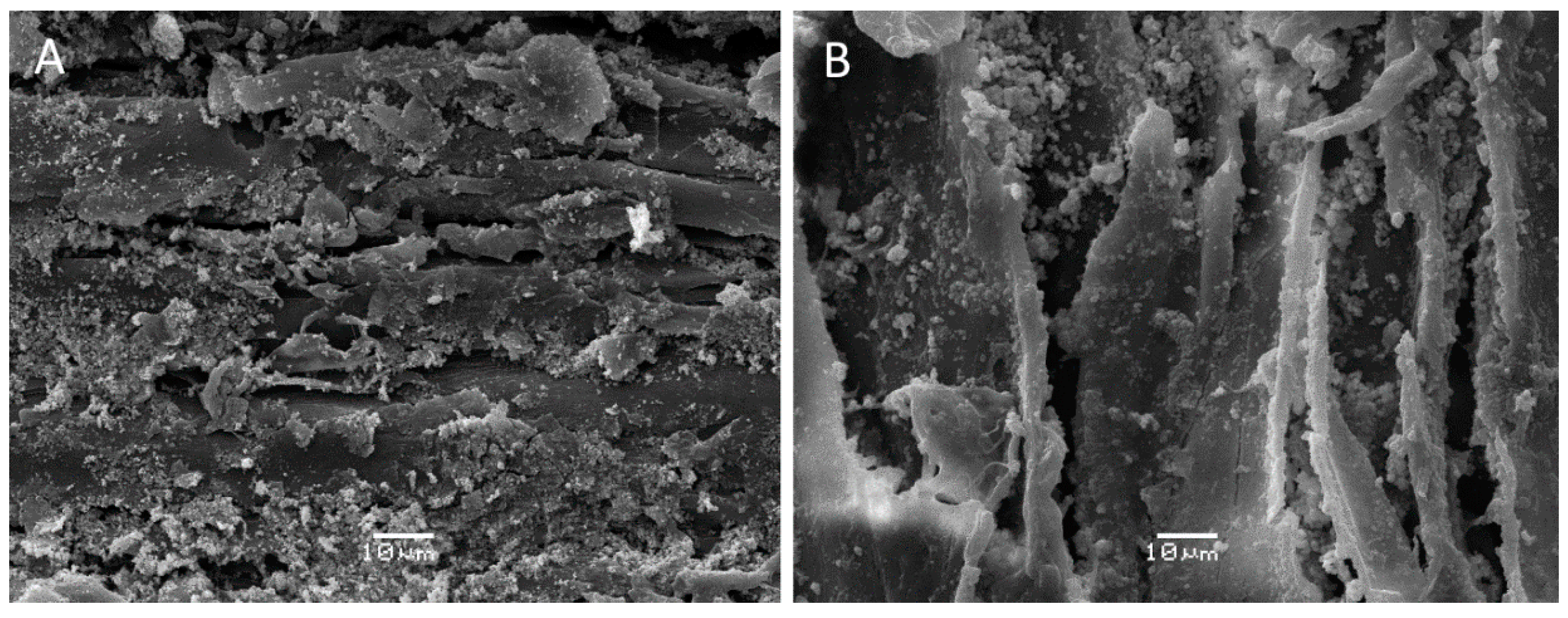
2.8. Scanning Electron Microscopy
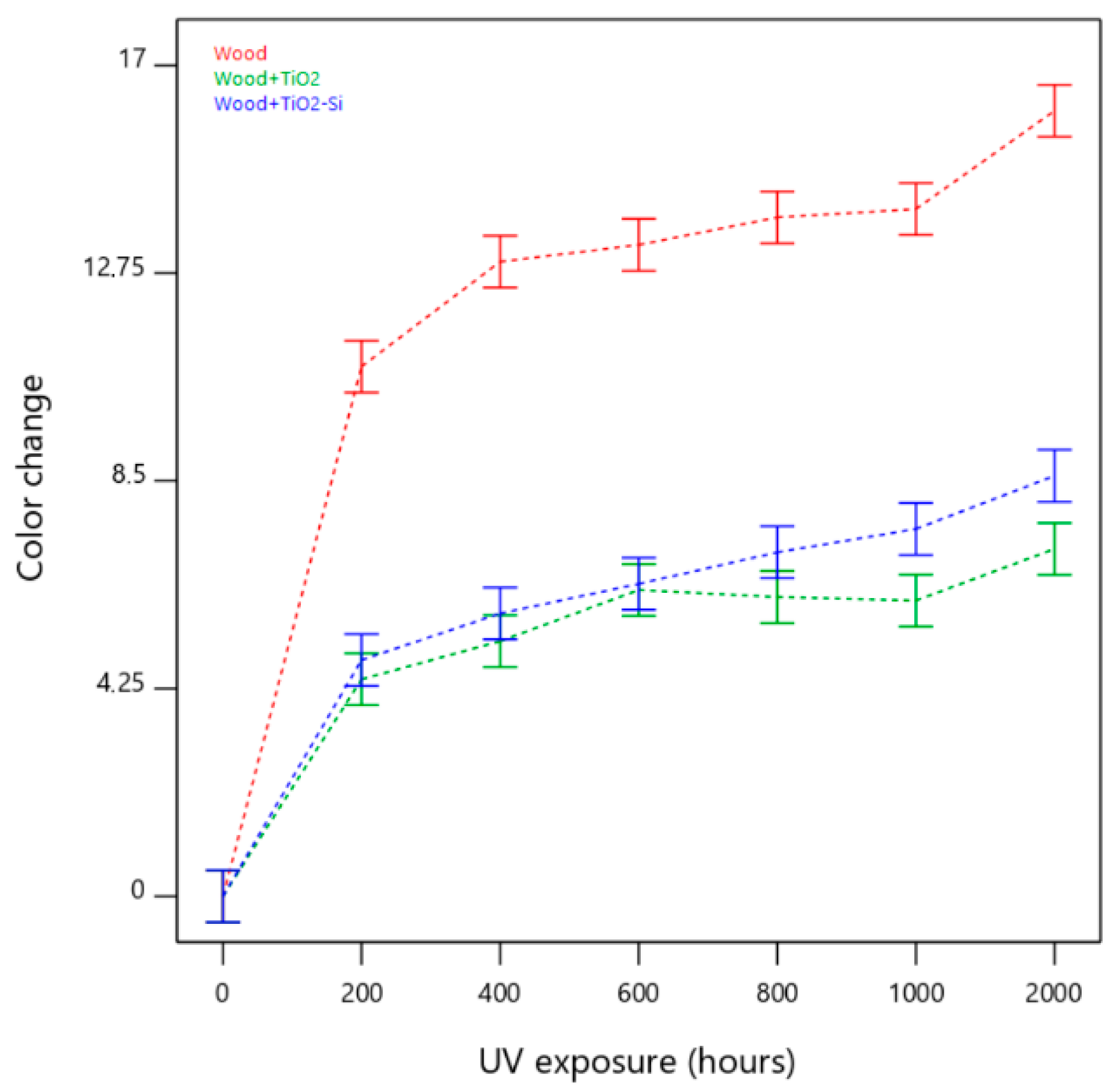
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fufa, S.; Jelle, B.; Hovde, P. Effects of TiO2 and clay nanoparticles loading on weathering performance of coated wood. Prog. Org. Coat. 2013, 76, 1425–1429. [Google Scholar] [CrossRef]
- Zanatta, P.; Lazarotto, M.; Gonzalez de Cademartori, P.; Cava, S.; Moreira, M.; Gatto, D. The effect of titanium dioxide nanoparticles obtained by microwave-assisted hydrothermal method on the color and decay resistance of pinewood. Maderas Cienc. Tecnol. 2017, 19, 495–506. [Google Scholar] [CrossRef]
- Egerton, T. UV-Absorption—The primary process in photocatalysis and some practical consequences. Molecules 2014, 19, 18192–18214. [Google Scholar] [CrossRef] [PubMed]
- Evans, P. Weathering and Photoprotection of Wood. In Development of Commercial Wood Preservatives; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2008; Volume 982, pp. 69–117. [Google Scholar]
- Beydoun, D.; Amal, R.; Low, G.; McEvoy, S. Role of nanoparticles in photocatalysis. J. Nanoparticle Res. 1999, 1, 439–458. [Google Scholar] [CrossRef]
- Brezová, V.; Gabčová, S.; Dvoranová, D.; Staško, A. Reactive oxygen species produced upon photoexcitation of sunscreens containing titanium dioxide (an EPR study). J. Photochem. Photobiol. B 2005, 79, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Gladis, F.; Schumann, R. A suggested standardised method for testing photocatalytic inactivation of aeroterrestrial algal growth on TiO2-coated glass. Int. Biodeterior. Biodegrad. 2011, 65, 415–422. [Google Scholar] [CrossRef]
- Jain, A.; Vaya, D.; Jain, A.; Vaya, D. Photocatalytic activity of TiO2 nanomaterials. J. Chil. Chem. Soc. 2017, 62, 3683–3690. [Google Scholar] [CrossRef]
- Nevárez-Martínez, M.; Espinoza-Montero, P.; Quiroz-Chávez, F.; Ohtani, B. Fotocatálisis: Inicio, actualidad y perspectivas a través del TiO2. Av. Quím. 2017, 12, 45–59. [Google Scholar]
- Hughes, W. Photodegradation of Paint Films Containing TiO2 Pigments; Verlag Chemie GmbH: Weinheim/Bergst, Germany, 1970; pp. 67–82. [Google Scholar]
- Allen, N.; McKellar, J.; Phillips, G.; Chapman, C. The TiO2 photosensitized degradation of nylon 6, 6: Stabilizing action of manganese ions. J. Polym. Sci. Lett. 1974, 12, 723–727. [Google Scholar] [CrossRef]
- Tsuzuki, T.; He, R.; Wang, J.; Sun, L.; Wang, X.; Hocking, R. Reduction of the photocatalytic activity of ZnO nanoparticles for UV protection applications. Int. J. Nanotechnol. 2012, 9, 1017–1029. [Google Scholar] [CrossRef]
- Hon, D.; Chang, S.; Feist, W. Participation of singlet oxygen in the photodegradation of wood surfaces. Wood Sci. Technol. 1982, 16, 193–201. [Google Scholar] [CrossRef]
- Baur, S.; Easteal, A. ESR studies on the free radical generation in wood by irradiation with selected sources from UV to IR wavelength regions. Holzforschung 2014, 68, 775–780. [Google Scholar] [CrossRef]
- Backa, S.; Gierer, J.; Reitberger, T.; Nilsson, T. Hydroxyl radical activity in brown-rot fungi studied by a new chemiluminescence method. Holzforschung 1992, 46, 61–67. [Google Scholar] [CrossRef]
- Tanaka, H.; Itakura, S.; Enoki, A. Hydroxyl radical generation by an extracellular low-molecular-weight substance and phenol oxidase activity during wood degradation by the white-rot basidiomycete Trametes versicolor. J. Biotechnol. 1999, 75, 57–70. [Google Scholar] [CrossRef]
- Hon, D.; Ifju, G.; Feist, W. Characteristics of free radicals in wood. Wood Fiber 1980, 12, 121–130. [Google Scholar]
- Chia, S.; Leong, D. Reducing ZnO nanoparticles toxicity through silica coating. Heliyon 2016, 2, e00177. [Google Scholar] [CrossRef]
- Yamazaki, I.; Piette, L. ESR spin-trapping studies on the reaction of Fe2+ ions with H2O2-reactive species in oxygen toxicity in biology. J. Biol. Chem. 1990, 265, 13589–13594. [Google Scholar] [PubMed]
- Hon, D.; Ifju, G. Measuring penetration of light into wood by detection of photo-induced free radicals. Wood Sci. 1978, 11, 118–127. [Google Scholar]
- Faix, O. Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 1991, 45, 21–28. [Google Scholar] [CrossRef]
- Harrington, K.; Higgins, H.; Michell, A. Infrared spectra of Eucalyptus regnans F. Muell and Pinus radiata D. Don. Holzforschung 1964, 18, 108–113. [Google Scholar] [CrossRef]
- Chazal, R.; Robert, P.; Durand, S.; Devaux, M.; Saulnier, L.; Lapierre, C.; Guillon, F. Investigating lignin key features in maize lignocelluloses using infrared spectroscopy. Appl. Spectrosc. 2014, 68, 1342–1347. [Google Scholar] [CrossRef]
- Lionetto, F.; Del Sole, R.; Cannoletta, D.; Vasapollo, G.; Maffezzoli, A. Monitoring wood degradation during weathering by cellulose crystallinity. Materials 2012, 5, 1910–1922. [Google Scholar] [CrossRef]
- Kringstad, K.; Lin, S. Mechanisms in the yellowing of high yield pulps by light: Structure and reactivity of free radical intermediates in the photodegradation of lignin. Tappi 1970, 53, 2296–2301. [Google Scholar]
- Gierer, J.; Lin, S. Photodegradation of lignin. A contribution to the mechanism of chromophore formation. Sven Papp. 1972, 75, 233–239. [Google Scholar]
- Scaiano, J.; Netto-Ferreira, J.; Wintgens, V. Fragmentation of ketyl radicals derived from α-phenoxyacetophenone: An important mode of decay for lignin-related radicals? J. Photochem. Photobiol. Chem. 1991, 59, 265–268. [Google Scholar] [CrossRef]
- Schmidt, J.; Heitner, C. Light-induced yellowing of mechanical and ultrahigh yield pulps. Part 2. Radical-induced cleavage of etherified guaiacylglycerol-β-arylether groups is the main degradative pathway. J. Wood Chem. Technol. 1993, 13, 309–325. [Google Scholar] [CrossRef]
- Schaller, C.; Rogez, D. New approaches in wood coating stabilization. J. Coat. Technol. Res. 2007, 4, 401–409. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Mahmoodi, N.; Arami, M.; Limaee, N.; Tabrizi, N. Kinetics of heterogeneous photocatalytic degradation of reactive dyes in an immobilized TiO2 photocatalytic reactor. J. Colloid Interface Sci. 2006, 295, 159–164. [Google Scholar] [CrossRef]
- Rassam, G.; Abdi, Y.; Abdi, A. Deposition of TiO2 nano-particles on wood surfaces for UV and moisture protection. J. Exp. Nanosci. 2012, 7, 468–476. [Google Scholar] [CrossRef]
- Zheng, R.; Tshabalala, M.; Wang, H. Photocatalytic degradation of wood coated with a combination of rutile TiO2 nanostructures and low-surface free-energy materials. Bioresources 2016, 11, 2393–2402. [Google Scholar] [CrossRef][Green Version]
- Hurum, D.; Agrios, A.; Gray, K.; Rajh, T.; Thurnauer, M. Explaining the enhanced photocatalytic activity of Degussa P25 mixed-phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Kataoka, Y.; Kiguchi, M.; Evans, P. Photodegradation depth profile and penetration of light in Japanese cedar earlywood (Cryptomeria japonica D.Don) exposed to artificial solar radiation. Surf. Coat. Int. Part B Coat. Trans. 2004, 87, 149–234. [Google Scholar] [CrossRef]
- Yang, K.; Dai, Y.; Huang, B. First-principles calculations for geometrical structures and electronic properties of Si-doped TiO2. Chem. Phys. Lett. 2008, 456, 71–75. [Google Scholar] [CrossRef]
- Long, R.; Dai, Y.; Meng, G.; Huang, B. Energetic and electronic properties of X- (Si, Ge, Sn, Pb) doped TiO2 from first-principles. Phys. Chem. Chem. Phys. 2009, 11, 8165–8172. [Google Scholar] [CrossRef]


| Factor | p-Value |
|---|---|
| A: Nanoparticle treatment | <0.0001 |
| B: Time of exposure A × B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, V.; Morales, C.; Sagredo, N.; Perez-Gonzalez, G.; Romero, R.; Contreras, D. Radical Species Production and Color Change Behavior of Wood Surfaces Treated with Suppressed Photoactivity and Photoactive TiO2 Nanoparticles. Coatings 2020, 10, 1033. https://doi.org/10.3390/coatings10111033
Hernandez V, Morales C, Sagredo N, Perez-Gonzalez G, Romero R, Contreras D. Radical Species Production and Color Change Behavior of Wood Surfaces Treated with Suppressed Photoactivity and Photoactive TiO2 Nanoparticles. Coatings. 2020; 10(11):1033. https://doi.org/10.3390/coatings10111033
Chicago/Turabian StyleHernandez, Vicente, Constanza Morales, Nicole Sagredo, Gabriel Perez-Gonzalez, Romina Romero, and David Contreras. 2020. "Radical Species Production and Color Change Behavior of Wood Surfaces Treated with Suppressed Photoactivity and Photoactive TiO2 Nanoparticles" Coatings 10, no. 11: 1033. https://doi.org/10.3390/coatings10111033
APA StyleHernandez, V., Morales, C., Sagredo, N., Perez-Gonzalez, G., Romero, R., & Contreras, D. (2020). Radical Species Production and Color Change Behavior of Wood Surfaces Treated with Suppressed Photoactivity and Photoactive TiO2 Nanoparticles. Coatings, 10(11), 1033. https://doi.org/10.3390/coatings10111033






