Multilayer Structure of Reduced Graphene Oxide and Copper Oxide as a Gas Sensor
Abstract
1. Introduction
2. Materials and Methods
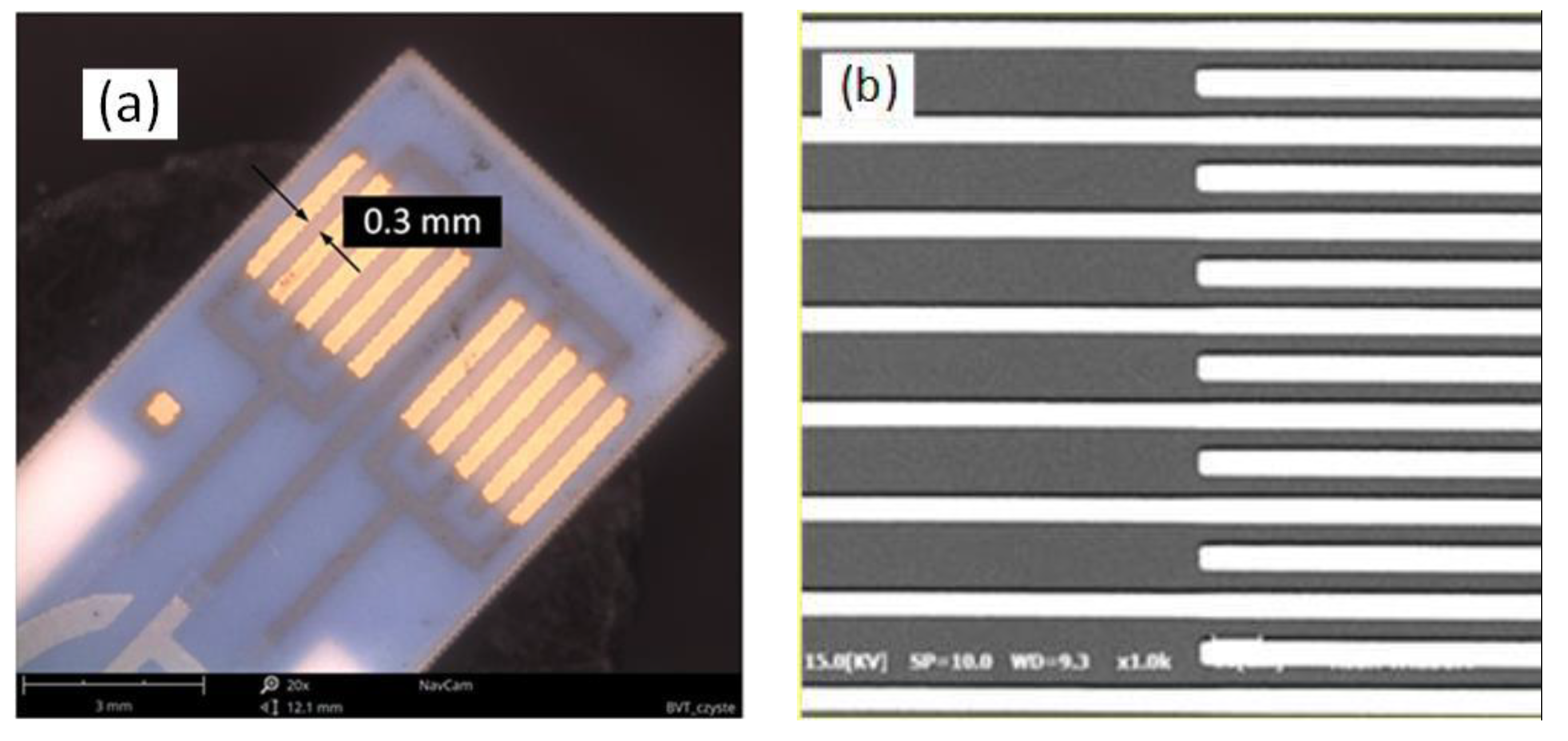
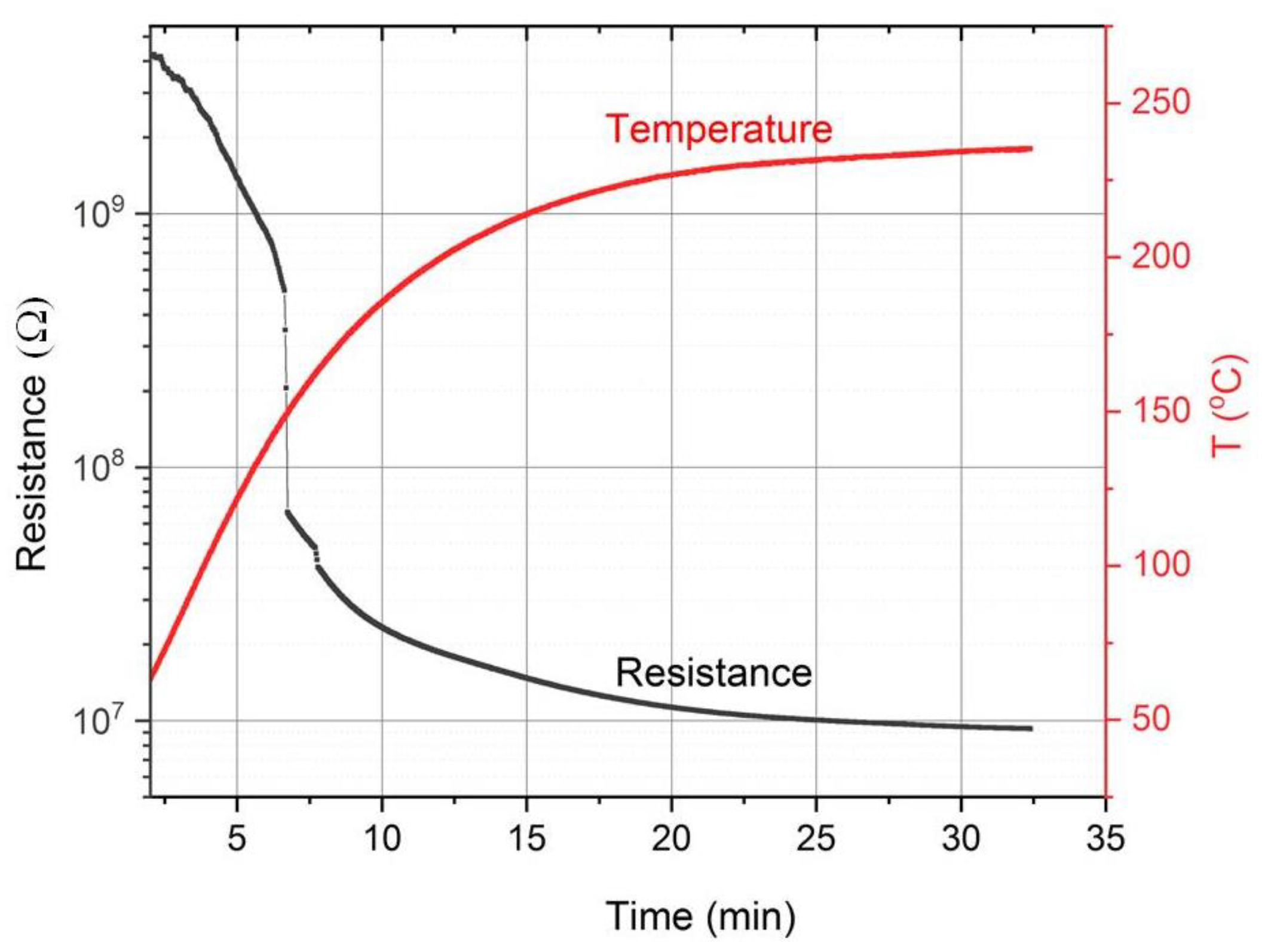
2.1. Sample Preparation
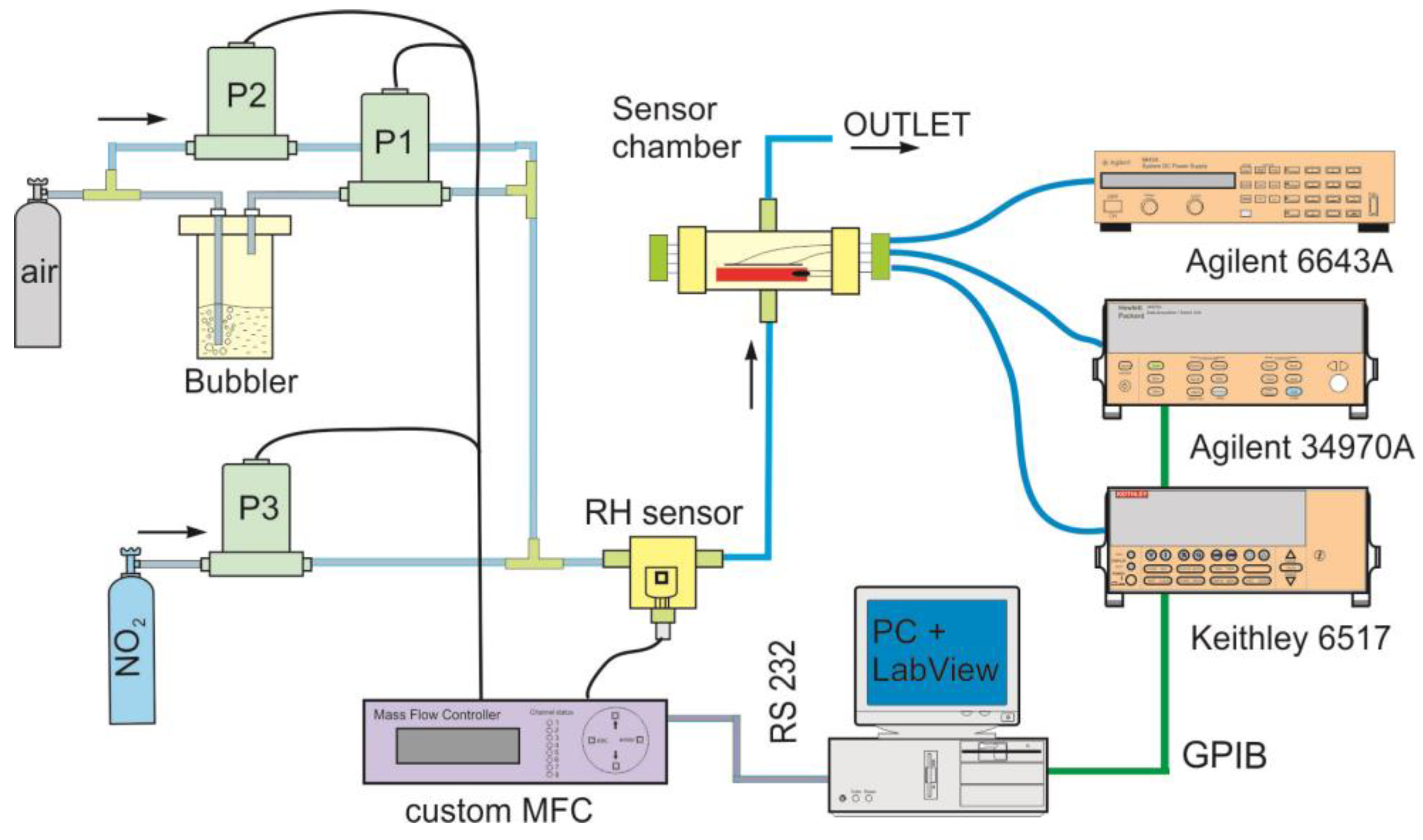
2.2. Gas Sensing Measurement Setup
3. Results and Discussion
3.1. Characterization of Thin Films
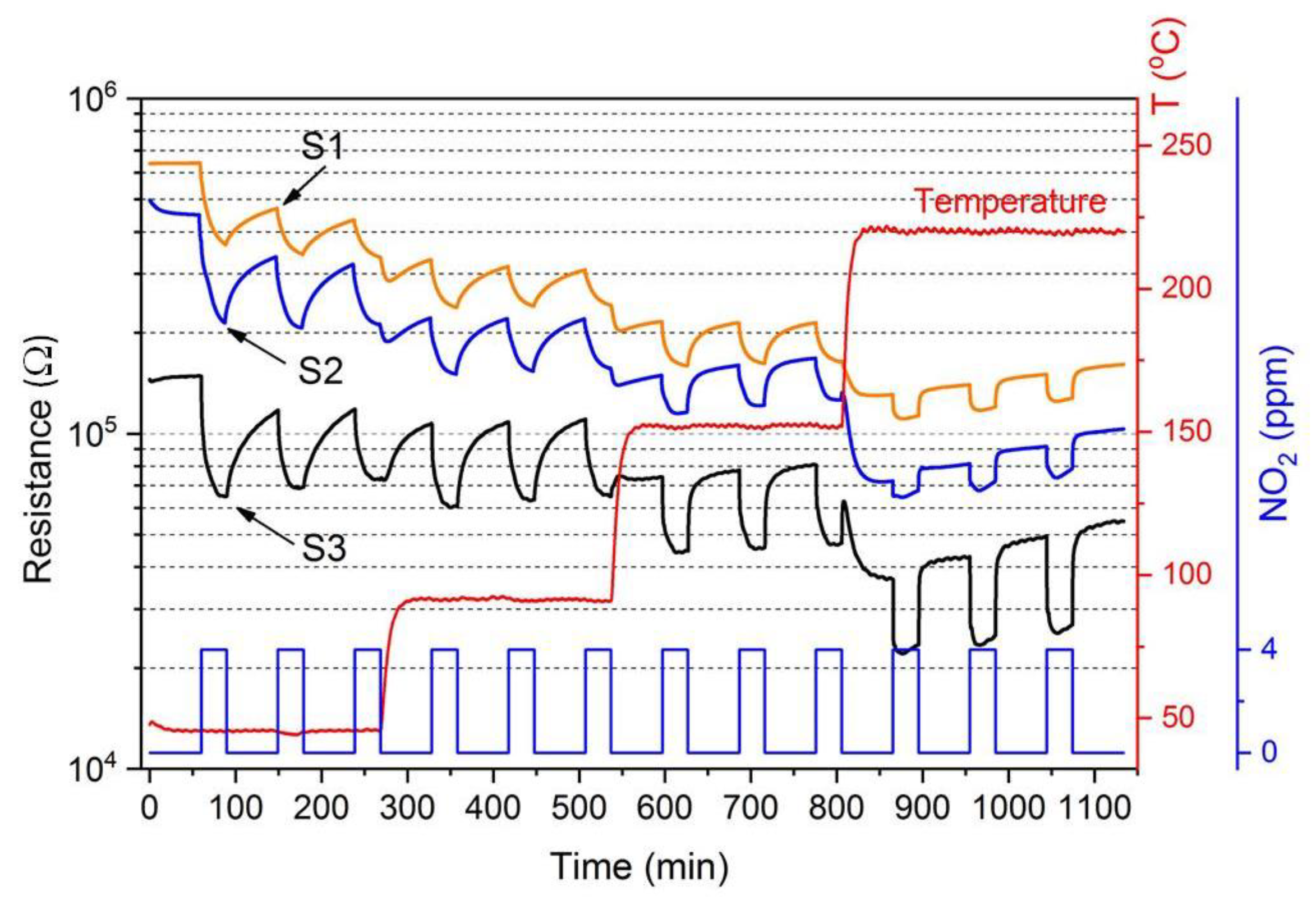
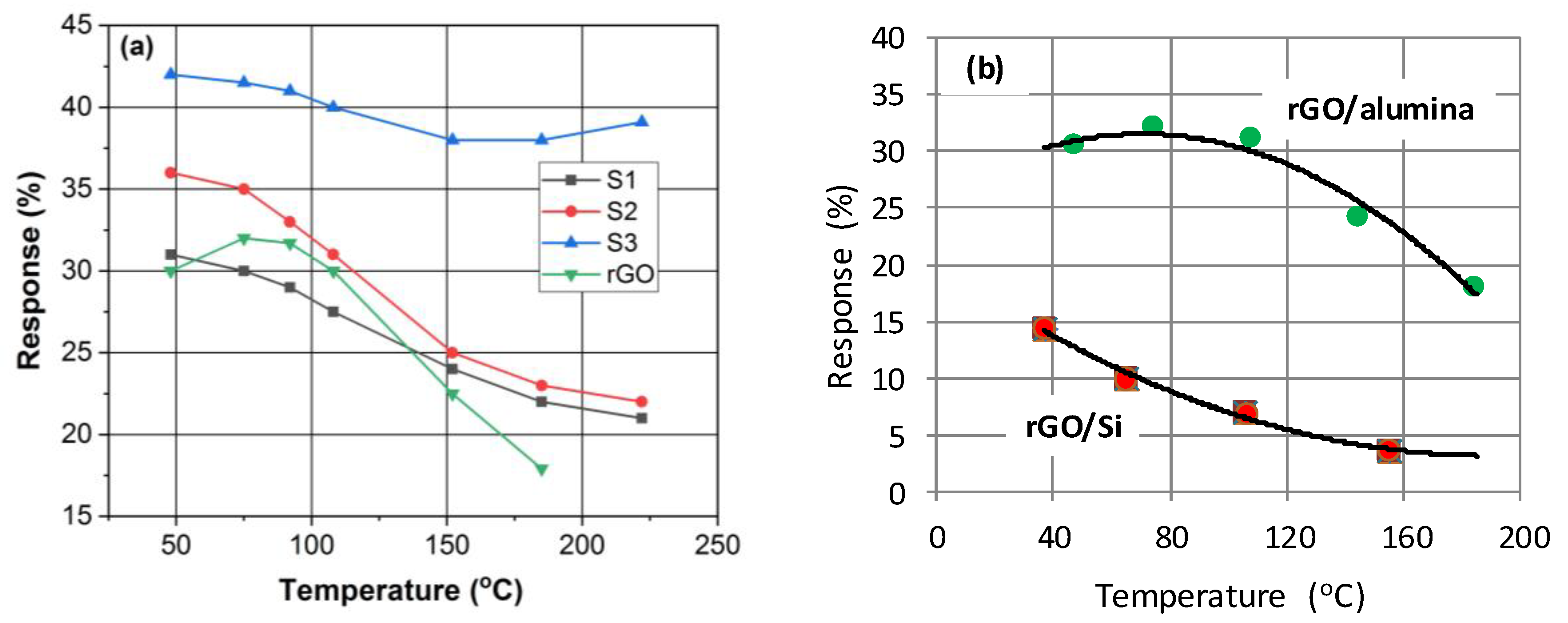
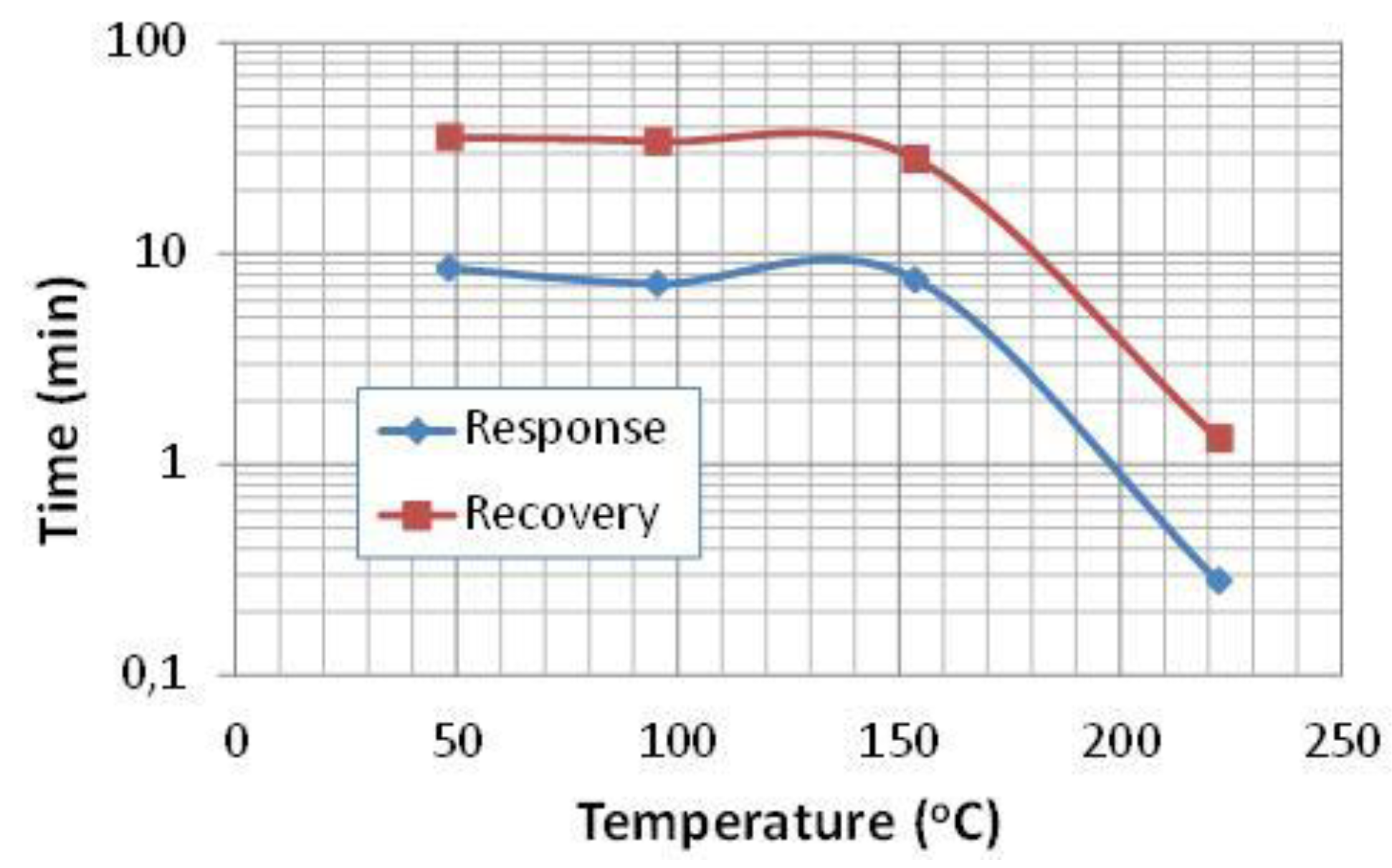
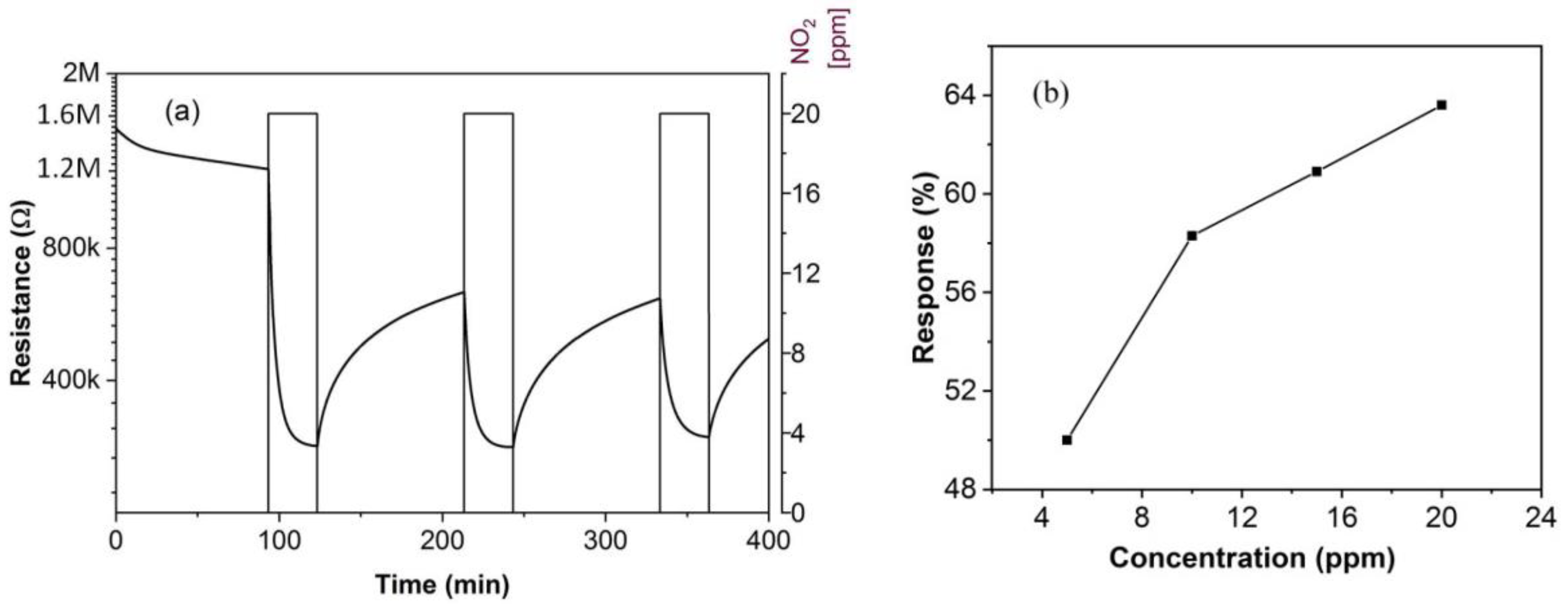
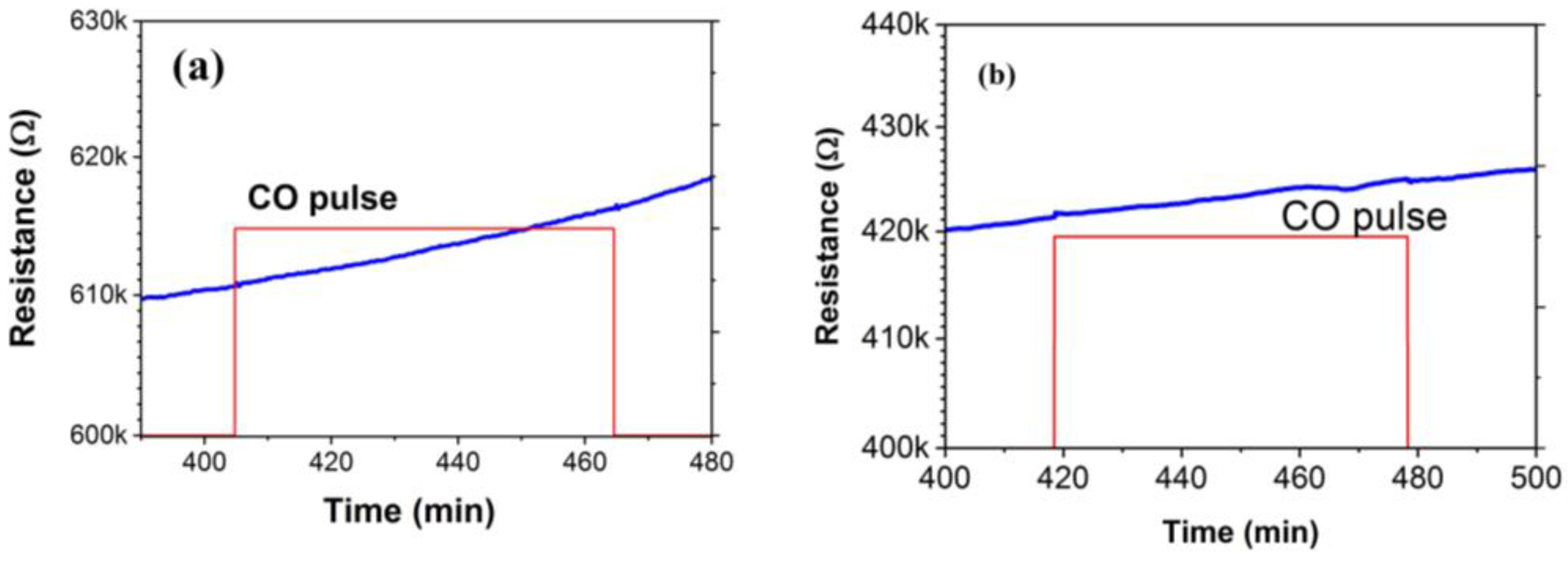
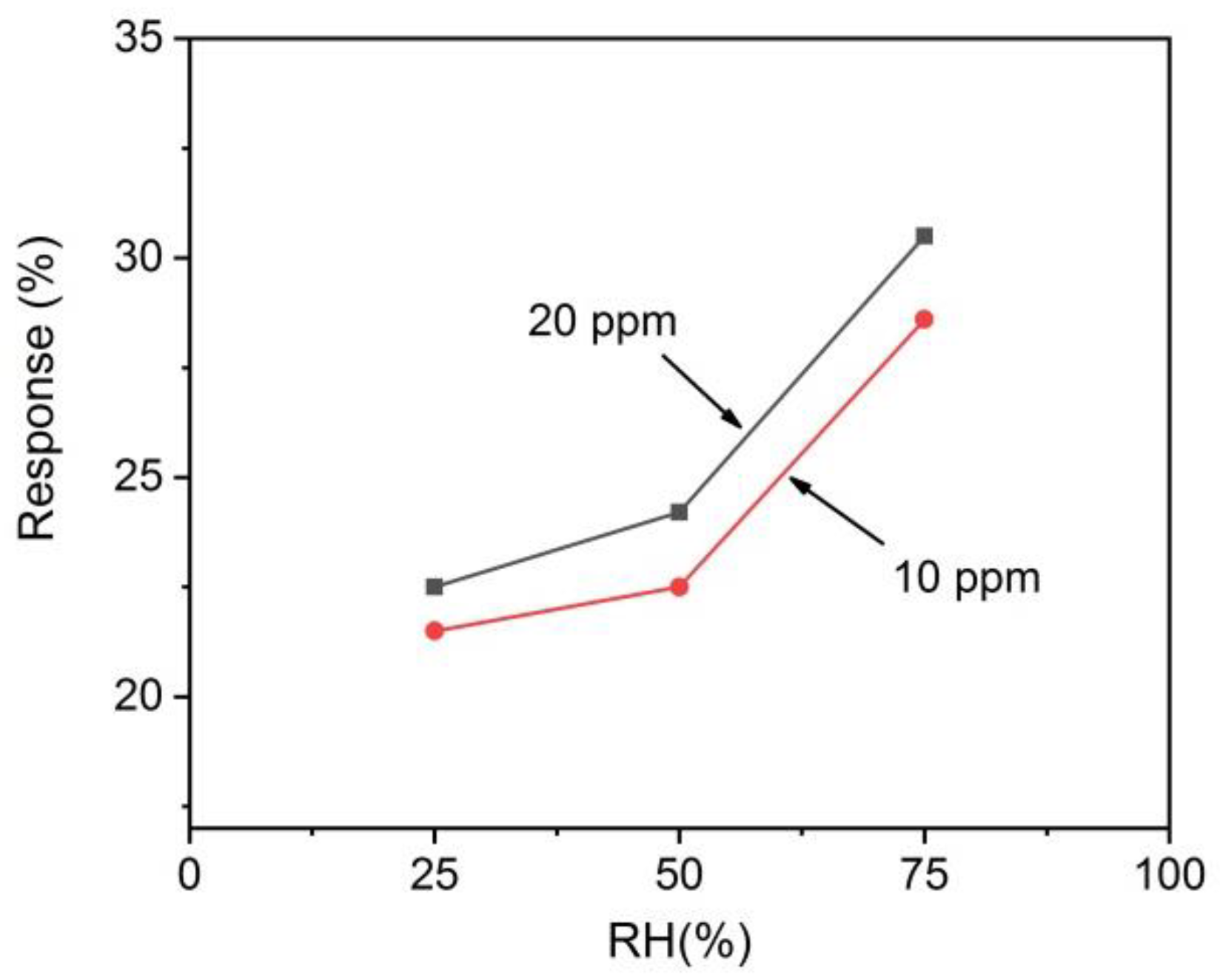
3.2. Gas Sensing Characteristics
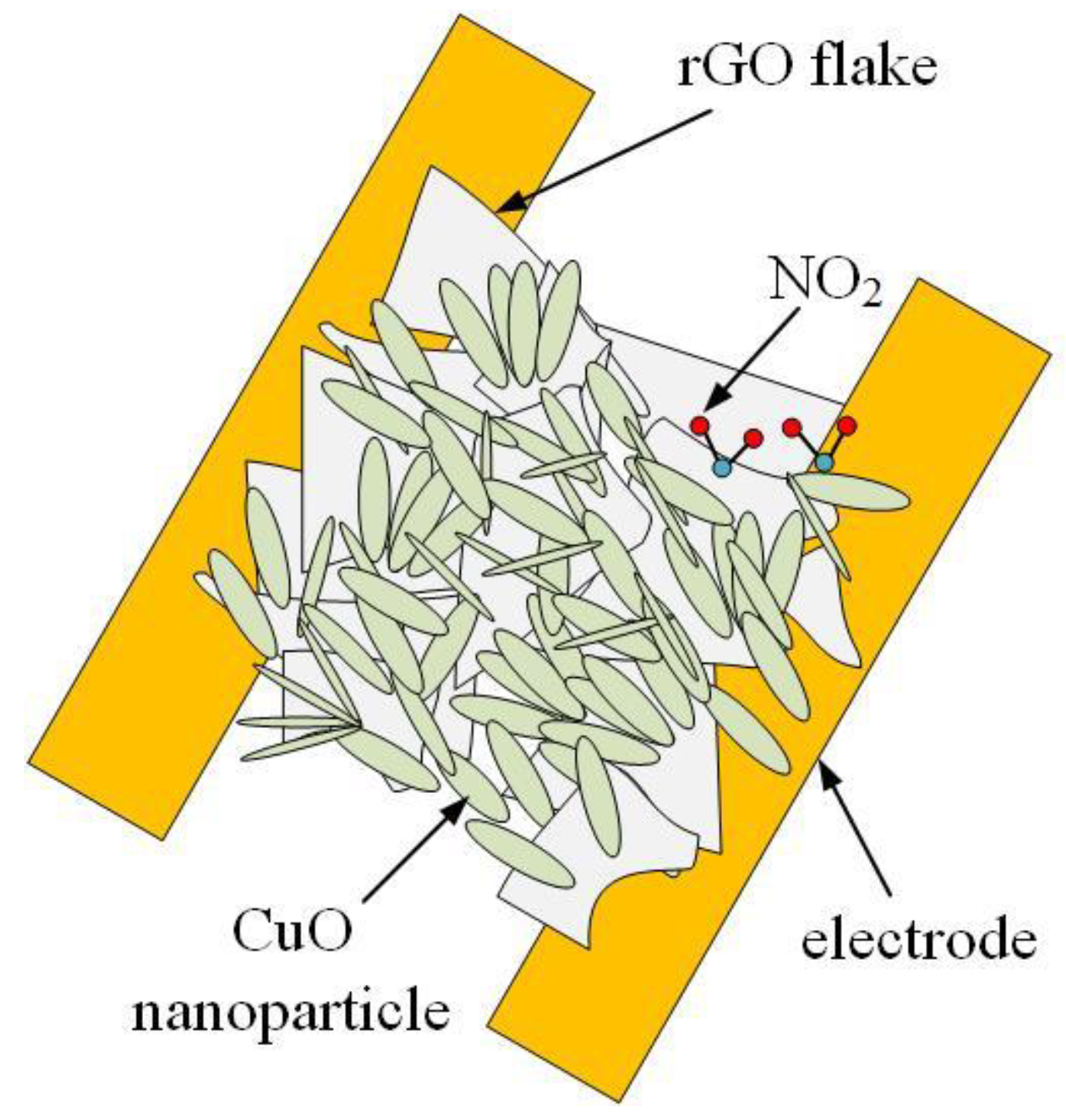
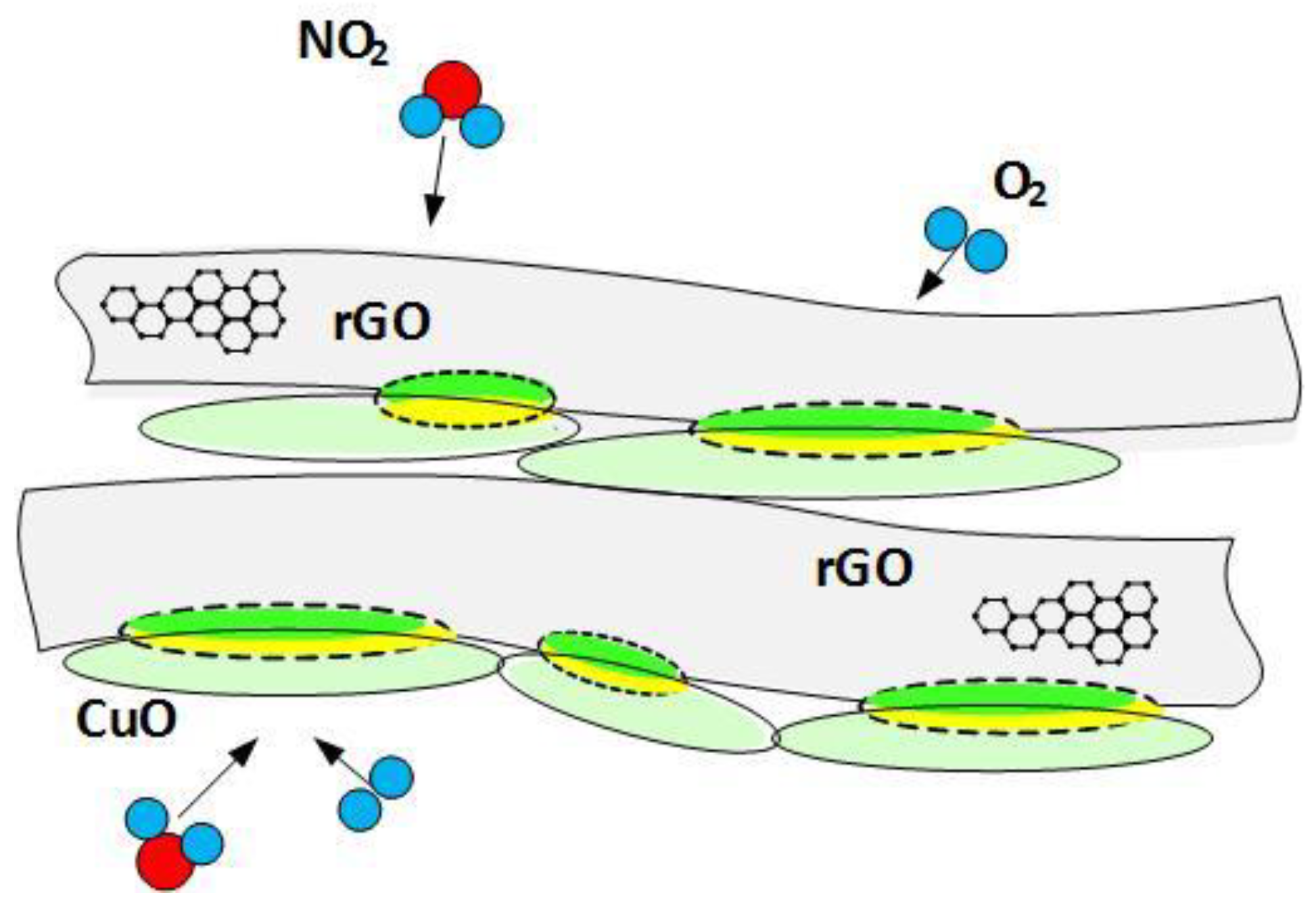
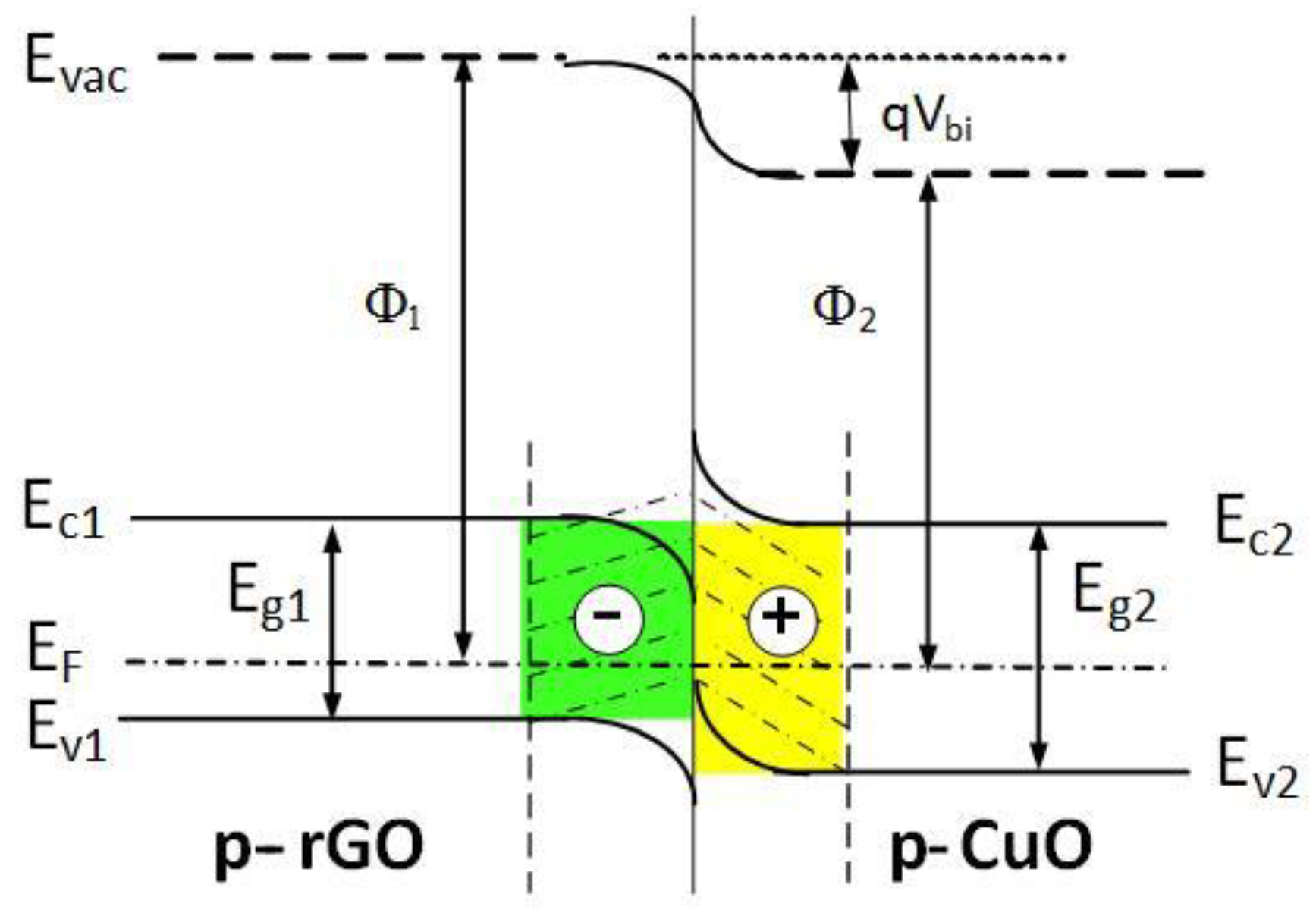
3.3. Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iezhokin, I.; Offermans, P.; Brongersma, S.H.; Giesbers, A.J.M.; Flipse, C.F.J. High sensitive quasi freestanding epitaxial graphene gas sensor on 6H-SiC. Appl. Phys. Lett. 2013, 103, 053514-1–053514-5. [Google Scholar] [CrossRef]
- Ovsianytskyi, O.; Nam, Y.-S.; Tsymbalenko, O.; Lan, P.-T.; Moon, M.-W.; Lee, K.-B. Highly sensitive chemiresistive H2S gas sensor based on graphene decorated with Ag nanoparticles and charged impurities. Sens. Actuators B 2018, 257, 278–285. [Google Scholar] [CrossRef]
- Niavol, S.S.; Budde, M.; Papadogianni, A.; Heilmann, M.; Moghaddan, H.M.; Aldao, C.M.; Ligorio, G.; List-Kratochvil, J.W.; Lopes, J.M.J.; Barsan, N.; et al. Conduction mechanisms in epitaxial NiO/Graphene gas sensors. Sens. Actuators B 2020, 325, 128797. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Y.; Xie, T.; Tai, H.; Xie, G.A. Novel sensing mechanism for resistive gas sensors based on layered reduced graphene oxide thin films at room temperature. Sens. Actuators B 2013, 203, 135–142. [Google Scholar] [CrossRef]
- Sun, D.; Luo, Y.; Debliquy, M.; Zhang, C. Graphene-enhanced metal oxide gas sensors at room temperature: A review. Beilstein J. Nanotechnol. 2018, 9, 2832–2844. [Google Scholar] [CrossRef]
- Zhou, L.; Shen, F.; Tian, X.; Wang, D.; Zhang, T.; Chen, W. Stable Cu2O nanocrystals grown on functionalized graphene sheets and room temperature H2S gas sensing with ultrahigh sensitivity. Nanoscale 2013, 5, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Ta, Q.T.H.; Namgung, G.; Noh, J.-S. Synthesis of Ag@rGO/g-C3N4 layered structures and their application to toxic gas sensors: Effect of Ag nanoparticles. Electron. Mater. Lett. 2019, 15, 750–759. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Kholmanov, I.; Ponzoni, A.; Sberveglieri, V.; Poli, N.; Faglia, G.; Sberveglieri, G. A composite structure based on reduced graphene oxide and metal oxide nanomaterials for chemical sensors. Beilstein J. Nanotechnol. 2016, 7, 1421–1427. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Jiang, C.; Li, P.; Sun, Y. High-performance sulfur dioxide sensing properties of layer-by-layer self-assembled titania-modified graphene hybrid nanocomposite. Sens. Actuators B 2017, 245, 560–567. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, C.; Liu, J.; Cao, Y. Carbon monoxide gas sensing at room temperature using copper oxide-decorated graphene hybrid nanocomposite prepared by layer-by-layer self-assembly. Sens. Actuators B 2017, 247, 875–882. [Google Scholar] [CrossRef]
- Neri, G.; Leonardi, S.G.; Latino, M.; Donato, N.; Baek, S.; Conte, D.E.; Russo, P.A.; Pinna, N. Sensing behaviour of SnO2/reduced graphene oxide nanocomposites toward NO2. Sens. Actuators B 2013, 179, 61–68. [Google Scholar] [CrossRef]
- Chen, Z.-W.; Hong, Y.-Y.; Lin, Z.-D.; Liu, L.-M.; Zhang, X.-W. Enhanced formaldehyde gas sensing properties of ZnO nanosheets modified with graphene. Electron. Mater. Lett. 2017, 13, 270–276. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Zhang, H.; Fei, T.; Zhang, T. Enhancing NO2 gas sensing performance at room temperature based on reduced graphene oxide-ZnO nanoparticles hybrids. Sens. Actuators B 2014, 202, 272–278. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, J.; Fei, T.; Liu, S.; Zhang, T. SnO2 nanoparticles-reduced graphene oxide nanocomposites for NO2 sensing at low operating temperature. Sens. Actuators B 2014, 190, 472–478. [Google Scholar] [CrossRef]
- Feng, Q.; Li, X.; Wang, J. Percolation effect of reduced graphene oxide (rGO) on ammonia sensing of rGO-SnO2 composite based sensor. Sens. Actuators B 2017, 243, 1115–1126. [Google Scholar] [CrossRef]
- Wang, W.H.; Han, W.; Pi, K.; McCreary, K.M.; Miao, F.; Bao, W.; Lau, C.N.; Kawakami, R.K. Growth of atomically smooth MgO films on graphene by molecular beam epitaxy. Appl. Phys. Lett. 2008, 93, 183107. [Google Scholar] [CrossRef]
- Punetha, D.; Kumar Pandey, S. Sensitivity enhancement of ammonia gas sensor based on hydrothermally synthesized rGO/WO3 nanocomposites. IEEE Sensors J. 2020, 20, 1738–1745. [Google Scholar] [CrossRef]
- Yin, F.; Li, Y.; Yue, W.; Gao, S.; Zhang, C.; Chen, Z. Sn3O4/rGO heterostructure as a material for formaldehyde gas sensor with a wide detecting range and low operating temperature. Sens. Actuators B 2020, 312, 127954. [Google Scholar] [CrossRef]
- Wu, J.; Ding, H.; Chen, J.; Wei, Y.; Wu, Z.; Wang, N.; Xie, X.; Shi, W.; Wang, X. Revealing the role of surface co-modification in boosting the gas sensing performance of graphene using experimental and theoretical evidences. Sens. Actuators B 2020, 316, 128162. [Google Scholar] [CrossRef]
- Shafiei, M.; Spizzirri, P.G.; Arsat, R.; Yu, J.; du Plessis, J.; Dubin, S.; Ianer, R.B.; Kalantar-Zadeh, K.; Wlodarski, W. Platinum/graphene nanosheet/SiC contacts and their application for hydrogen gas sensing. J. Phys. Chem. C1 2010, 114, 13796–13801. [Google Scholar] [CrossRef]
- Cho, B.; Yoon, J.; Hahm, M.G.; Kim, D.H.; Kim, A.R.; Khang, Y.H.; Park, S.-W.; Lee, Y.-J.; Park, S.-G.; Kwon, J.-D.; et al. Graphene-based gas sensor: Metal decoration effect and application to a flexible device. J. Mater. Chem. C 2014, 2, 5280–5285. [Google Scholar] [CrossRef]
- Kumar, R.; Varandani, D.; Mehta, B.R.; Singh, V.N.; Wen, Z.; Feng, X.; Muellen, K. Fast response and recovery of hydrogen sensing in Pd–Pt nanoparticle-graphene composite layers. Nanotechnology 2011, 22, 275719–275726. [Google Scholar] [CrossRef] [PubMed]
- Toh, R.J.; Poh, H.I.; Sofer, Z.; Pumera, M. Transition metal (Mn, Fe, Co, Ni) doped graphen hybrides for electrocatalysis. Chem. Asian. J. 2013, 8, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Luo, X.; Song, H.; Lin, X.; Lu, X.; Tang, Y. DFT study of adsorption and dissociation behaviour of H2S on Fe-doped graphene. Appl. Surf. Sci. 2014, 317, 511–516. [Google Scholar] [CrossRef]
- Na, H.G.; Cho, H.J.; Kwon, Y.J.; Kang, S.Y.; Lee, C.; Jung, T.K.; Lee, H.-S.; Kim, H.W. Reduced graphene oxide functionalized with Cu nanoparticles: Fabrication, structure, and sensing properties. Thin Solid Films 2015, 588, 11–18. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Chen, Y.-B.; Zhou, K.-G.; Liu, C.-H.; Zeng, J.; Zhang, H.-L.; Peng, Y. Improving gas sensing properties of graphene by introducing dopants and defects: A first-principles study. Nanotechnology 2009, 20, 185504. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Chen, X.Q.; Wu, N.Q.; Nguyen, S.T.; Ruoff, R. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly (sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Pisarkiewicz, T.; Maziarz, W.; Małolepszy, A.; Stobiński, L.; Michoń, D.; Rydosz, A. Gas sensing properties of reduced graphene oxide modified by copper oxide. In Proceedings of the 15th International Conference on Optical and Electronic Sensors COE 2018, Warsaw, Poland, 18–20 June 2018. [Google Scholar]
- Geng, W.; Ma, Z.; Zhao, Y.; Yang, J.; He, X.; Duan, L.; Li, F.; Hou, H.; Zhang, Q. Morphology-dependent gas sensing properties of CuO microstructures self-assembled from nanorod. Sens. Actuators B 2020, 325, 128775. [Google Scholar] [CrossRef]
- Stobiński, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron. Spectr. Related Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Lipatov, A.; Verezhnikov, A.; Wilson, P.; Sysoev, V.; Kolmakov, A.; Sinitskii, A. Highly selective gas sensor arrays based on thermally reduced graphene oxide. Nanoscale 2013, 5, 5426–5434. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Ocola, L.G.; Chen, J. Reduced graphene oxide for room temperature gas sensors. Nanotechnology 2009, 20, 445502. [Google Scholar] [CrossRef] [PubMed]
- Rasch, F.; Postica, V.; Schuett, F.; Mishra, Y.K.; Nia, A.S.; Lohe, M.R.; Feng, X.; Adelung, R.; Lupan, O. Highly selective and ultra-low power consumption metal oxide based hydrogen gas sensor employing graphene oxide as molecular sieve. Sens. Actuators B 2020, 320, 128363. [Google Scholar] [CrossRef]
- Yang, Y.; Tian, C.; Wang, J.; Sun, I.; Shi, K.; Zhou, W.; Fu, H. Facile synthesis of novel 3D nanoflower-like CuxO multilayer graphen composites for room temperature NOx gas sensor application. Nanoscale 2014, 6, 7369–7378. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, Y.; Yu, D.; Li, L. Seed/catalyst free growth and self-powered photoresponse of vertically aligned ZnO nanorods on reduced graphene oxide nanosheets. Cryst. Growth Des. 2016, 16, 4831–4838. [Google Scholar] [CrossRef]
- Huang, H.; Li, Z.; She, J.; Wang, W. Oxygen density dependent band gap of reduced graphene oxide. J. Appl. Phys. 2012, 111, 054317. [Google Scholar] [CrossRef]
- Lin, H.H.; Wang, C.Y.; Shih, H.C.; Chen, J.M.; Hsieh, C.T. Characterizing well-oriented CuO nanofibrils synthesized through gas-solid reactions. J. Appl. Phys. 2004, 95, 5889–5894. [Google Scholar] [CrossRef]
- Park, W.J.; Choi, K.J.; Kim, M.H.; Koo, B.H.; Lee, J.-L.; Baik, J.M. Self-assembled and highly selective sensors based on air-bridge-structured nanowire junction arrays. ACS Appl. Mater. Interfaces 2013, 5, 6802–6807. [Google Scholar] [CrossRef]


| Sample | C (wt %) | O (wt %) | N (wt %) | H (wt %) | Others (wt %) |
|---|---|---|---|---|---|
| GO | 45.51 | 47.81 | 0.23 | 2.45 | 4.0 |
| rGO | 85.55 | 9.95 | 3.20 | 1.05 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisarkiewicz, T.; Maziarz, W.; Małolepszy, A.; Stobiński, L.; Michoń, D.; Rydosz, A. Multilayer Structure of Reduced Graphene Oxide and Copper Oxide as a Gas Sensor. Coatings 2020, 10, 1015. https://doi.org/10.3390/coatings10111015
Pisarkiewicz T, Maziarz W, Małolepszy A, Stobiński L, Michoń D, Rydosz A. Multilayer Structure of Reduced Graphene Oxide and Copper Oxide as a Gas Sensor. Coatings. 2020; 10(11):1015. https://doi.org/10.3390/coatings10111015
Chicago/Turabian StylePisarkiewicz, Tadeusz, Wojciech Maziarz, Artur Małolepszy, Leszek Stobiński, Dagmara Michoń, and Artur Rydosz. 2020. "Multilayer Structure of Reduced Graphene Oxide and Copper Oxide as a Gas Sensor" Coatings 10, no. 11: 1015. https://doi.org/10.3390/coatings10111015
APA StylePisarkiewicz, T., Maziarz, W., Małolepszy, A., Stobiński, L., Michoń, D., & Rydosz, A. (2020). Multilayer Structure of Reduced Graphene Oxide and Copper Oxide as a Gas Sensor. Coatings, 10(11), 1015. https://doi.org/10.3390/coatings10111015






