Development of a Fluorine-Free Polymer-Assisted-Deposition Route for YBa2Cu3O7−x Superconducting Films
Abstract
1. Introduction
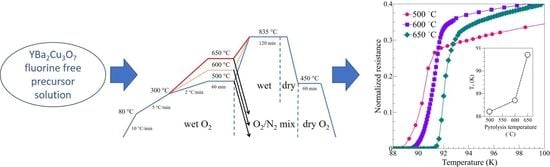
2. Materials and Methods
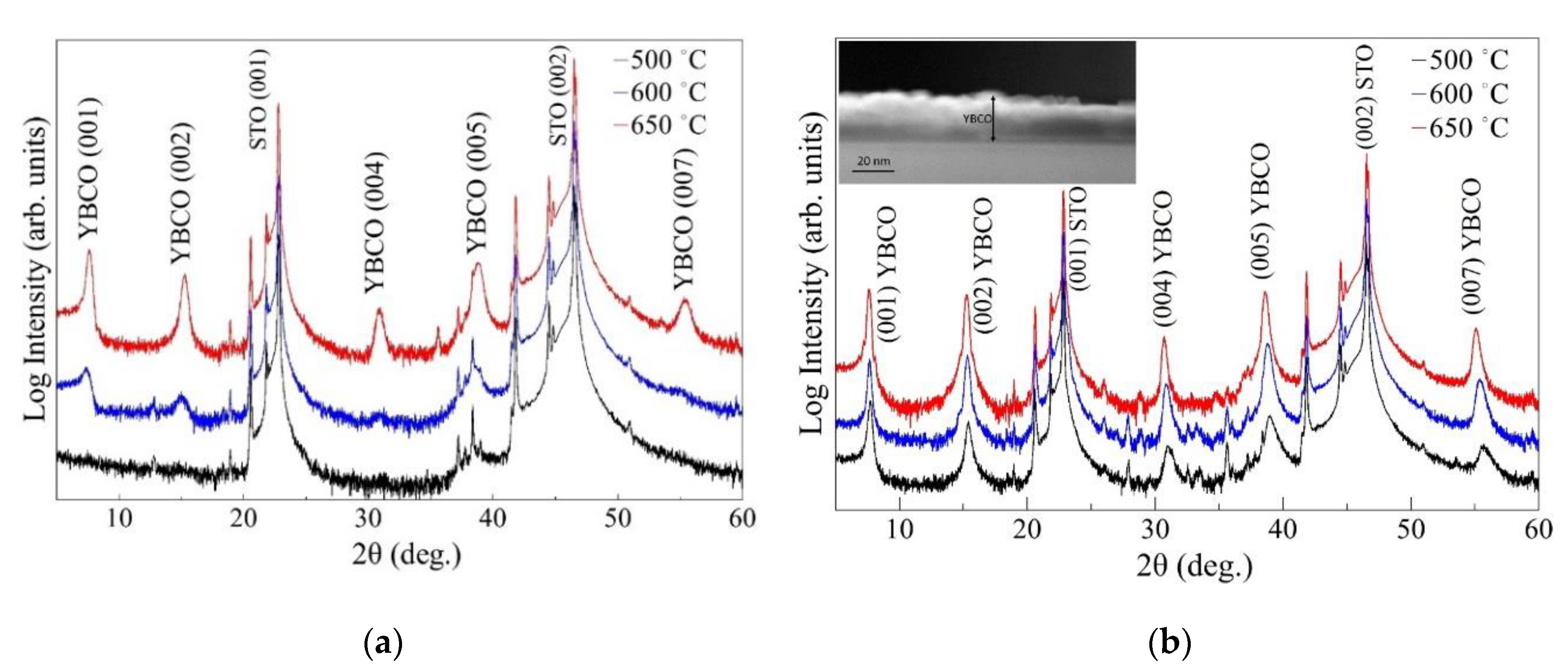
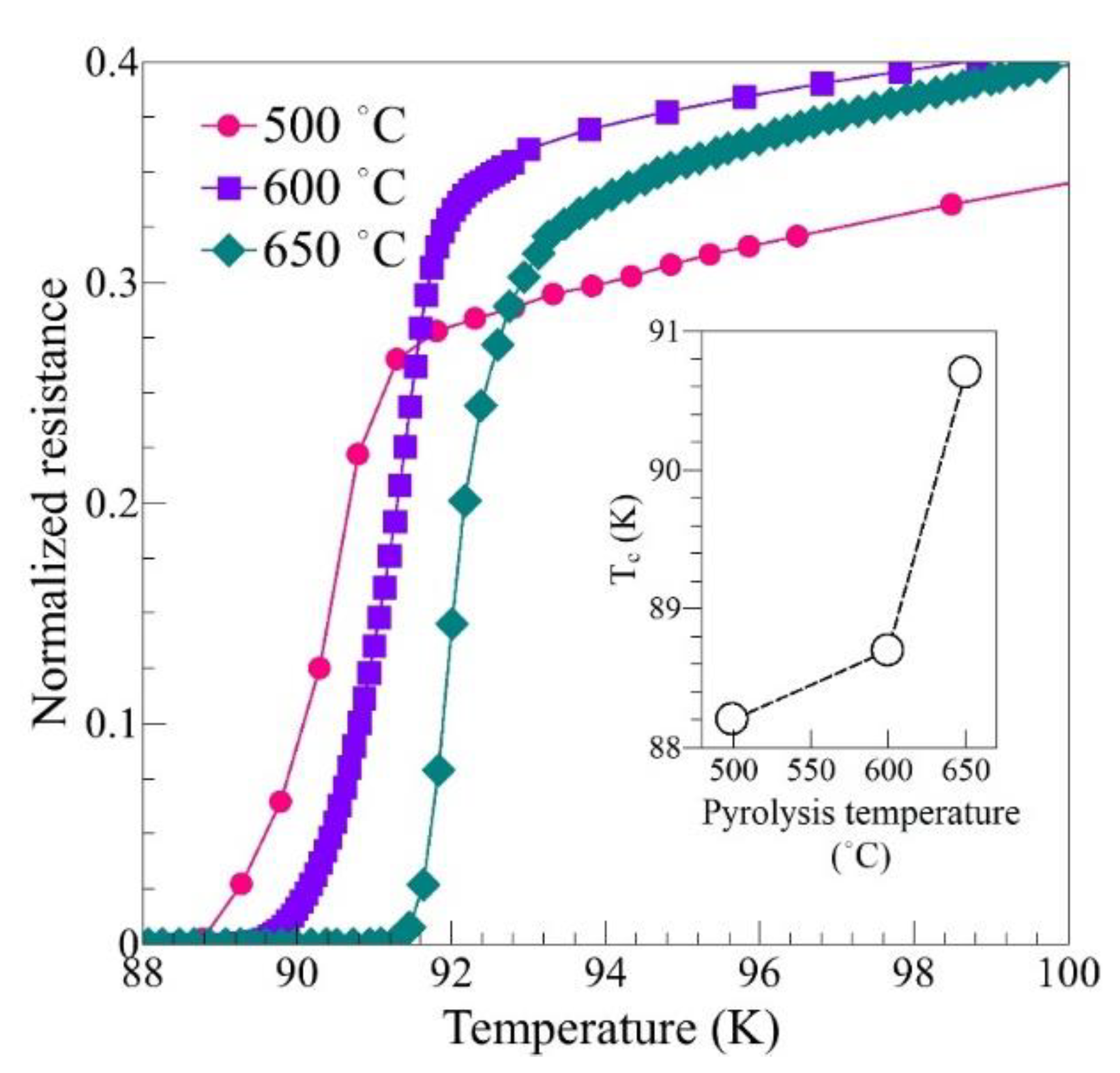
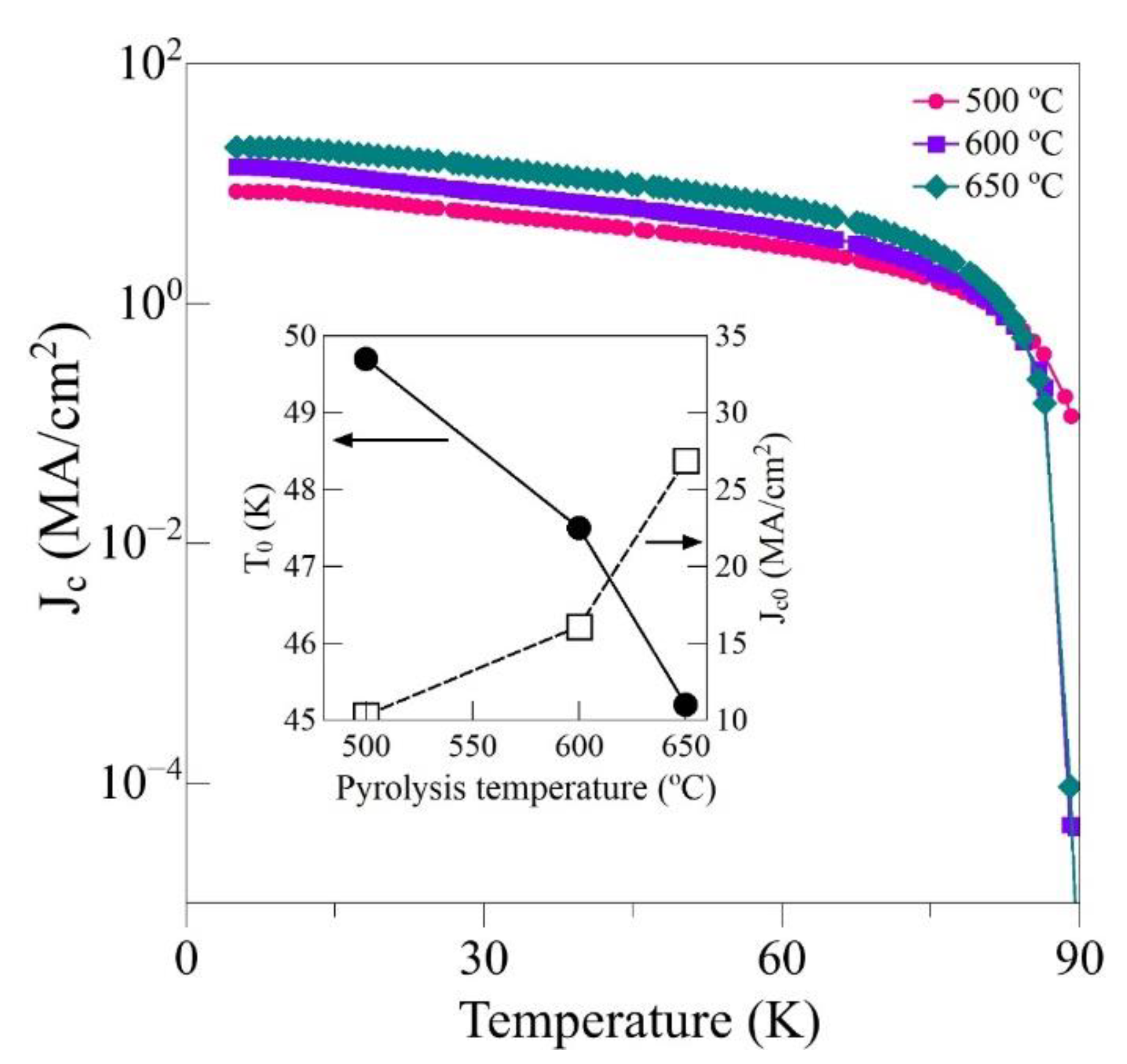
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Larbalestier, D.C.; Gurevich, A.; Feldmann, D.M.; Polyanskii, A. High-Tc superconducting materials for electric power applications. Nat. Cell Biol. 2001, 414, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Obradors, X.; Puig, T.; Pomar, A.; Sandiumenge, F.; Mestres, N.; Coll, M.; Cavallaro, A.; Roma, N.; Gazquez, J.; Gonzalez, J.C. Progress towards all-chemical superconducting YBa2Cu3O7-coated conductors. Supercond. Sci. Technol. 2006, 19, S13–S26. [Google Scholar] [CrossRef]
- Pop, C.; Villarejo, B.; Pino, F.; Mundet, B.; Ricart, S.; De Palau, M.; Puig, T.; Obradors, X. Growth of all-chemical high critical current YBa2Cu3O7−δ thick films and coated conductors. Supercond. Sci. Technol. 2018, 32, 015004. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Wang, L.; Gao, L.; Liu, J.; Zang, Z. High quality uniform YBCO film growth by the metalorganic deposition using trifluoroacetates. Phys. C Supercond. 2017, 534, 68–72. [Google Scholar] [CrossRef]
- Palmer, X.; Pop, C.; Eloussifi, H.; Villarejo, B.; Roura, P.; Farjas, J.; Calleja, A.; Palau, A.; Obradors, X.; Puig, T.; et al. Solution design for low-fluorine trifluoroacetate route to YBa2Cu3O7 films. Supercond. Sci. Technol. 2015, 29, 024002. [Google Scholar] [CrossRef]
- Li, M.; Cayado, P.; Erbe, M.; Jung, A.; Hänisch, J.; Holzapfel, B.; Liu, Z.; Cai, C. Rapid Pyrolysis of SmBa2Cu3O7-δ Films in CSD-MOD Using Extremely-Low-Fluorine Solutions. Coatings 2020, 10, 31. [Google Scholar] [CrossRef]
- Pinto, V.; Vannozzi, A.; Armenio, A.A.; Rizzo, F.; Masi, A.; Santoni, A.; Meledin, A.; Ferrarese, F.M.; Orlanducci, S.; Celentano, G. Chemical Solution Deposition of YBCO Films with Gd Excess. Coatings 2020, 10, 860. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.; Bai, C.; Lu, Y.; Guo, Y.; Cai, C. Artificial control for nucleation and growth rate of YBa2Cu3O7−δ coated conductors prepared by low fluorine chemical solution deposition. Phys. C 2017, 537, 29–33. [Google Scholar] [CrossRef]
- Moriya, K.; Igarashi, K.; Watanabe, H.; Hasegawa, H.; Sasaki, T.; Yasuda, A. Growth of YBa2Cu3O7 superconductor thin films using ethanolamine-based solutions via simple spin coating. Results Phys. 2018, 11, 364–367. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, Y.; Wu, W.; Andersen, N.; Grivel, J.-C. High-Jc YBa2Cu3O7−x-Ag superconducting thin films synthesized through a fluorine-free MOD method. J. Eur. Ceram. Soc. 2015, 35, 1761–1769. [Google Scholar] [CrossRef]
- Nasui, M.; Petrisor, T., Jr.; Mos, R.B.; Gabor, M.S.; Mesaros, A.; Goga, F.; Ciontea, L.; Petrisor, T. Fluorine-free propionate route for the chemical solution deposition of YBa2Cu3O7−x superconducting films. Ceram. Int. 2015, 41, 4416–4421. [Google Scholar] [CrossRef]
- Zhao, Y.; Chu, J.; Qureishy, T.; Wu, W.; Zhang, Z.; Mikheenko, P.; Johansen, T.H.; Grivel, J.-C. Structural and superconducting characteristics of YBa2Cu3O7 films grown by fluorine-free metal-organic deposition route. Acta Mater. 2018, 144, 844–852. [Google Scholar] [CrossRef]
- Huang, R.X.; Feng, F.; Wu, W.; Xue, Y.R.; Zhang, Y.Y.; Shi, K.; Qu, T.M.; Zhao, V.; Wang, X.H.; Zhang, X.W.; et al. A water-free metal organic deposition method for YBa2Cu3O7−δ thin film fabrication. Supercond. Sci. Technol. 2013, 26, 115010. [Google Scholar] [CrossRef]
- Vermeir, P.; Feys, J.; Schaubroeck, J.; Verbeken, K.; Lommens, P.; Driessche, I. Van Influence of sintering conditions in the preparation of acetate-based fluorine-free CSD YBCO films using a direct sintering method. Mater. Res. Bull. 2012, 47, 4376–4382. [Google Scholar] [CrossRef]
- Soler, L.; Jareño, J.; Banchewski, J.; Rasi, S.; Chamorro, N.; Guzmán, R.; Yáñez, R.; Mocuta, C.; Ricart, S.; Farjas, J.; et al. Ultrafast transient liquid assisted growth of high current density superconducting films. Nat. Commun. 2020, 11, 344–348. [Google Scholar] [CrossRef]
- Schoofs, B.; Cloet, V.; Vermeir, P.; Schaubroeck, J.; Hoste, S.; Van Driessche, I. A water-based sol–gel technique for chemical solution deposition of (RE)Ba2Cu3O7−y(RE = Nd and Y) superconducting thin films. Supercond. Sci. Technol. 2006, 19, 1178–1184. [Google Scholar] [CrossRef]
- Lei, L.; Zhao, G.; Bai, Y.; Wu, C.; Zhang, X. Carbon Dioxide Controlled Thermal-Decomposition Reactions for Preparation of High Quality YBCO Films Derived from Fluorine-Free Solution. J. Supercond. Nov. Magn. 2013, 27, 23–26. [Google Scholar] [CrossRef]
- Calleja, A.; Mos, R.B.; Roura, P.; Farjas, J.; Arbiol, J.; Ciontea, L.; Obradors, X.; Puig, T. Growth of epitaxial CeO2 buffer layers by polymer assisted deposition. MRS Proc. 2012, 1449, 31–39. [Google Scholar] [CrossRef]
- Patta, Y.; Wesolowski, D.; Cima, M. Aqueous polymer–nitrate solution deposition of YBCO films. Phys. C Supercond. 2009, 469, 129–134. [Google Scholar] [CrossRef]
- Wang, W.; Li, G.; Pu, M.; Sun, R.; Zhou, H.; Zhang, Y.; Zhang, H.; Yang, Y.; Cheng, C.; Zhao, Y. Chemical solution deposition of YBCO thin film by different polymer additives. Phys. C Supercond. 2008, 468, 1563–1566. [Google Scholar] [CrossRef]
- Wang, W.T.; Wang, Z.; Pu, M.H.; Wang, M.J.; Zhang, X.; Lei, M.; Zhao, Y. A Novel Partial Melting Process for YBa2Cu3O7−z Superconducting Films by Fluorine-Free Polymer-Assisted Metal Organic Deposition Approach. J. Supercond. Nov. Magn. 2015, 28, 3249–3253. [Google Scholar] [CrossRef]
- Jia, Q.X.; McCleskey, T.M.; Burrell, A.K.; Lin, Y.; Collis, G.E.; Wang, H.; Li, A.D.Q.; Foltyn, S.R. Polymer-assisted deposition of metal-oxide films. Nat. Mater. 2004, 3, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.W. Chemical Solution Deposition of Perovskite Thin Films. Chem. Mater. 1997, 9, 2325–2340. [Google Scholar] [CrossRef]
- Garcia, M.A.; Ali, M.; Parsons-Moss, T.; Ashby, P.D.; Nitsche, H. Metal oxide films produced by polymer-assisted deposition (PAD) for nuclear science applications. Thin Solid Film. 2008, 516, 6261–6265. [Google Scholar] [CrossRef][Green Version]
- Vikram, L.; Raju, B.; Ragul, R.; Sivasankar, B.N. Thermal degradation studies on some lanthanide—edta complexes containing hydrazinium cation. J. Therm. Anal. Calorim. 2008, 93, 987–991. [Google Scholar] [CrossRef]
- Kalagi, S.; Dalavi, D.; Pawar, R.; Tarwal, N.; Mali, S.S.; Patil, P. Polymer assisted deposition of electrochromic tungsten oxide thin films. J. Alloy. Compd. 2010, 493, 335–339. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Yan, Y.; Wang, J.; Huang, J. Elegant cooperativity of noncovalent interactions in effective removal of Cu–EDTA from water via stepwise addition of polymer and surfactant. RSC Adv. 2016, 6, 101725–101730. [Google Scholar] [CrossRef]
- Zou, G.; Zhao, J.; Luo, H.M.; McCleskey, T.M.; Burrell, A.K.; Jia, Q.X. Polymer-assisted-deposition: A chemical solution route for a wide range of materials. Chem. Soc. Rev. 2013, 42, 439–449. [Google Scholar] [CrossRef]
- Ramanavičius, S.; Tereshchenko, A.; Karpicz, R.; Ratautaite, V.; Bubniene, U.; Maneikis, A.; Jagminas, A.; Ramanavicius, A. TiO2−x/TiO2-Structure Based ‘Self-Heated’ Sensor for the Determination of Some Reducing Gases. Sensors 2019, 20, 74. [Google Scholar] [CrossRef]
- Zhai, H.Y.; Chu, W.K. Effect of interfacial strain on critical temperature of YBa2Cu3O7−δ thin films. Appl. Phys. Lett. 2000, 76, 3469–3471. [Google Scholar] [CrossRef]
- Puig, T.; Gutiérrez, J.; Pomar, A.; Llordés, A.; Gázquez, J.; Ricart, S.; Sandiumenge, F.; Obradors, X. Vortex pinning in chemical solution nanostructured YBCO films. Supercond. Sci. Technol. 2008, 21, 034008. [Google Scholar] [CrossRef]
- Maroni, V.A.; Li, Y.; Feldmann, D.M.; Jia, Q.X. Correlation between cation disorder and flux pinning in the YBa2Cu3O7 coated conductor. J. Appl. Phys. 2007, 102, 113909. [Google Scholar] [CrossRef]


| Pyrolysis Temperature (°C) | 550 | 600 | 650 |
|---|---|---|---|
| c lattice parameter (Å) | 11.553 | 11.604 | 11.665 |
| I(007) YBCO/I(002) STO (%) | 2.41 × 10−5 | 7.96 × 10−5 | 11.1 × 10−5 |
| FWHM (°) | 0.836 | 0.659 | 0.467 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasui, M.; Sonher, R.B.; Petrisor, T., Jr.; Varodi, S.; Pop, C.; Ciontea, L.; Petrisor, T. Development of a Fluorine-Free Polymer-Assisted-Deposition Route for YBa2Cu3O7−x Superconducting Films. Coatings 2020, 10, 966. https://doi.org/10.3390/coatings10100966
Nasui M, Sonher RB, Petrisor T Jr., Varodi S, Pop C, Ciontea L, Petrisor T. Development of a Fluorine-Free Polymer-Assisted-Deposition Route for YBa2Cu3O7−x Superconducting Films. Coatings. 2020; 10(10):966. https://doi.org/10.3390/coatings10100966
Chicago/Turabian StyleNasui, Mircea, Ramona Bianca Sonher, Traian Petrisor, Jr., Sorin Varodi, Cornelia Pop, Lelia Ciontea, and Traian Petrisor. 2020. "Development of a Fluorine-Free Polymer-Assisted-Deposition Route for YBa2Cu3O7−x Superconducting Films" Coatings 10, no. 10: 966. https://doi.org/10.3390/coatings10100966
APA StyleNasui, M., Sonher, R. B., Petrisor, T., Jr., Varodi, S., Pop, C., Ciontea, L., & Petrisor, T. (2020). Development of a Fluorine-Free Polymer-Assisted-Deposition Route for YBa2Cu3O7−x Superconducting Films. Coatings, 10(10), 966. https://doi.org/10.3390/coatings10100966