Reactive Sputtering Deposition of Epitaxial TiC Film on Si (100) Substrate
Abstract
1. Introduction
2. Material and Methods
3. Results and Discussion
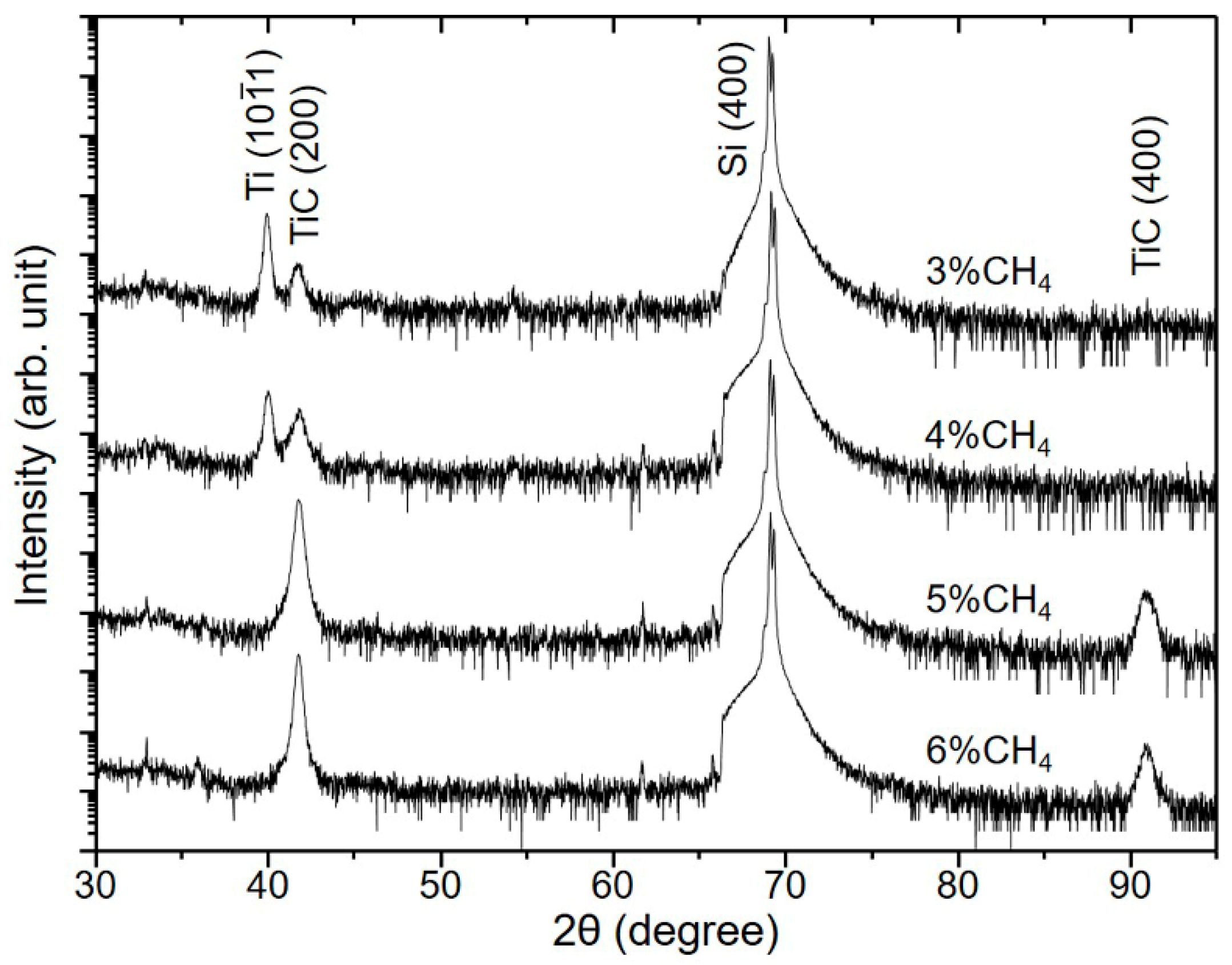
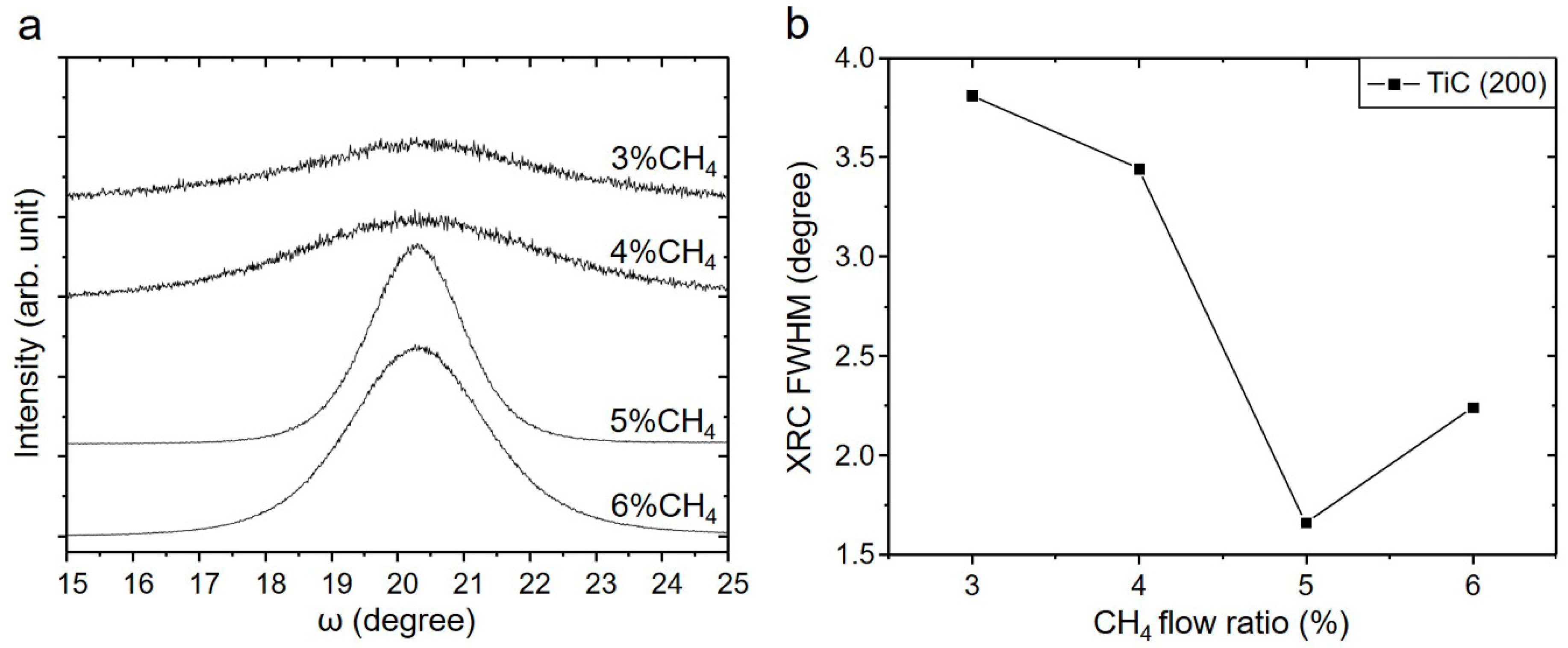
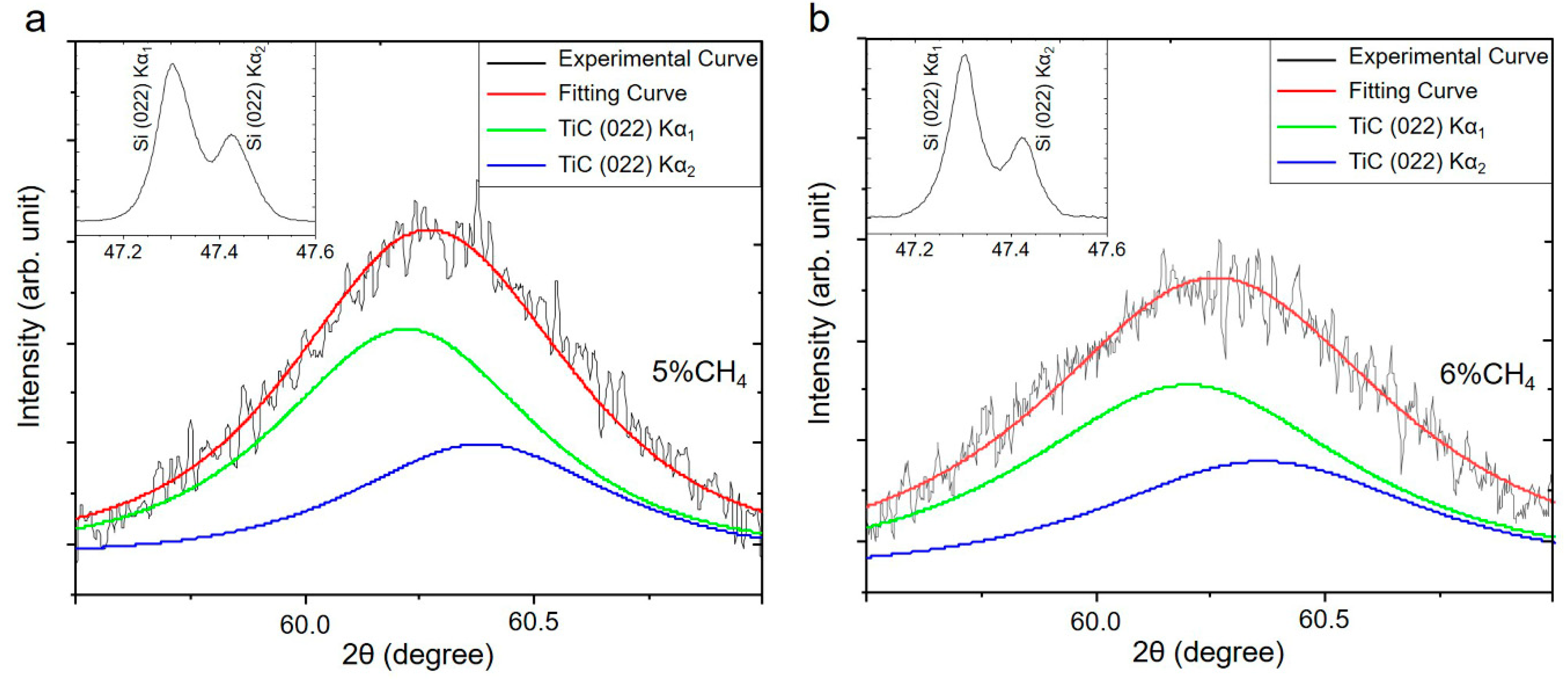
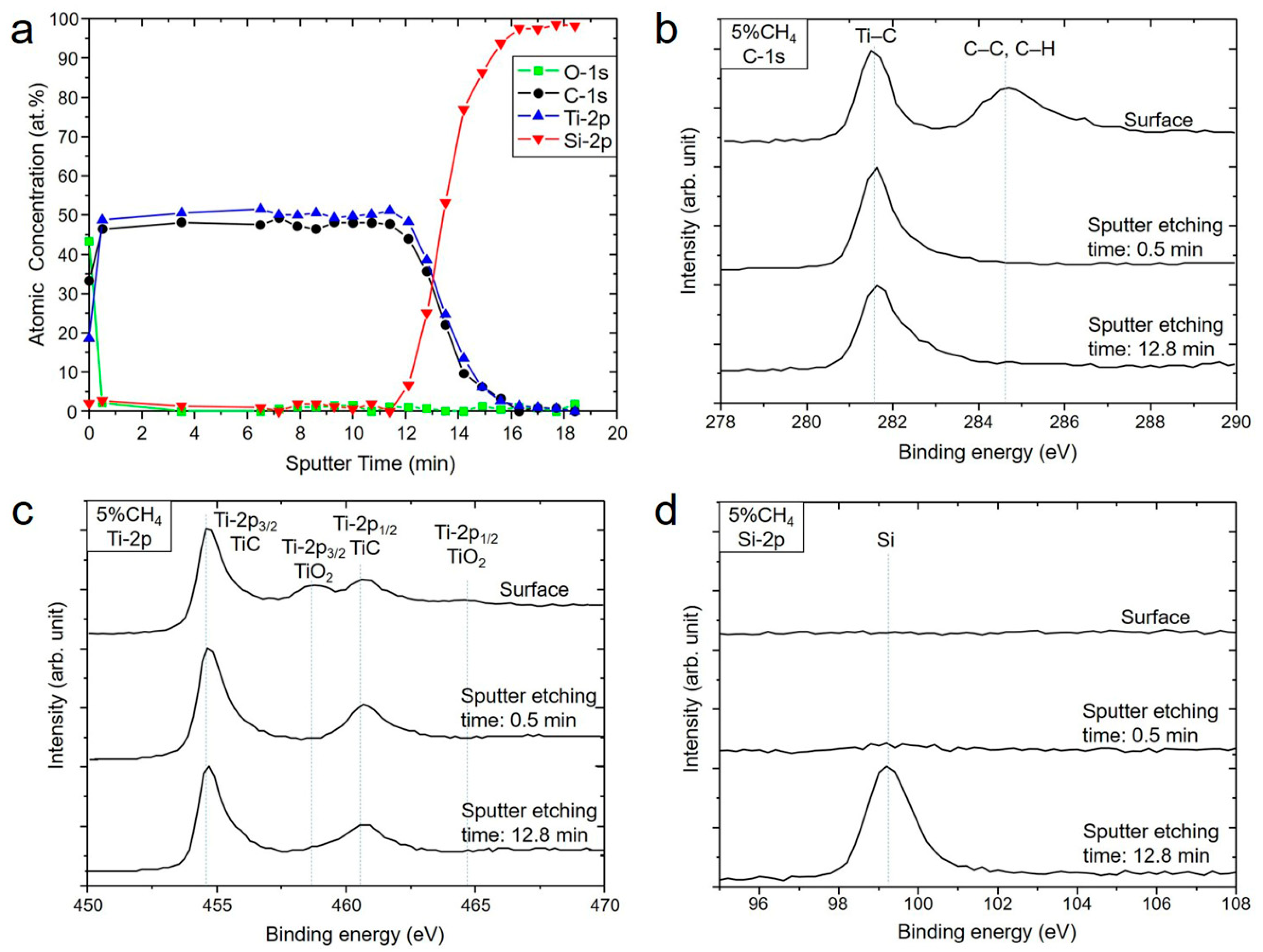
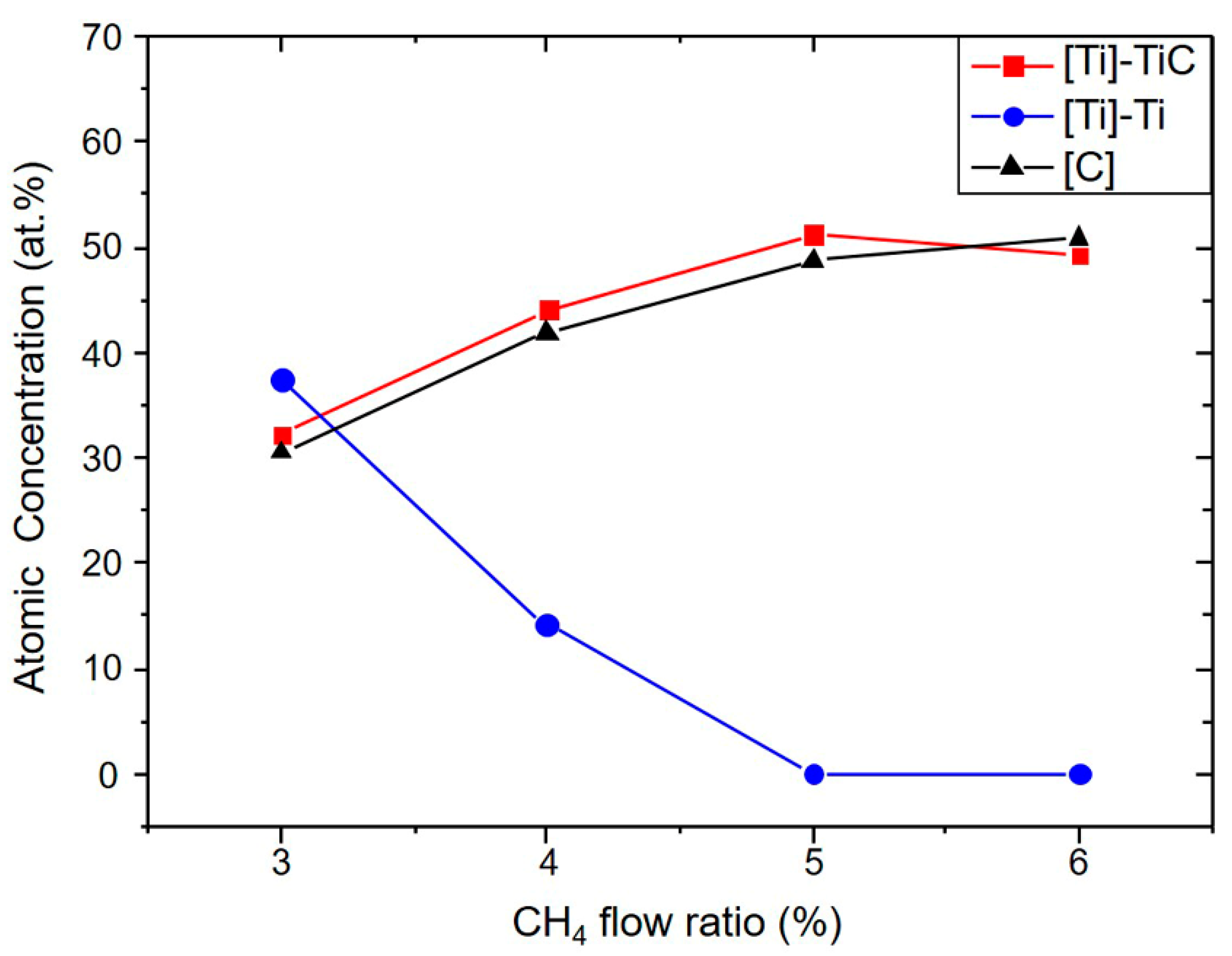
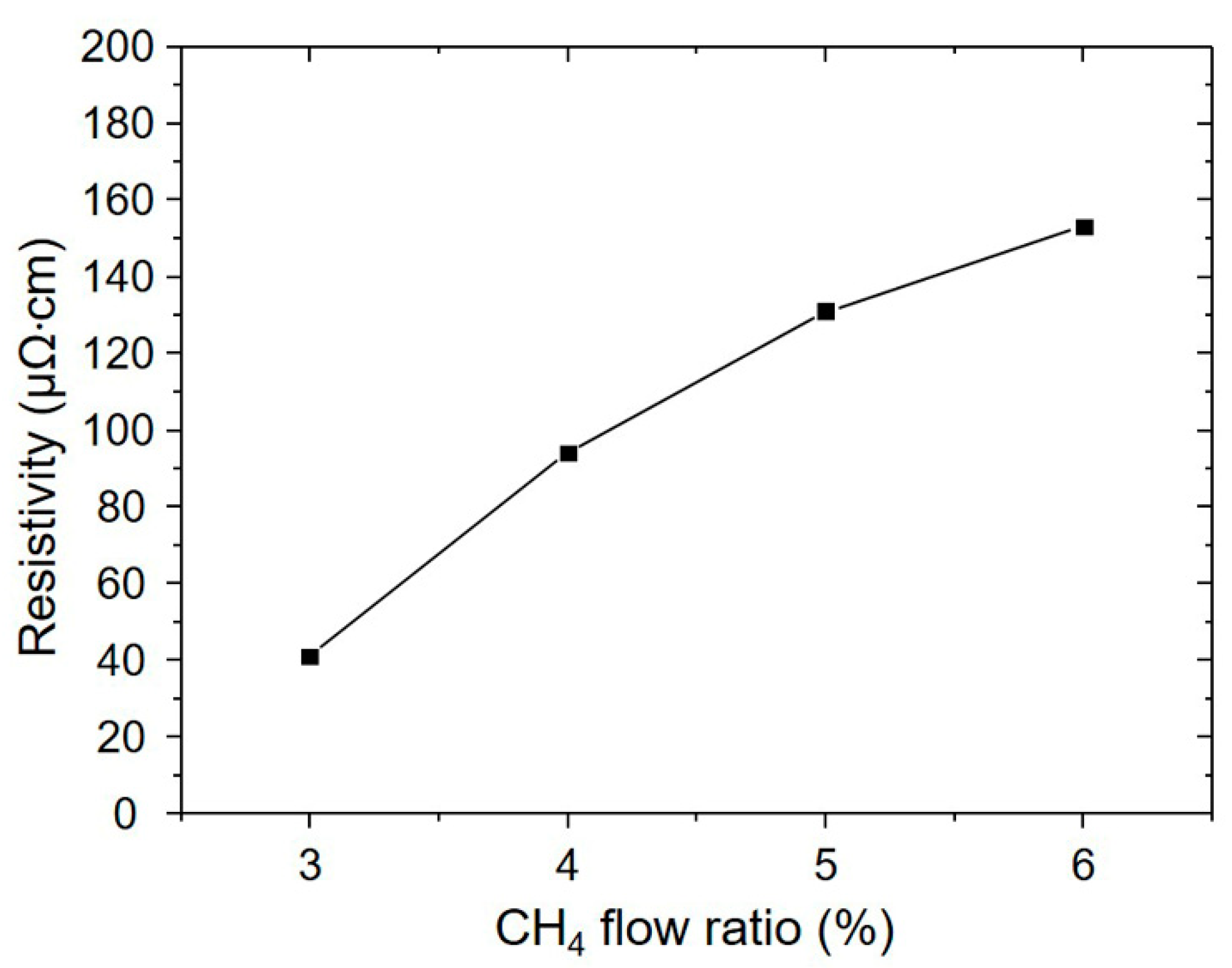
3.1. Influence of the CH4 Flow Ratio on Film Characteristics
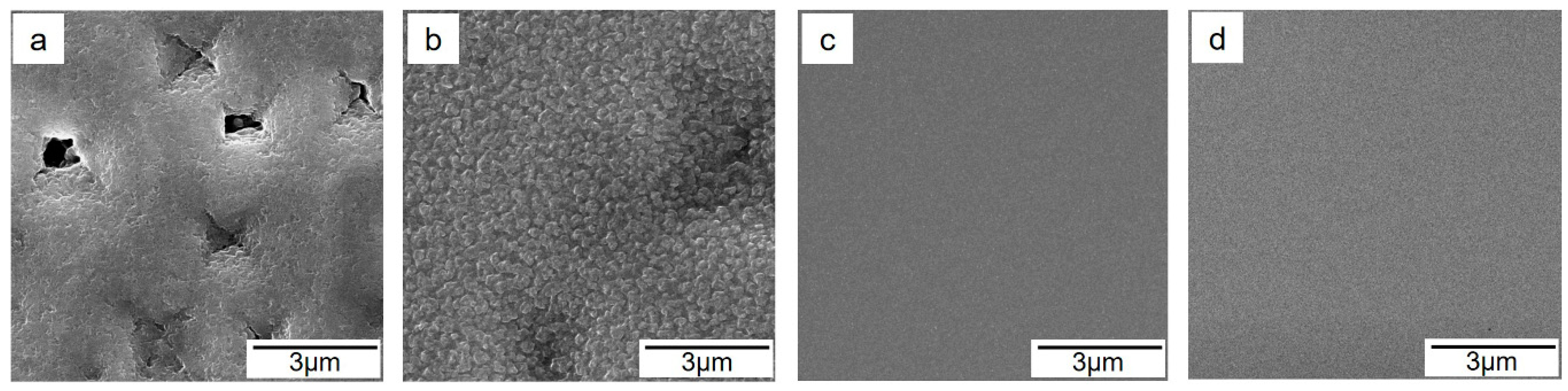
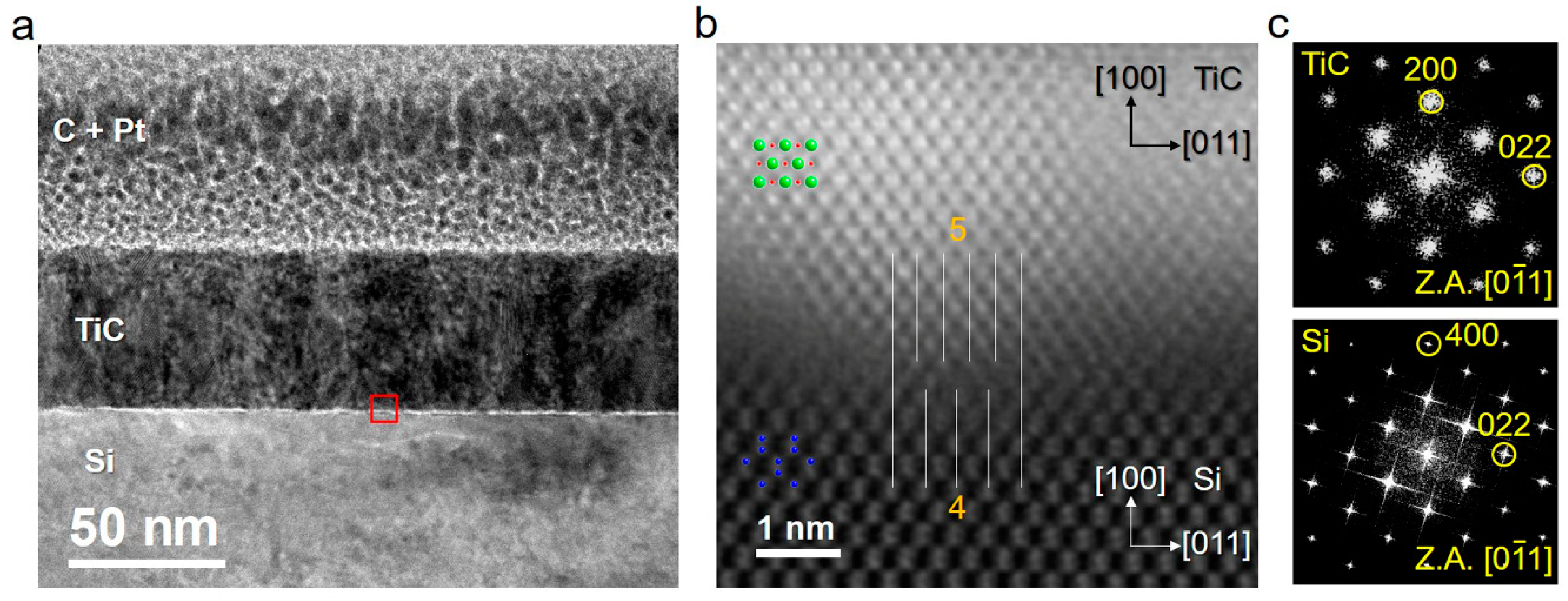
3.2. Evolution of Microstructure with Film Thickness
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pierson, H.O. Handbook of Refractory Carbides and Nitrides; Noyes Press: Park Ridge, NJ, USA, 1996; pp. 71–72. [Google Scholar]
- Perry, D.L. Handbook of Inorganic Compounds, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011; p. 431. [Google Scholar]
- Lipatnikov, V.N.; Zueva, L.V.; Gusev, A.I.; Kottar, A. Disorder-order phase transformations and electrical resistivity of nonstoichiometric titanium carbide. Phys. Solid State 1998, 40, 1211–1218. [Google Scholar] [CrossRef]
- Oakes, J.J. A comparative evaluation of HfN, Al2O3, TiC and TiN coatings on cemented carbide tools. Thin Solid Films 1983, 108, 173. [Google Scholar] [CrossRef]
- Boving, H.J.; Zintermann, H.E. Wear-resistant hard titanium carbide coatings for space applications. Tribol. Int. 1990, 23, 129–133. [Google Scholar] [CrossRef]
- Shanaghi, A.; Chu, P.K.; Rouhaghdam, A.R.S.; Xu, R.; Hu, T. Structure and corrosion resistance of Ti/TiC coatings fabricated by plasma immersion ion implantation and deposition on nickel–titanium. Surf. Coat. Technol. 2013, 229, 151–155. [Google Scholar] [CrossRef]
- Palma, R.H.; Sepúlveda, A.H.; Espinoza, R.A.; Montiglio, R.C. Performance of Cu-TiC alloy electrodes developed by reaction milling for electrical-resistance welding. J. Mater. Process. Technol. 2005, 169, 62–66. [Google Scholar] [CrossRef]
- Gao, Y.; Presser, V.; Zhang, L.; Niu, J.J.; McDonough, J.K.; Pérez, C.R.; Lin, H.; Fong, H.; Gogotsi, Y. High power supercapacitor electrodes based on flexible TiC-CDC nano-felts. J. Power Sources 2012, 201, 368–375. [Google Scholar] [CrossRef]
- Pan, L.; Shoji, T.; Nagataki, A.; Nakayama, Y. Field emission properties of titanium carbide coated carbon nanotube arrays. Adv. Eng. Mater. 2007, 9, 584–587. [Google Scholar] [CrossRef]
- Qin, Y.; Hu, M. Characterization and field emission characteristics of carbon nanotubes modified by titanium carbide. Appl. Surf. Sci. 2008, 254, 3313–3317. [Google Scholar] [CrossRef]
- Lee, S.-K.; Zetterling, C.-M.; Östling, M.; Palmquist, J.-P.; Jansson, U. Low resistivity ohmic contacts on 4H-silicon carbide for high power and high temperature device applications. Microelectron. Eng. 2002, 60, 261–268. [Google Scholar] [CrossRef]
- Wilhelmsson, O.; Palmquist, J.-P.; Lewin, E.; Emmerlich, J.; Eklund, P.; Persson, P.O.A.; Hogberg, H.; Li, S.; Ahuja, R.; Eriksson, O.; et al. Deposition and characterization of ternary thin films within the Ti–Al–C system by DC magnetron sputtering. J. Cryst. Growth 2006, 291, 290–300. [Google Scholar] [CrossRef]
- Palmquist, J.-P.; Jansson, U.; Seppänen, T.; Persson, P.O.A.; Birch, J.; Hultman, L.; Isberg, P. Magnetron sputtered epitaxial single-phase Ti3SiC2 thin films. Appl. Phys. Lett. 2002, 81, 835. [Google Scholar] [CrossRef]
- Eklund, P.; Högberg, H.; Hultman, L. Epitaxial TiC/SiC multilayers. Phys. Status Solidi Rapid Res. Lett. 2007, 1, 113–115. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Cheng, X.; Dong, L.; Zhang, Y.; Zang, J. Platinum nanoparticles supported on epitaxial TiC/nanodiamond as an electrocatalyst with enhanced durability for fuel cells. Carbon 2014, 67, 409–416. [Google Scholar] [CrossRef]
- Zhao, Q.H.; Parsons, J.D.; Chen, H.S.; Chaddha, A.K.; Wu, J.; Kruaval, G.B.; Downham, D. Single crystal titanium carbide, epitaxially grown on zincblend and wurtzite structures of silicon carbide. Mater. Res. Bull. 1995, 30, 761–769. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, W.Z.; Shi, L.Q.; Zhang, W.Y.; Zhang, W.; Zhang, B. Preparation of single-crystal TiC (111) by radio frequency magnetron sputtering at low temperature. Thin Solid Films 2012, 520, 6882–6887. [Google Scholar] [CrossRef]
- Braic, M.; Zoita, N.C.; Danila, M.; Grigorescu, C.E.A.; Logofatu, C. Hetero-epitaxial growth of TiC films on MgO (001) at 100 °C by DC reactive magnetron sputtering. Thin Solid Films 2015, 589, 590–596. [Google Scholar] [CrossRef]
- Ferro, D.; Rau, J.V.; Albertini, V.R.; Generosi, A.; Teghil, R.; Barinov, S.M. Pulsed laser deposited hard TiC, ZrC, HfC and TaC films on titanium: Hardness and an energy dispersive X-ray diffraction study. Surf. Coat. Technol. 2008, 202, 1455–1461. [Google Scholar] [CrossRef]
- Norin, L.; McGinnis, S.; Jansson, U.; Carlsson, J.-O. Low temperature deposition of epitaxial titanium carbide on MgO (001) by co-evaporation of C60 and Ti. J. Vac. Sci. Technol. A 1997, 15, 3082. [Google Scholar] [CrossRef]
- Archer, N.J. The plasma-assisted chemical vapour deposition of TiC, TiN and TiCxN1−x. Thin Solid Films 1981, 80, 221–225. [Google Scholar] [CrossRef]
- Sarkar, J. Sputtering Materials for VLSI and Thin Film Devices; William Andrew Publishing: Boston, MA, USA, 2010; pp. 93–170. [Google Scholar]
- Zoita, N.C.; Braic, V.; Danila, M.; Vlaicu, A.M.; Logofatu, C.; Grigorescu, C.E.A.; Braic, M. Influence of film thickness on the morphological and electrical properties of epitaxial TiC films deposited by reactive magnetron sputtering on MgO substrates. J. Cryst. Growth 2014, 389, 92–98. [Google Scholar] [CrossRef]
- Narayan, J.; Larson, B.C. Domain epitaxy: A unified paradigm for thin film growth. J. Appl. Phys. 2003, 93, 278. [Google Scholar] [CrossRef]
- Sheu, W.-H.; Wu, S.-T. Epitaxial Growth of TiC (002) on Si (001) by Reactive Magnetron Sputtering at Low Temperatures. Jpn. J. Appl. Phys. 1998, 37, 6094–6097. [Google Scholar] [CrossRef]
- Haase, V.; Kirschstein, G.; List, H.; Ruprecht, S.; Sangster, R.; Schröder, F.; Töpper, W.; Vanecek, H.; Heit, W.; Schlichting, J. Gmelin Handbook of Inorganic and Organometallic Chemistry: Si Silicon; Springer: Berlin/Heidelberg, Germany, 1985; pp. 1–5. [Google Scholar]
- Wakelkamp, W.J.J.; van Loo, F.J.J.; Metselaar, R. Phase Relations in the Ti-Si-C System. J. Eur. Ceram. Soc. 1991, 8, 135–139. [Google Scholar] [CrossRef]
- Bandyopadhyay, D. The Ti-Si-C system (Titanium-Silicon-Carbon). J. Phase Equilibria Diffus. 2004, 25, 415–420. [Google Scholar] [CrossRef]
- Gulbiński, W.; Mathur, S.; Shen, H.; Suszko, T.; Gilewicz, A.; Warcholiński, B. Evaluation of phase, composition, microstructure and properties in TiC/a-C:H thin films deposited by magnetron sputtering. Appl. Surf. Sci. 2005, 239, 302–310. [Google Scholar] [CrossRef]
- Riley, D.P.; Connor, D.J.O.; Dastoor, P.; Brack, N.; Pigram, P.J. Comparative analysis of Ti3SiC2 and associated compounds using X-ray diffraction and X-ray photoelectron spectroscopy. J. Phys. D 2002, 35, 1603. [Google Scholar] [CrossRef]
- Shin, C.-S.; Gall, D.; Kim, Y.-W.; Desjardins, P.; Petrov, I.; Greene, J.E. Epitaxial NaCl structure δ-TaNx(001): Electronic transport properties, elastic modulus, and hardness versus N/Ta ratio. J. Appl. Phys. 2001, 90, 2879. [Google Scholar] [CrossRef]
- Adachi, S. Properties of Semiconductor Alloys: Group-IV, III–V and II–VI Semiconductors; John Wiley & Sons Ltd.: Gumma, Japan, 2009; p. 36. [Google Scholar]
- Suhail, M.H.; Mohan Rao, G.; Mohan, S. Dc reactive magnetron sputtering of titanium-structural and optical characterization of TiO2 films. J. Appl. Phys. 1992, 71, 1421. [Google Scholar] [CrossRef]
- Combadiere, L.; Machet, J. Study and control of both target-poisoning mechanisms and reactive phenomenon in reactive planar magnetron cathodic sputtering of TiN. Surf. Coat. Technol. 1996, 82, 145–157. [Google Scholar] [CrossRef]
- Nyberga, T.; Högberg, H.; Greczynski, G.; Berg, S. A simple model for non-saturated reactive sputtering processes. Thin Solid Films 2019, 688, 137413. [Google Scholar] [CrossRef]
- Sundgren, J.-E.; Johansson, B.-O.; Karlsson, S.-E. Mechanisms of reactive sputtering of titanium nitride and titanium carbide I: Influence of process parameters on film composition. Thin Solid Films 1983, 105, 353–366. [Google Scholar] [CrossRef]
- Kisi, E.H.; Crossley, J.A.A.; Myhra, S.; Barsoum, M.W. Structure and crystal chemistry of Ti3SiC2. J. Phys. Chem. Solids 1998, 59, 1437–1443. [Google Scholar] [CrossRef]
- Chan, C.-M.; Trigwell, S.; Duerig, T. Oxidation of an NiTi alloy. Surf. Interface Anal. 1990, 15, 349–354. [Google Scholar] [CrossRef]
- Johansson, L.I.; Hagström, A.L.; Jacobson, B.E.; Hagström, S.B.M. ESCA studies of core level shifts and valence band structure in nonstoichiometric single crystals of titanium carbide. J. Electron Spectros. Relat. Phenom. 1977, 10, 259–271. [Google Scholar] [CrossRef]
- Ramqvist, L.; Ekstig, B.; Källne, E.; Noreland, E.; Manne, R. X-ray study of inner level shifts and band structure of TiC and related compounds. J. Phys. Chem. Solids 1969, 30, 1849–1860. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Zhao, Q.; Li, C.; He, X. The effect of O2 partial pressure on the structure and photocatalytic property of TiO2 films prepared by sputtering. Mater. Chem. Phys. 2005, 90, 207–212. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V., Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Holt, J.B.; Munir, Z.A. Combustion synthesis of titanium carbide: Theory and experiment. J. Mater. Sci. 1986, 21, 251–259. [Google Scholar] [CrossRef]
- Barsoum, M.W. The MN+1AXN phases: A new class of solids: Thermodynamically stable nanolaminates. Prog. Solid State Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Yang, S.; Sun, Z.M.; Hashimoto, H. Synthesis of Ti3SiC2 powder from 1Ti/(1+x)Si/2TiC powder mixtures. J. Alloys Compd. 2004, 368, 318–325. [Google Scholar] [CrossRef]
- Otani, S.; Tanaka, T.; Ishizawa, Y. Electrical resistivities in single crystals of TiCx and VCx. J. Mater. Sci. 1986, 21, 1001–1014. [Google Scholar] [CrossRef]
- Morelli, D.T. Thermal conductivity and thermoelectric power of titanium carbide single crystals. Phys. Rev. B 1991, 44, 5453–5458. [Google Scholar] [CrossRef]
- Petrov, I.; Barna, P.B.; Hultman, L.; Greene, J.E. Microstructural evolution during film growth. J. Vac. Sci. Technol. A 2003, 21, 117–128. [Google Scholar] [CrossRef]
- McKenzie, D.R.; Bilek, M.M.M. Electron diffraction from polycrystalline materials showing stress induced preferred orientation. J. Appl. Phys. 1999, 86, 230–236. [Google Scholar] [CrossRef]
- Djafer, A.Z.A.; Saoula, N.; Madaoui, N.; Zerizer, A. Deposition and characterization of titanium carbide thin films by magnetron sputtering using Ti and TiC targets. Appl. Surf. Sci. 2014, 312, 57–62. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Li, Y.; Sun, D. Bias effect on microstructure and mechanical properties of magnetron sputtered nanocrystalline titanium carbide thin films. Thin Solid Films 2008, 516, 5419–5423. [Google Scholar] [CrossRef]
- Su, Y.; Wang, X.; Wang, H.; Wen, M.; Zheng, W. Grain-size effect on the preferred orientation of TiC/α-C:H thin films. Appl. Surf. Sci. 2012, 258, 6800–6806. [Google Scholar] [CrossRef]
- Lee, C.W.; Nam, S.W.; Chun, J.S. Effect of experimental parameters on the preferred orientation of chemically vapor deposited TiC on cemented carbides. J. Vac. Sci. Technol. 1982, 21, 42–46. [Google Scholar] [CrossRef]
- Pelleg, J.; Zevin, L.Z.; Lungo, S.; Croitoru, N. Reactive-sputter-deposited TiN films on glass substrates. Thin Solid Films 1991, 197, 117–128. [Google Scholar] [CrossRef]
- Oh, U.C.; Je, J.H. Effects of strain energy on the preferred orientation of TiN thin films. J. Appl. Phys. 1993, 74, 1692. [Google Scholar] [CrossRef]
- Je, J.H.; Noh, D.Y.; Kim, H.K.; Liang, K.S. Preferred orientation of TiN films studied by a real time synchrotron X-ray scattering. J. Appl. Phys. 1997, 81, 1692. [Google Scholar] [CrossRef][Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Y.-S.; Do, T.H.; Chiu, K.-A.; Chen, W.-C.; Chang, L. Reactive Sputtering Deposition of Epitaxial TiC Film on Si (100) Substrate. Coatings 2020, 10, 647. https://doi.org/10.3390/coatings10070647
Fang Y-S, Do TH, Chiu K-A, Chen W-C, Chang L. Reactive Sputtering Deposition of Epitaxial TiC Film on Si (100) Substrate. Coatings. 2020; 10(7):647. https://doi.org/10.3390/coatings10070647
Chicago/Turabian StyleFang, Yu-Siang, Thi Hien Do, Kun-An Chiu, Wei-Chun Chen, and Li Chang. 2020. "Reactive Sputtering Deposition of Epitaxial TiC Film on Si (100) Substrate" Coatings 10, no. 7: 647. https://doi.org/10.3390/coatings10070647
APA StyleFang, Y.-S., Do, T. H., Chiu, K.-A., Chen, W.-C., & Chang, L. (2020). Reactive Sputtering Deposition of Epitaxial TiC Film on Si (100) Substrate. Coatings, 10(7), 647. https://doi.org/10.3390/coatings10070647





