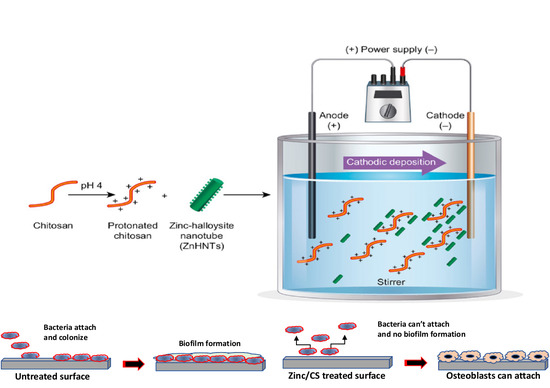
Electrophoretic Deposition of Gentamicin-Loaded ZnHNTs-Chitosan on Titanium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
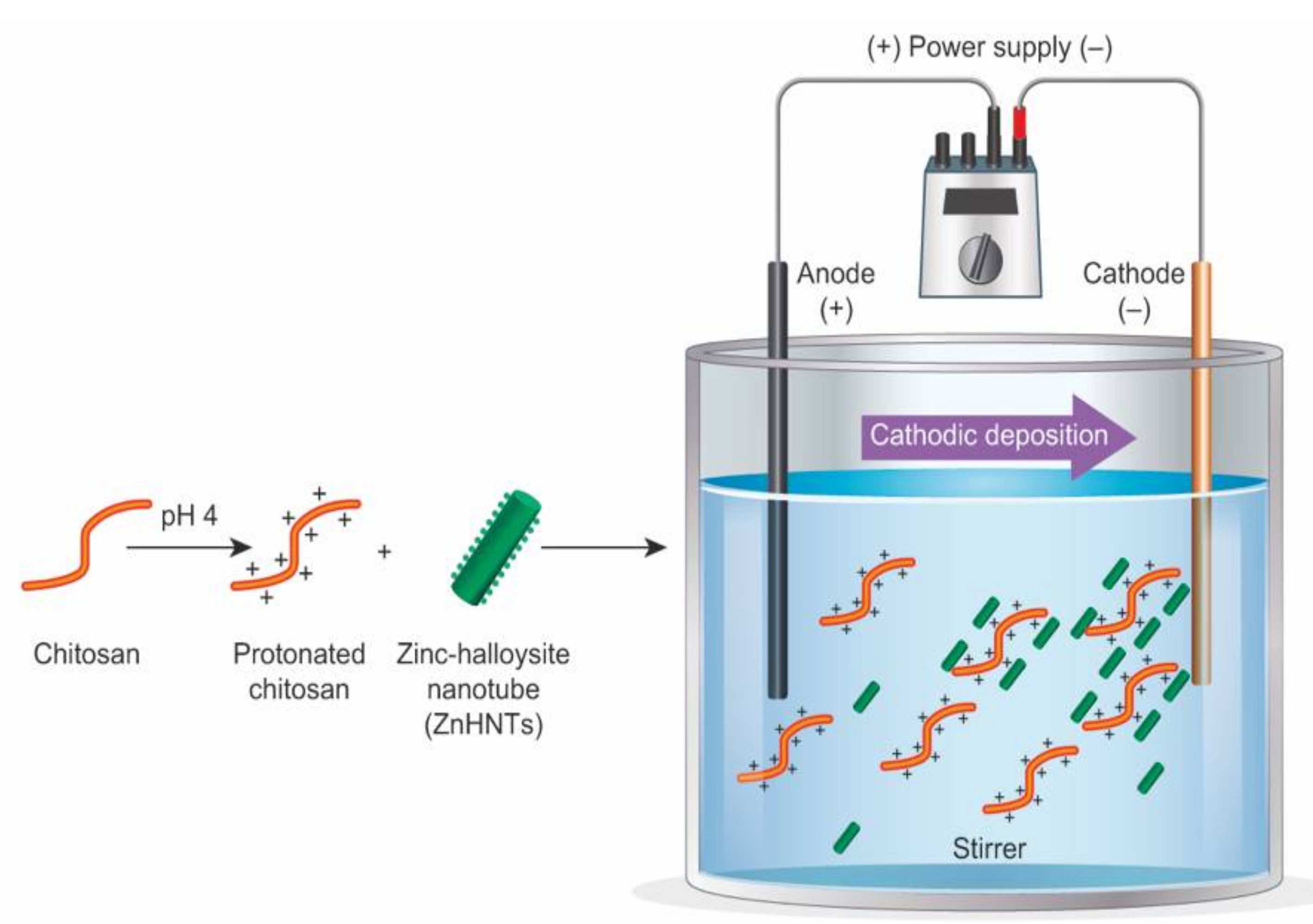
2.2. Electrolytic Metallization of HNTs
2.3. Loading of HNTs with Gentamicin
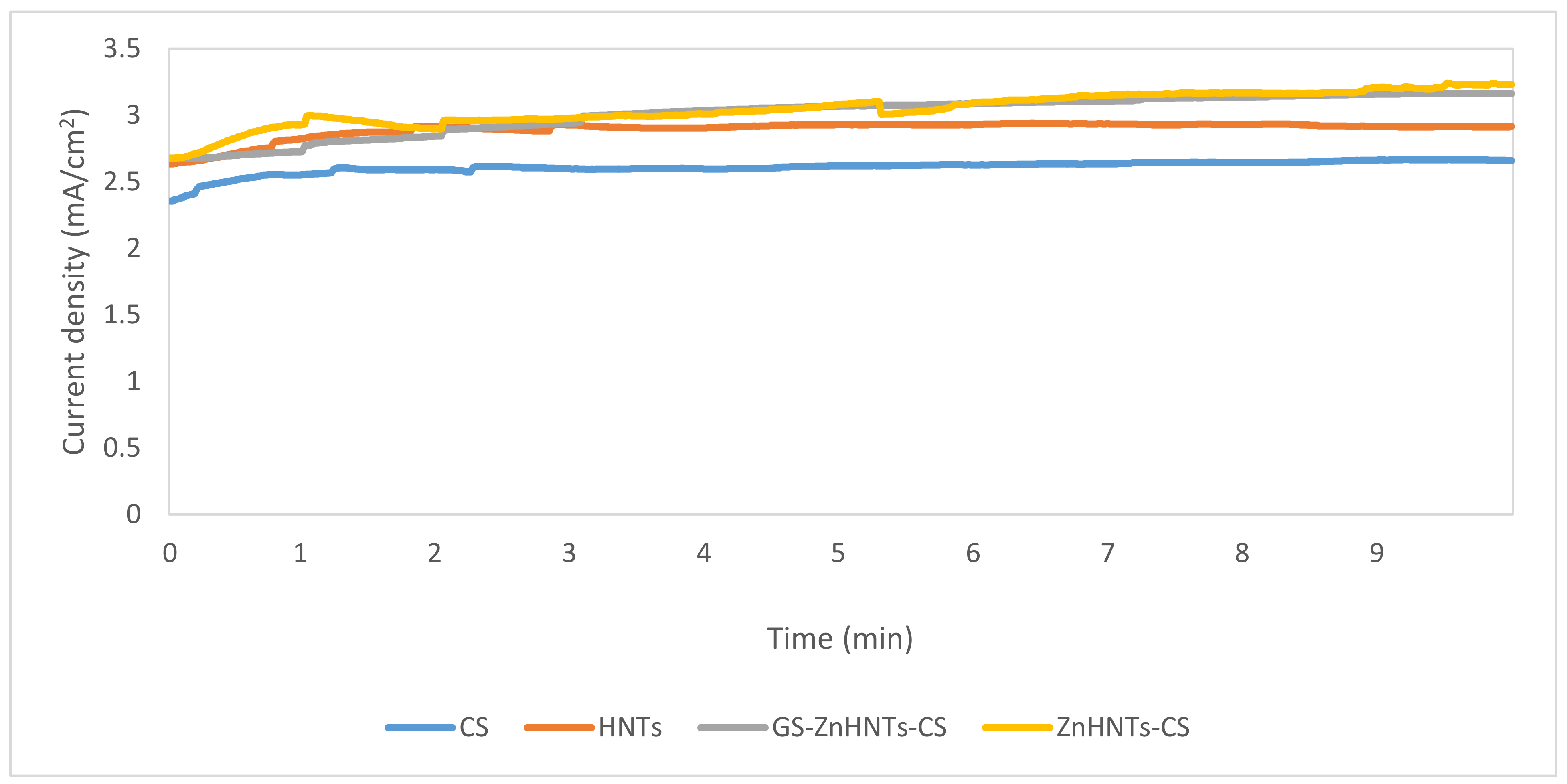
2.4. Electrophoretic Deposition of the Coatings on Titanium
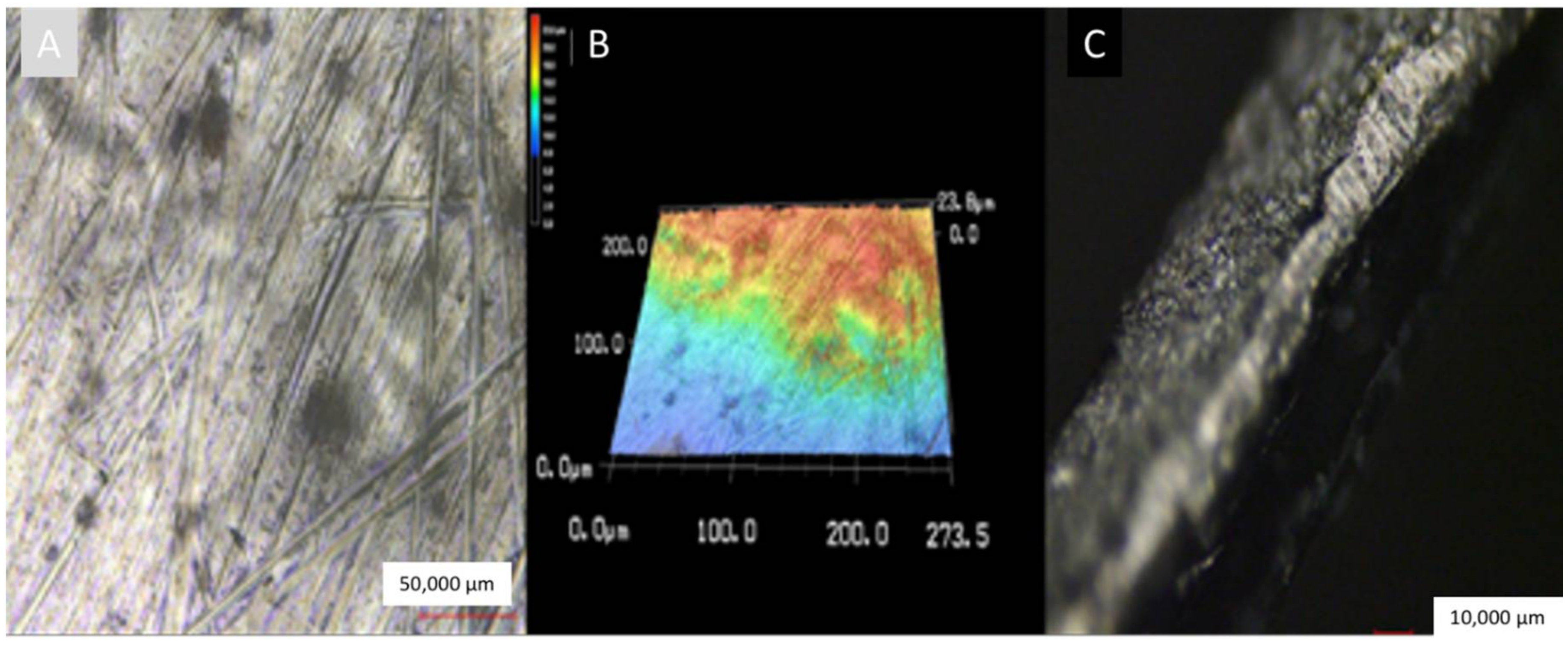
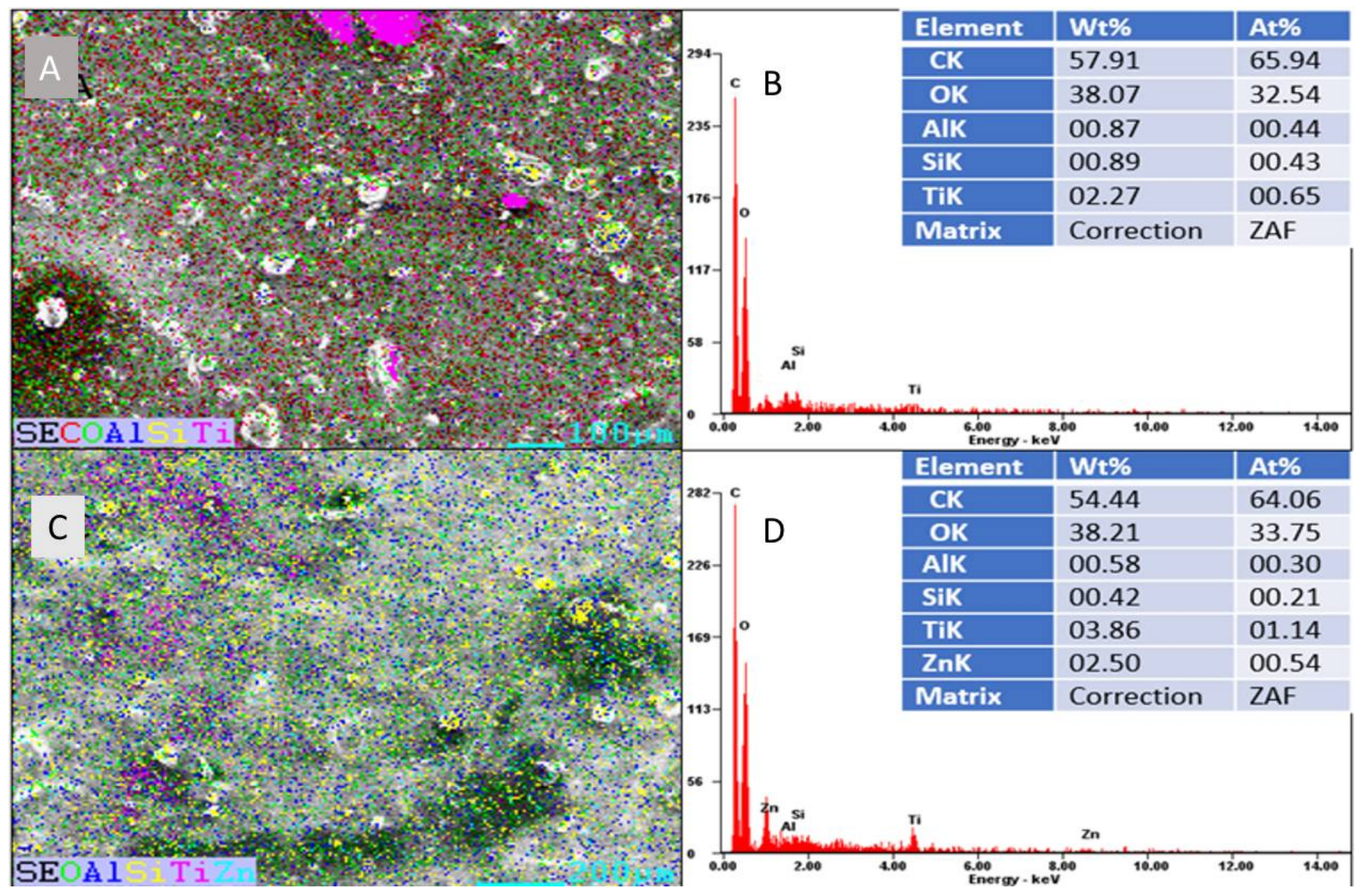
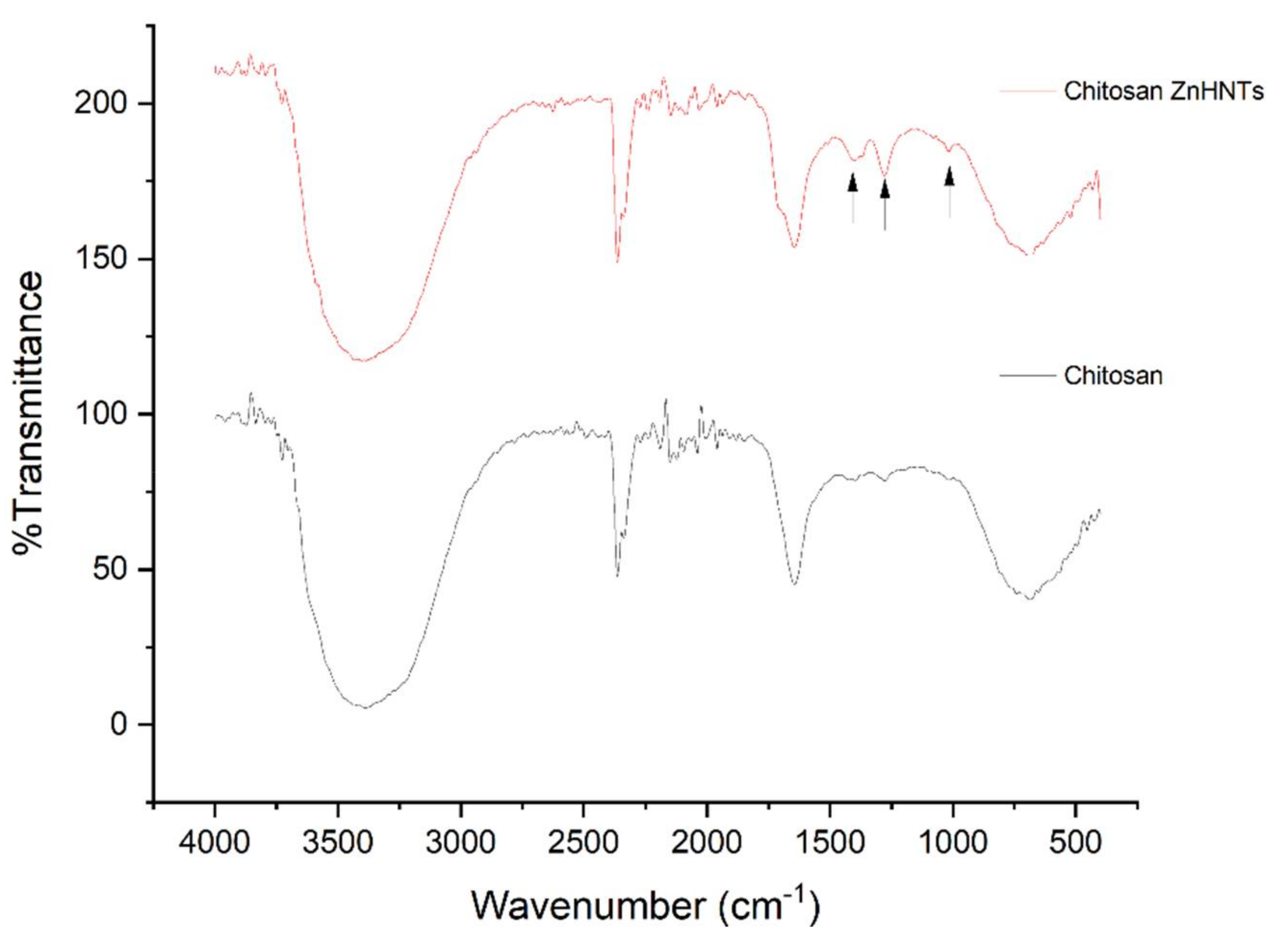
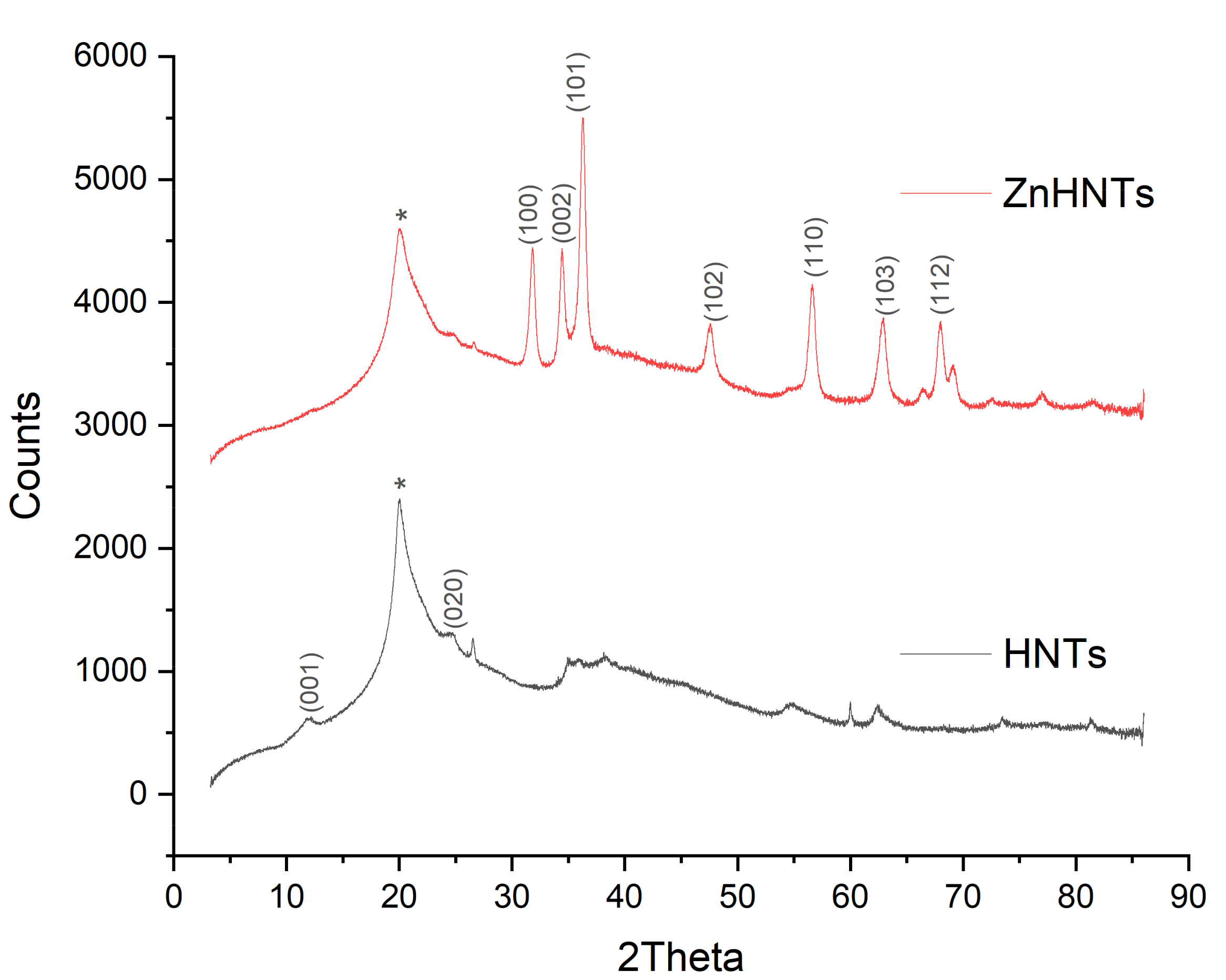
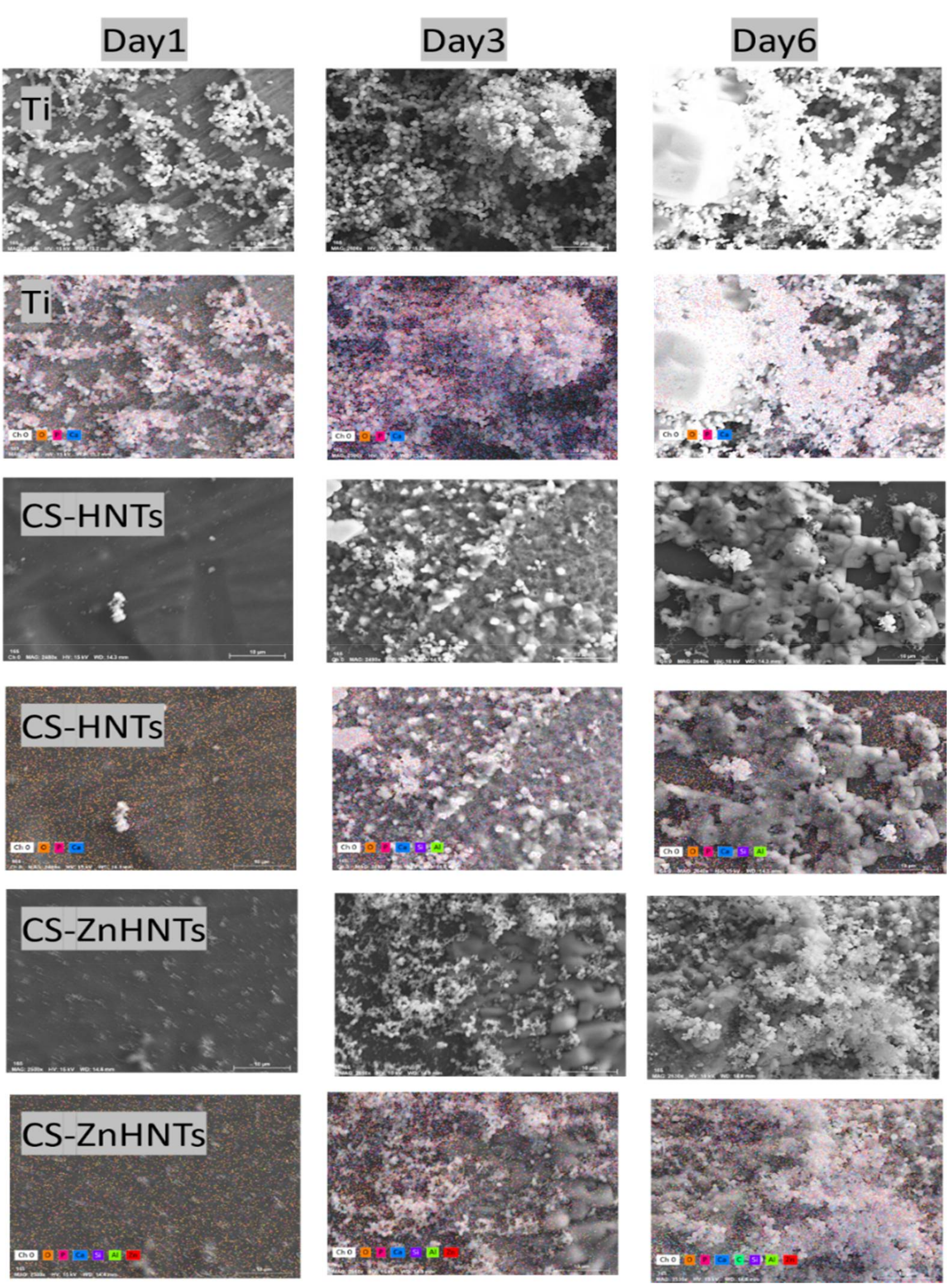
2.5. Characterization of Titanium Coatings
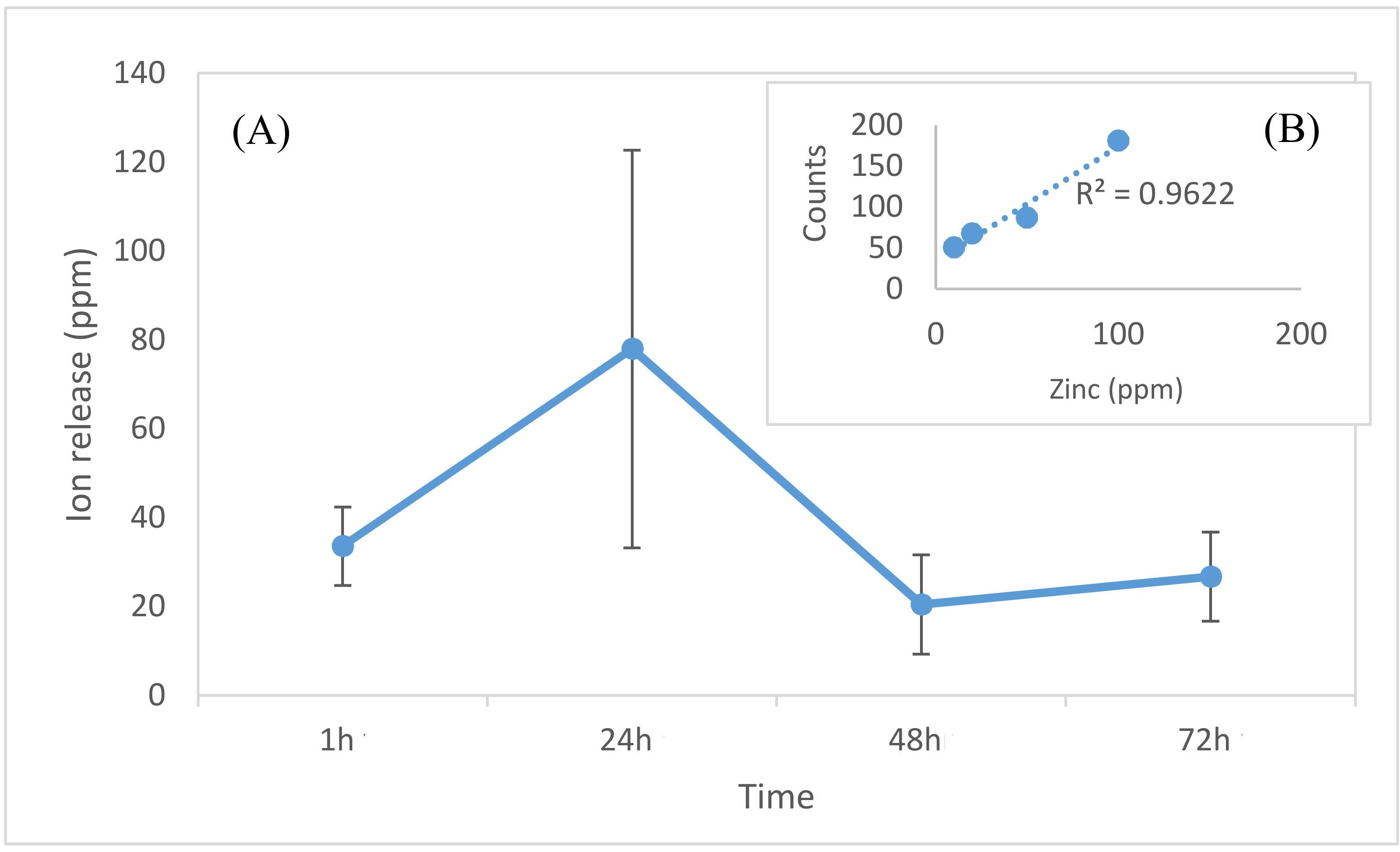
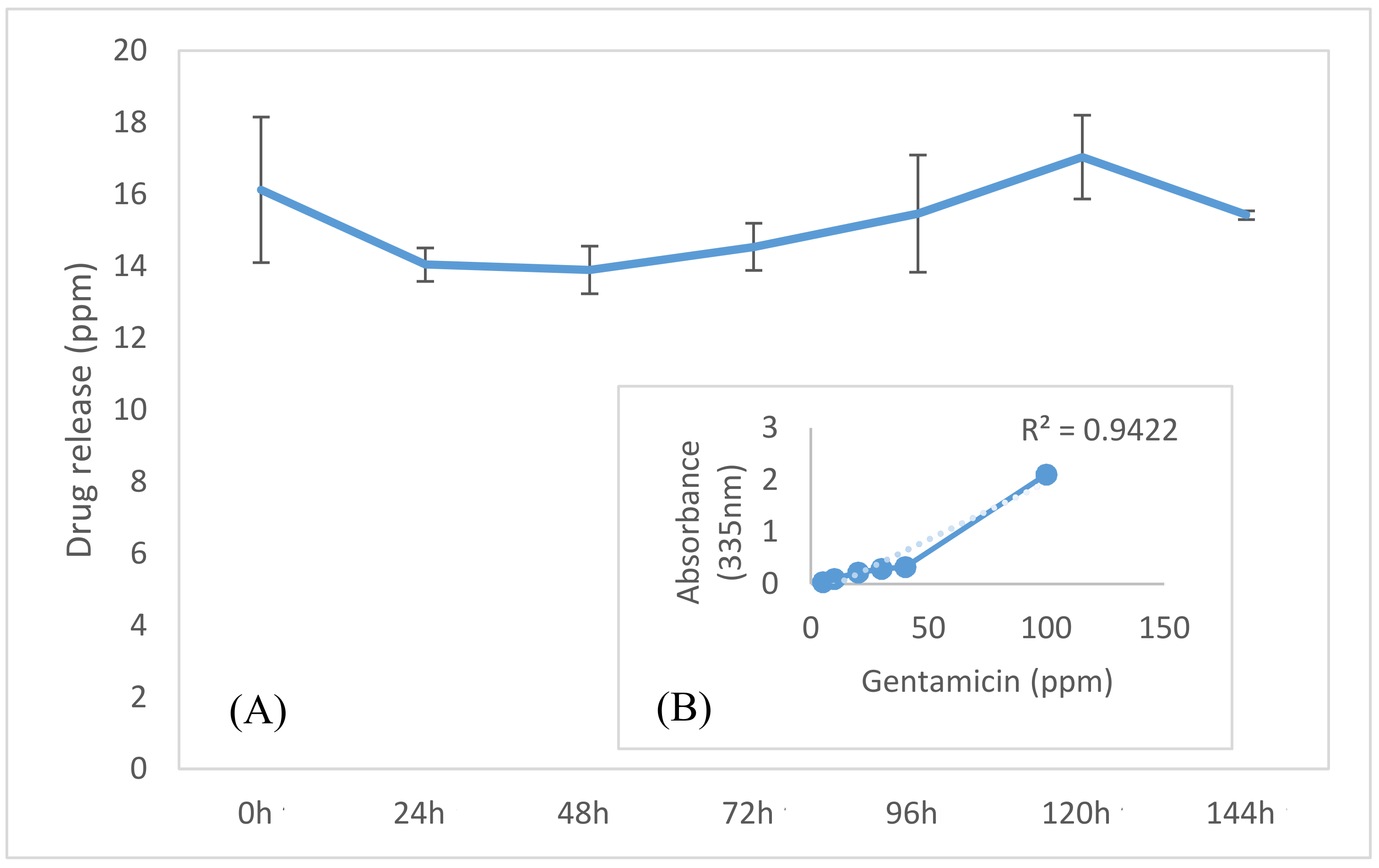
2.6. Gentamicin Release Analysis
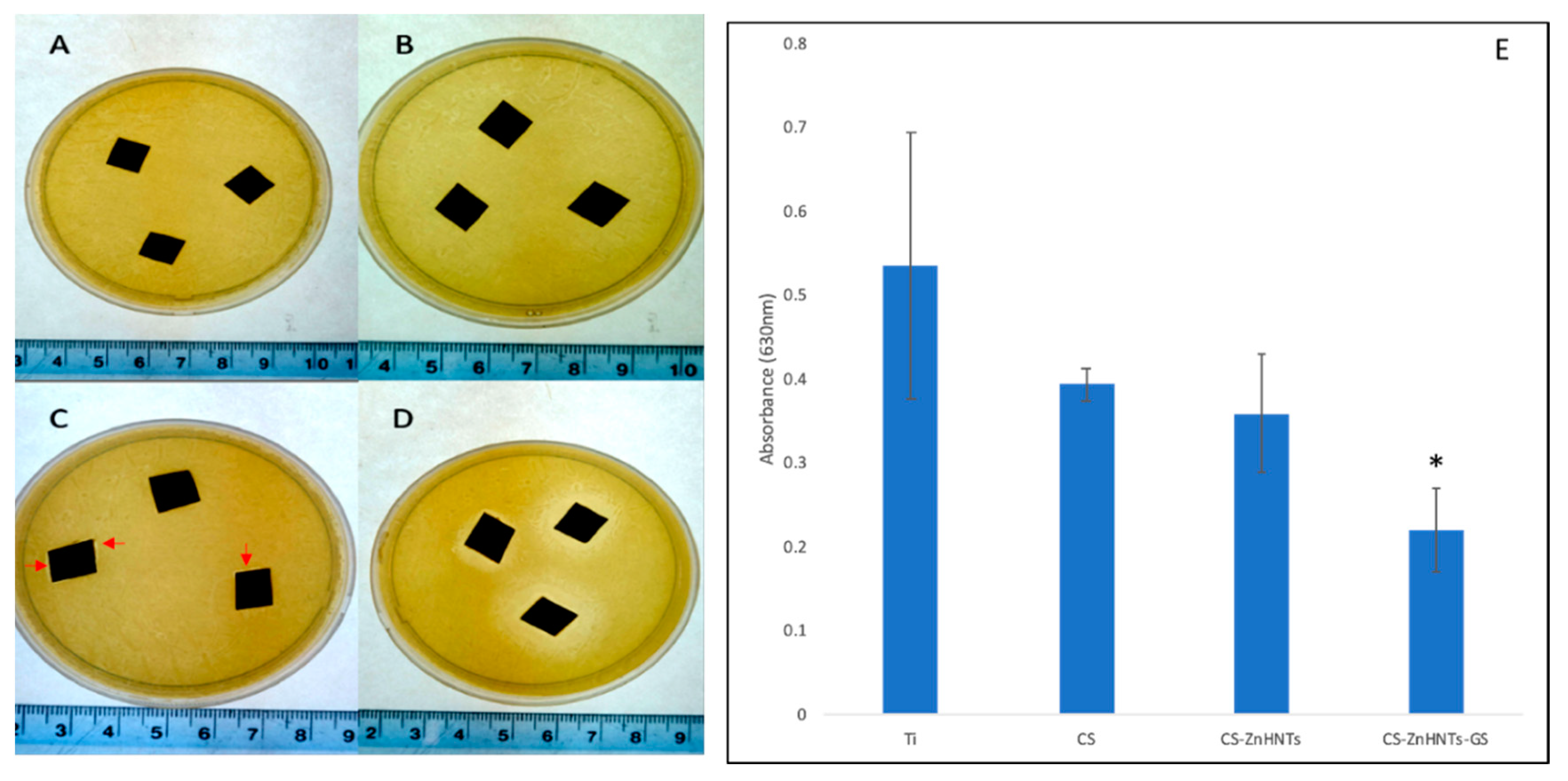
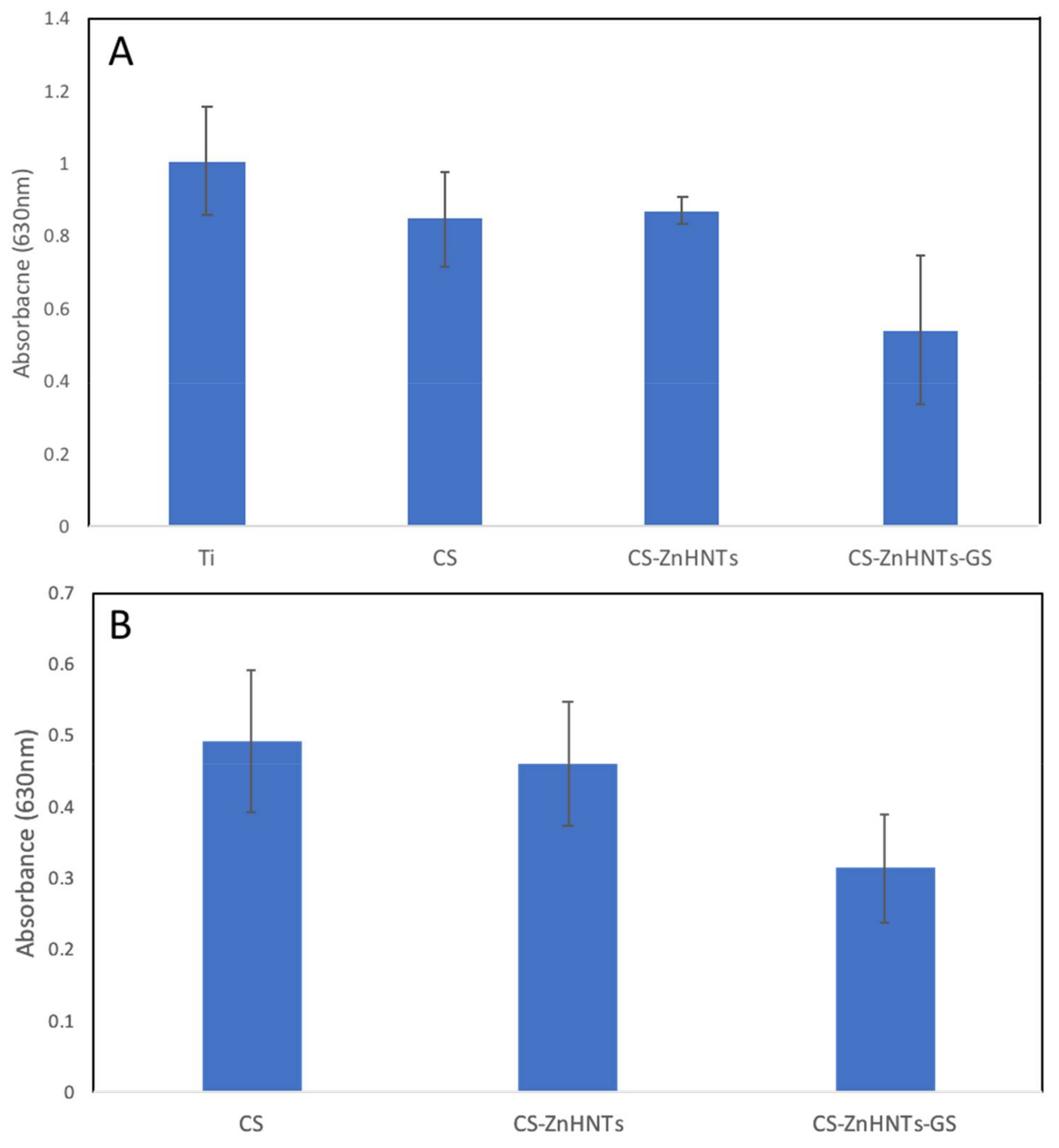
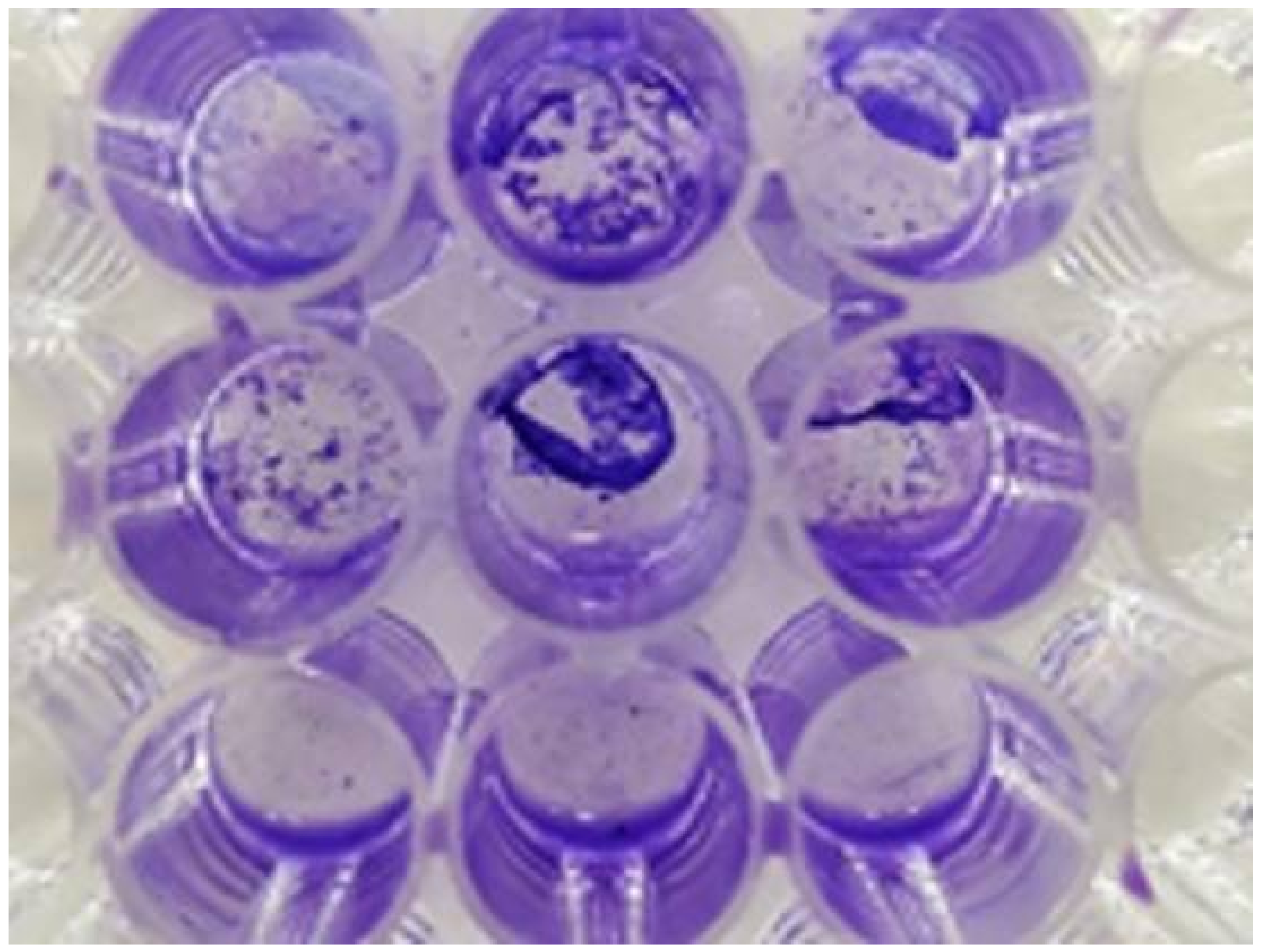
2.7. Antibacterial Analysis
2.8. In Vitro Bioactivity Tests
2.9. Cell Culture
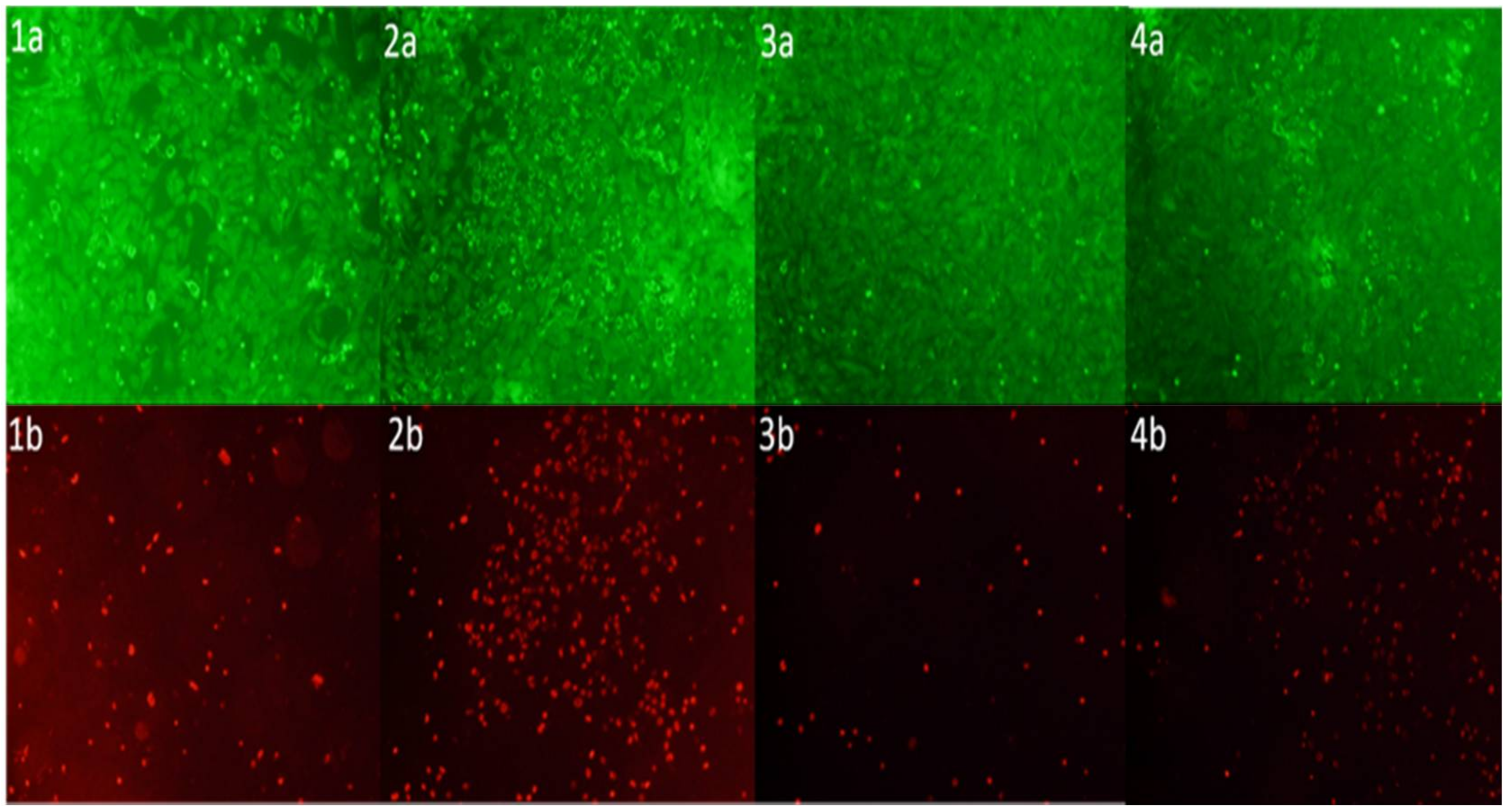
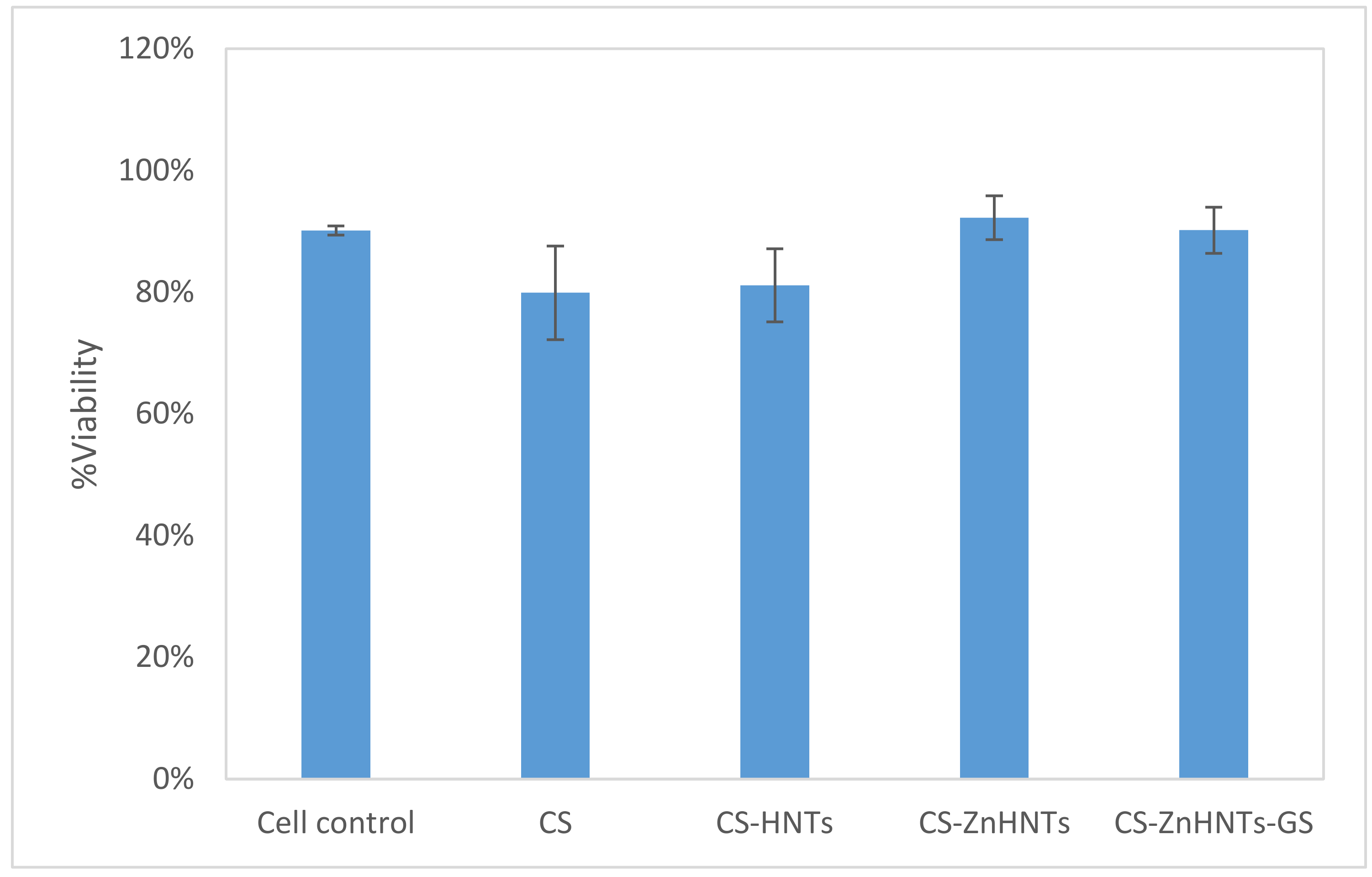
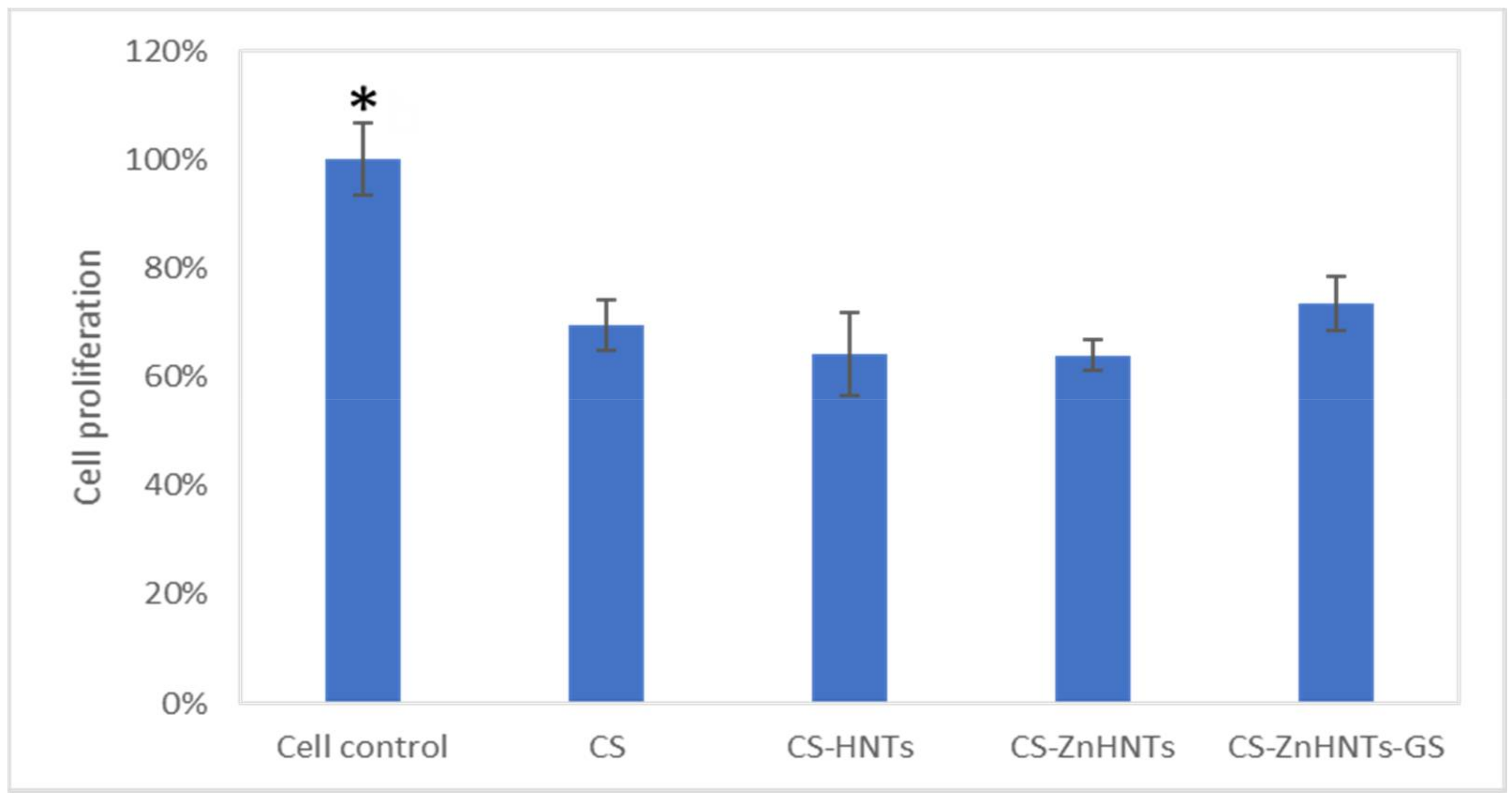
2.10. Live/Dead Cytotoxicity Tests and Cell Proliferation Studies
2.11. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mattioli-Belmonte, M.; Cometa, S.; Ferretti, C.; Iatta, R.; Trapani, A.; Ceci, E.; Falconi, M.; De Giglio, E. Characterization and cytocompatibility of an antibiotic/chitosan/cyclodextrins nanocoating on titanium implants. Carbohydr. Polym. 2014, 110, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Ordikhani, F.; Dehghani, M.; Simchi, A. Antibiotic-loaded chitosan–Laponite films for local drug delivery by titanium implants: Cell proliferation and drug release studies. J. Mater. Sci. Mater. Electron. 2015, 26, 269. [Google Scholar] [CrossRef] [PubMed]
- Norowski, P.A.; Courtney, H.S.; Babu, J.; Haggard, W.O.; Bumgardner, J.D. Chitosan coatings deliver antimicrobials from titanium implants: A preliminary study. Implant. Dent. 2011, 20, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Swanson, T.E.; Cheng, X.; Friedrich, C. Development of chitosan-vancomycin antimicrobial coatings on titanium implants. J. Biomed. Mater. Res. Part A 2011, 97, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Farrokhi-Rad, M.; Fateh, A.; Shahrabi, T. Electrophoretic deposition of vancomycin loaded halloysite nanotubes-chitosan nanocomposite coatings. Surf. Coat. Technol. 2018, 349, 144–156. [Google Scholar] [CrossRef]
- Radda’A, N.S.; Goldmann, W.H.; Detsch, R.; Roether, J.A.; Cordero-Arias, L.; Virtanen, S.; Moskalewicz, T.; Boccaccini, A.R. Electrophoretic deposition of tetracycline hydrochloride loaded halloysite nanotubes chitosan/bioactive glass composite coatings for orthopedic implants. Surf. Coat. Technol. 2017, 327, 146–157. [Google Scholar] [CrossRef]
- Besinis, A.; Hadi, S.D.; Le, H.R.; Tredwin, C.; Handy, R.D. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology 2017, 11, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Ewald, A.; Glückermann, S.K.; Thull, D.-I.R.; Gbureck, U. Antimicrobial titanium/silver PVD coatings on titanium. Biomed. Eng. Online 2006, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Kazemzadeh-Narbat, M.; Lai, B.F.; Ding, C.; Kizhakkedathu, J.N.; Hancock, R.E.W.; Wang, R. Multilayered coating on titanium for controlled release of antimicrobial peptides for the prevention of implant-associated infections. Biomaterials 2013, 34, 5969–5977. [Google Scholar] [CrossRef]
- Peng, Z.-X.; Tu, B.; Shen, Y.; Du, L.; Wang, L.; Guo, S.-R.; Tang, T.-T. Quaternized chitosan inhibits icaA transcription and biofilm formation by Staphylococcus on a titanium surface. Antimicrob. Agents Chemother. 2011, 55, 860–866. [Google Scholar] [CrossRef] [Green Version]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic biomaterials: current challenges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Sowmya, S.; Jayakumar, R.; Deepthi, S.; Chennazhi, K.; Ehrlich, H.; Tsurkan, M.V.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Massaro, M.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Covalently modified halloysite clay nanotubes: Synthesis, properties, biological and medical applications. J. Mater. Chem. B 2017, 5, 2867–2882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augello, C.; Liu, H. Surface modification of magnesium by functional polymer coatings for neural applications. Surf. Modif. Magnes. Alloys Biomed. Appl. 2015, 335–353. [Google Scholar] [CrossRef]
- Farrokhi-Rad, M.; Ghorbani, M. Electrophoretic deposition of titania nanoparticles in different alcohols: Kinetics of deposition. J. Am. Ceram. Soc. 2011, 94, 2354–2361. [Google Scholar] [CrossRef]
- Chozhanathmisra, M.; Ramya, S.; Kavitha, L.; Gopi, D. Development of zinc-halloysite nanotube/minerals substituted hydroxyapatite bilayer coatings on titanium alloy for orthopedic applications. Colloids Surf. A Physicochem. Eng. Asp. 2016, 511, 357–365. [Google Scholar] [CrossRef]
- Mills, D.; Boyer, C. Method for Metalizing Nanotubes through Electrolysis. U.S. Patent 9,981,074, 29 May 2018. [Google Scholar]
- Khaydarov, R.A.; Khaydarov, R.R.; Gapurova, O.; Estrin, Y.; Scheper, T. Electrochemical method for the synthesis of silver nanoparticles. J. Nanopart. Res. 2008, 11, 1193–1200. [Google Scholar] [CrossRef]
- Mahmoodi, S.; Sorkhi, L.; Farrokhi-Rad, M.; Shahrabi, T. Electrophoretic deposition of hydroxyapatite–chitosan nanocomposite coatings in different alcohols. Surf. Coat. Technol. 2013, 216, 106–114. [Google Scholar] [CrossRef]
- Ismail, A.F.H.; Mohamed, F.; Rosli, L.M.M.; Shafri, M.A.M.; Haris, M.S.; Adina, A.B. Spectrophotometric determination of gentamicin loaded PLGA microparticles and method validation via ninhydrin-gentamicin complex as a rapid quantification approach. J. Appl. Pharm. Sci. 2016, 6, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Weisman, J.A.; Jammalamadaka, U.; Tappa, K.; Mills, D.K. Doped halloysite nanotubes for use in the 3D printing of medical devices. Bioengineering 2017, 4, 96. [Google Scholar] [CrossRef] [Green Version]
- Lima, E.M.C.X.; Koo, H.; Smith, A.M.V.; Rosalen, P.L.; Cury, A.A.D.B. Adsorption of salivary and serum proteins, and bacterial adherence on titanium and zirconia ceramic surfaces. Clin. Oral Implant Res. 2008, 19, 780–785. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, e2437. [Google Scholar] [CrossRef] [PubMed]
- Barrere, F.; Van Blitterswijk, C.; De Groot, K.; Layrolle, P.; Van Blitterswijk, C.A. Nucleation of biomimetic Ca–P coatings on Ti6Al4V from a SBF×5 solution: Influence of magnesium. Biomaterials 2002, 23, 2211–2220. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Jammalamadaka, U.; Sun, L.; Tappa, K.; Mills, D.K. Sustained telease of antibacterial agents from doped halloysite nanotubes. Bioengineering 2015, 3, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhitomarsky, I.; Gal-Or, L. Electrophoretic deposition of hydroxyapatite. J. Mater. Sci. Mater. Med. 1997, 8, 213–219. [Google Scholar] [CrossRef]
- Pang, X.; Zhitomirsky, I. Electrophoretic deposition of composite hydroxyapatite-chitosan coatings. Mater. Charact. 2007, 58, 339–348. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite nanotubes coated by chitosan for the controlled release of Khellin. Polymers 2020, 12, 1766. [Google Scholar] [CrossRef]
- Rapacz-Kmita, A.; Ewa, S.-Z.; Ziąbka, M.; Różycka, A.; Dudek, M. Instrumental characterization of the smectite clay–gentamicin hybrids. Bull. Mater. Sci. 2015, 38, 1069–1078. [Google Scholar] [CrossRef]
- Raoufi, D. Synthesis and microstructural properties of ZnO nanoparticles prepared by precipitation method. Renew. Energy 2013, 50, 932–937. [Google Scholar] [CrossRef]
- Sakka, S.; Coulthard, P. Implant failure: Etiology and complications. Medicina Oral Patología Oral y Cirugia Bucal 2011, 16, e42–e44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Jayaramudu, T.; Varaprasad, K.; Pyarasani, R.D.; Reddy, K.K.; Kumar, K.D.; Akbari-Fakhrabadi, A.; Mangalaraja, R.; Amalraj, J. Chitosan capped copper oxide/copper nanoparticles encapsulated microbial resistant nanocomposite films. Int. J. Biol. Macromol. 2019, 128, 499–508. [Google Scholar] [CrossRef]
- Mishra, S.K.; Ferreira, J.M.F.; Kannan, S. Mechanically stable antimicrobial chitosan–PVA–silver nanocomposite coatings deposited on titanium implants. Carbohydr. Polym. 2015, 121, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Esparza, L.M.; Ruvalcaba-Gómez, J.M.; Maytorena-Verdugo, C.I.; González-Silva, N.; Romero-Toledo, R.; Aguilera-Aguirre, S.; Pérez-Larios, A.; Montalvo-González, E. Chitosan-TiO2: A versatile hybrid composite. Materials 2020, 13, 811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Silva, S.; Henriques, M.; Martins, A.; Oliveira, R.; Williams, D.W.; Azeredo, J. Biofilms of non-Candida albicans Candidaspecies: Quantification, structure and matrix composition. Med. Mycol. 2009, 47, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.S. Zinc in human health: effect of zinc on immune Cells. Mol. Med. 2008, 14, 353–357. [Google Scholar] [CrossRef]
- Prasad, A.S.; Beck, F.W.; Endre, L.; Handschu, W.; Kukuruga, M.; Kumar, G. Zinc deficiency affects cell cycle and deoxythymidine kinase gene expression in HUT-78 cells. J. Lab. Clin. Med. 1996, 128, 51–60. [Google Scholar] [CrossRef]
- Luna, S.M.; Silva, S.S.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Cell adhesion and proliferation onto chitosan-based membranes treated by plasma surface modification. J. Biomater. Appl. 2010, 26, 101–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.; Zhang, W.; Qiao, Y.; Jiang, X.; Liu, X.; Ding, C. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta Biomater. 2012, 8, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Cao, H.; Qiao, Y.; Meng, F.; Zhu, H.; Liu, X. Osteogenic activity and antibacterial effect of zinc ion implanted titanium. Colloids Surf. B Biointerfaces 2014, 117, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jin, G.; Xue, Y.; Wang, N.; Liu, X.; Sun, J. Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017, 49, 590–603. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, J.P.; Kanjilal, D.; Teitelbaum, M.; Lin, S.S.; Cottrell, J. Zinc as a Therapeutic Agent in Bone Regeneration. Materials 2020, 13, 2211. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, W.; Tian, P.; Meng, F.; Zhu, H.; Jiang, X.; Liu, X.; Chu, P.K. Stimulation of bone growth following zinc incorporation into biomaterials. Biomaterials 2014, 35, 6882–6897. [Google Scholar] [CrossRef]
- Yu, J.; Xu, L.; Li, K.; Xie, N.; Xi, Y.; Wang, Y.; Zheng, X.; Chen, X.; Wang, M.; Ye, X. Zinc-modified calcium silicate coatings promote osteogenic differentiation through TGF-β/Smad pathway and osseointegration in osteopenic rabbits. Sci. Rep. 2017, 7, 3440. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Zhang, B.; Shao, L.; Feng, W.; Jiang, L.; Zhao, B. Biomechanical and histological studies of the effects of active zinc-coated implants by plasma electrolytic oxidation method on osseointegration in rabbit osteoporotic jaw. Surf. Coat. Technol. 2020, 396, 125848. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humayun, A.; Luo, Y.; Mills, D.K. Electrophoretic Deposition of Gentamicin-Loaded ZnHNTs-Chitosan on Titanium. Coatings 2020, 10, 944. https://doi.org/10.3390/coatings10100944
Humayun A, Luo Y, Mills DK. Electrophoretic Deposition of Gentamicin-Loaded ZnHNTs-Chitosan on Titanium. Coatings. 2020; 10(10):944. https://doi.org/10.3390/coatings10100944
Chicago/Turabian StyleHumayun, Ahmed, Yangyang Luo, and David K. Mills. 2020. "Electrophoretic Deposition of Gentamicin-Loaded ZnHNTs-Chitosan on Titanium" Coatings 10, no. 10: 944. https://doi.org/10.3390/coatings10100944
APA StyleHumayun, A., Luo, Y., & Mills, D. K. (2020). Electrophoretic Deposition of Gentamicin-Loaded ZnHNTs-Chitosan on Titanium. Coatings, 10(10), 944. https://doi.org/10.3390/coatings10100944