Effects of Inorganic ZnO Particle Doping on Crystalline Polymer Morphology and Space Charge Behavior
Abstract
:1. Introduction
2. Sample Preparation and Methods
2.1. Experimental Materials and Sample Preparation
2.2. Experimental Methods
2.2.1. PLM Test
2.2.2. DSC Test
2.2.3. SEM Test
2.2.4. AC/DC Breakdown Test
2.2.5. Space Charge Test
3. Results and Analysis
3.1. PLM Characterization Results and Analysis
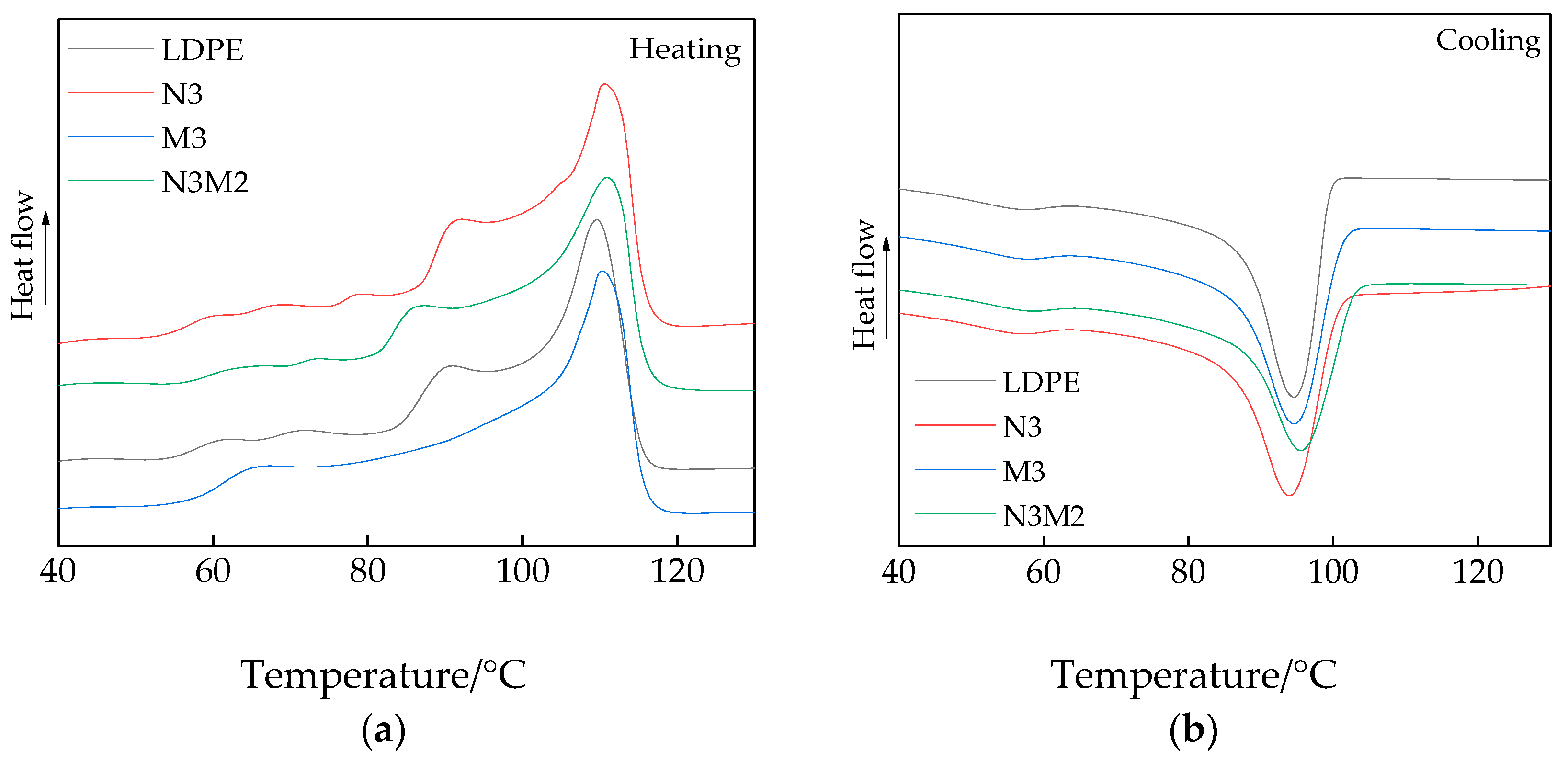
3.2. DSC Characterization Results and Analysis
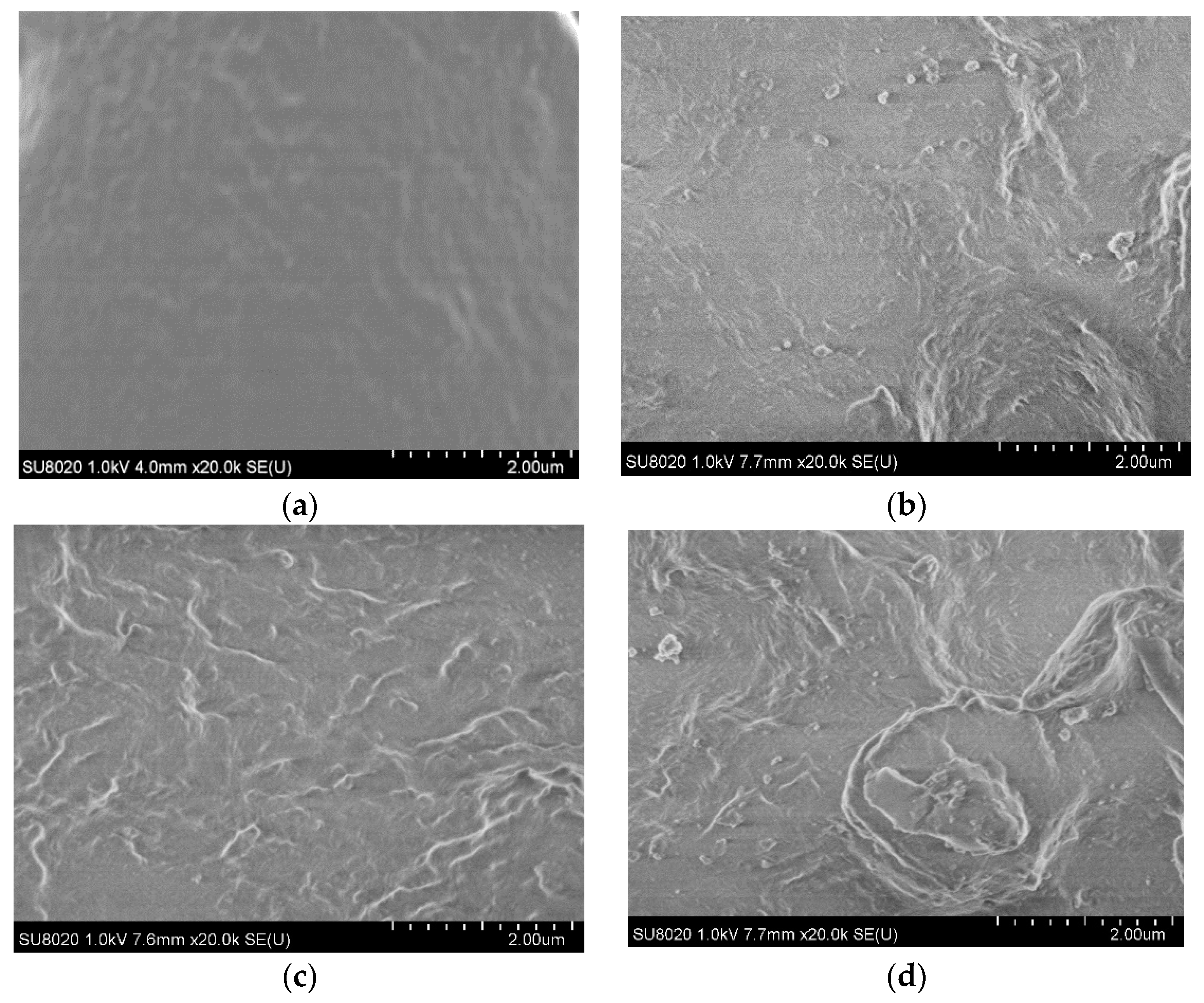
3.3. SEM Characterization Results and Analysis
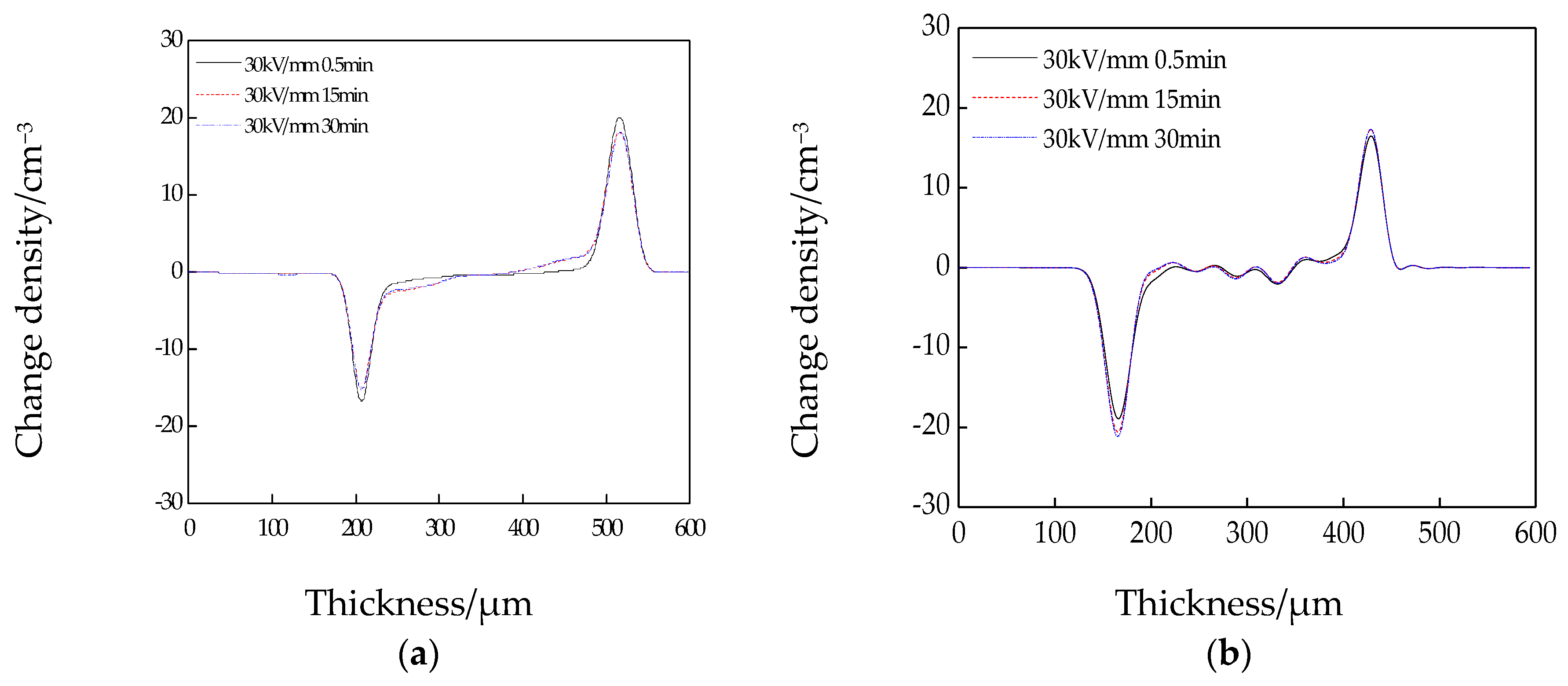
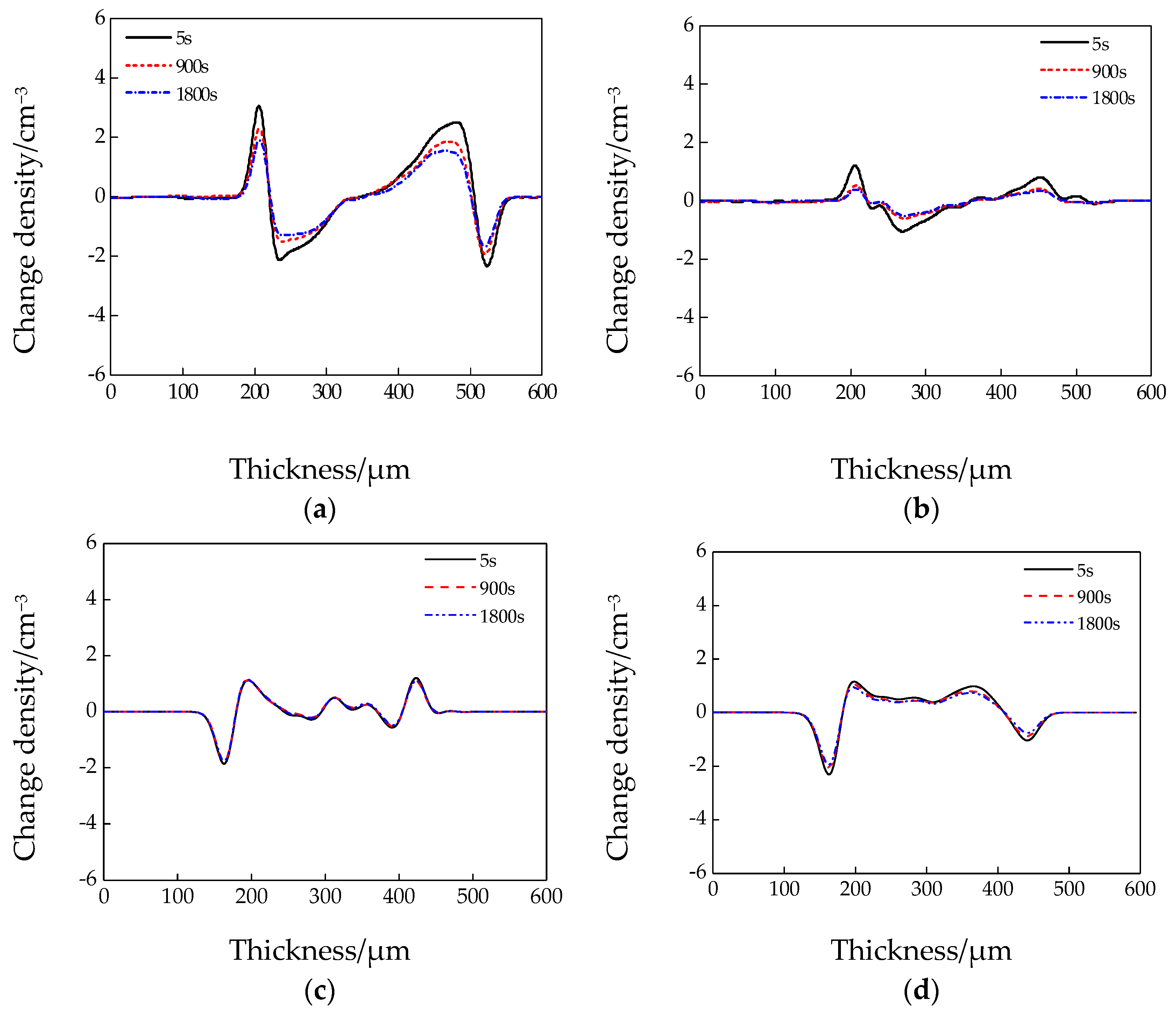
3.4. Space Charge Test Results and Analysis
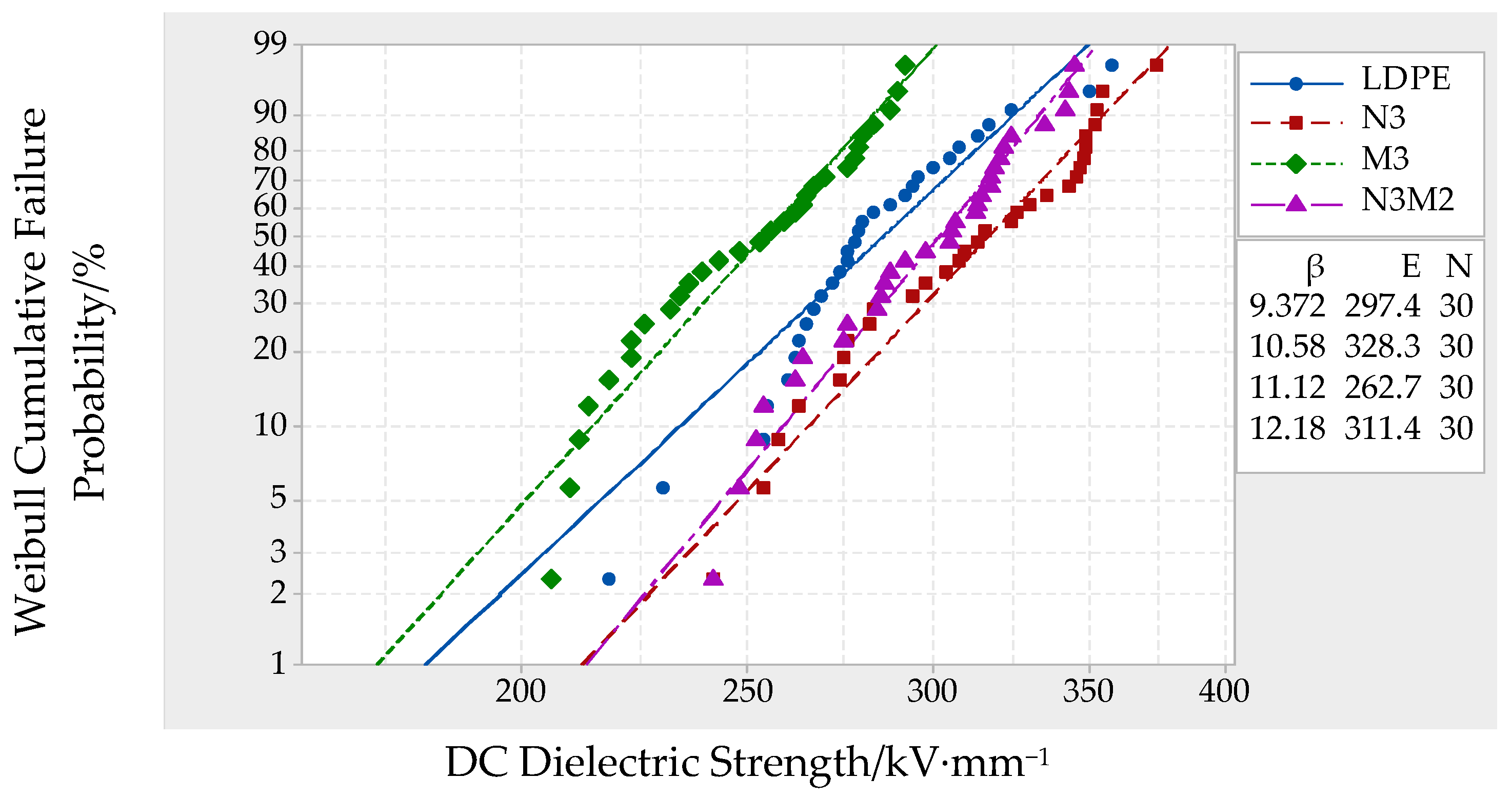
3.5. AC/DC Breakdown Test Results and Analysis
3.6. Dielectric Spectrum Test Results and Analysis
4. Conclusions
- (1)
- Based on the SEM test results, the inorganic ZnO particles were uniformly dispersed in the matrix. Based on the results of DSC and PLM crystallization characterization, the doping of the inorganic ZnO particles improved the crystallinity and crystallization rate of the polymers. In addition, the inorganic particles possessed appropriate thermal conductivity. Therefore, the melting temperatures of the polymer composites were high. The nanocomposite particle size was small, and the grain arrangement was dense and regular. The crystallinity of the micro–nanocomposites was highest.
- (2)
- Based on the AC/DC breakdown test results, the doping of the ZnO particles with different sizes generated varying effects on the composite breakdown field strength. The nano-ZnO particle doping substantially improved the AC/DC breakdown field strength. The breakdown field strength of the microcomposites was slightly lower than that of the pure LDPE. Moreover, the DC breakdown field strengths of all the samples were higher than their AC breakdown field strengths. Based on the dielectric spectrum, the dielectric constants of the nanocomposites were lower than that of the pure LDPE. However, the dielectric constants of other test composites were higher than that of the pure LDPE. Moreover, the dielectric loss of all the composites was lower than that of LDPE.
- (3)
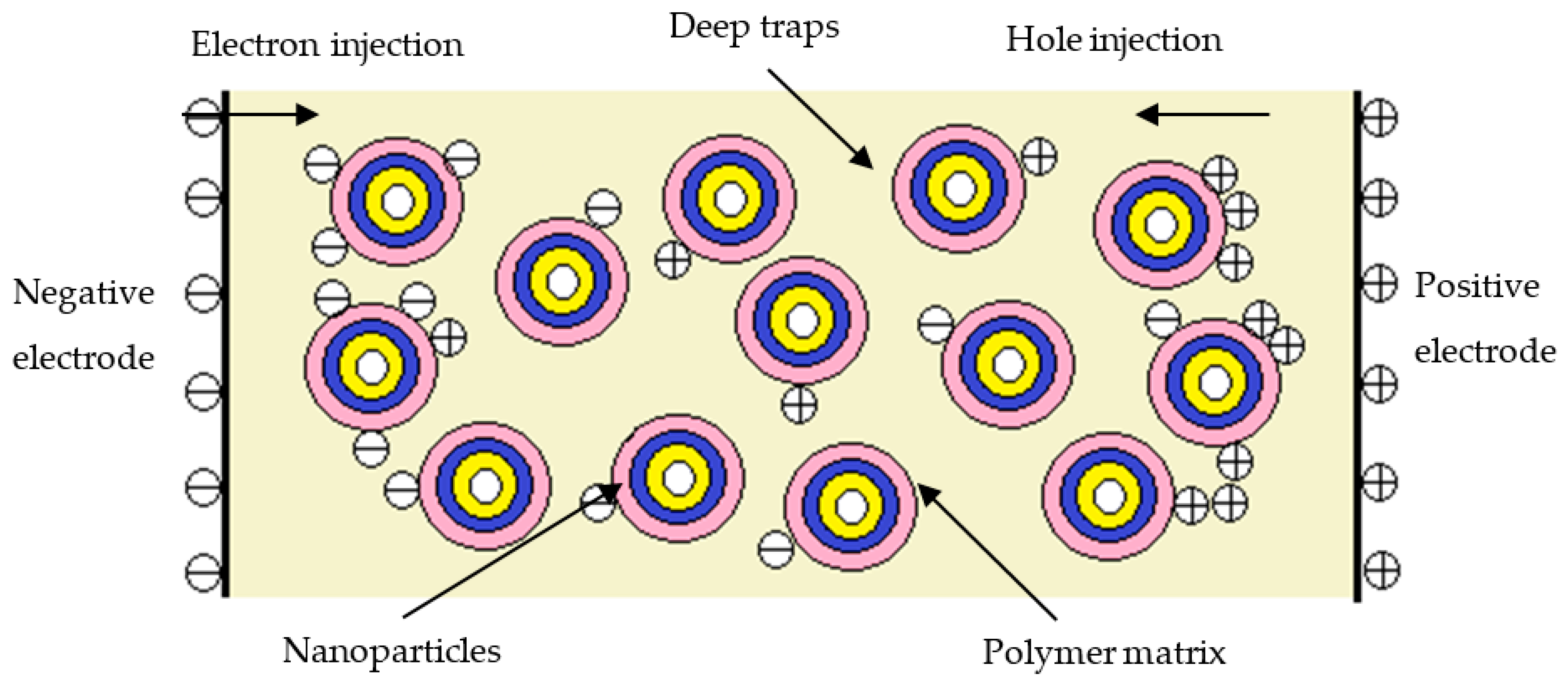
- Based on the results of the space charge test, considerable time was required to stabilize the pure LDPE. By contrast, the steady state of the composites was reached in the first few seconds of voltage application. After 1800 s of short-circuit treatment, there were fewer residual space charges in the three composites than in the pure LDPE. The residual space charges of the nanocomposites were fewest, followed by those of the micro–nanocomposites. Thus, inorganic ZnO particle doping can restrain the accumulation of space charges, and the space charges can be released by the dielectric double-layer effect.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sethi, G.; Furman, E.; Koch, B.; Lanagan, M. Influence of impedance contrast on field distribution and tree growth in laminate dielectrics. Model. Simul. Mater. Sci. Eng. 2014, 22, 025024. [Google Scholar] [CrossRef]
- Zhang, H.; Shang, Y.; Zhao, H.; Han, B.; Li, Z.-S. Mechanisms on inhibition of polyethylene electrical tree aging: A theoretical study. J. Mol. Modeling 2013, 19, 3035–3044. [Google Scholar] [CrossRef] [PubMed]
- Li, C.M.; Zhang, G.X.; Li, C.Y.; Han, B.Z. Inhibition of electrical tree initiation inside high-voltage cross-linked polyethylene cable with nonlinear shielding layer. Adv. Mater. Res. 2014, 873, 406–410. [Google Scholar] [CrossRef]
- Nelson, J.K.; Fothergill, J.C.; Dissado, L.A.; Peasgood, W. Towards an understanding of nanometric dielectrics. In Proceedings of the Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Cancun, Quintana Roo, Mexico, 20–24 October 2002; pp. 295–298. [Google Scholar]
- Cheng, Y.; Zhang, X.; Zhou, X.; Ning, G.; Ru, C.; Tian, Z. Effects of Cooling Methods on Dielectric Properties of MMT/LDPE. J. Inorg. Mater. 2015, 30, 1295–1302. [Google Scholar]
- Hajibeygi, M.; Mousavi, M.; Shabanian, M.; Vahabi, H. The effect of phosphorus based melamine-terephthaldehyde resin and Mg-Al layered double hydroxide on the thermal stability, flame retardancy and mechanical properties of polypropylene MgO composites. Mater. Today Commun. 2020, 23, 100880. [Google Scholar] [CrossRef]
- Yan, H.D.; Zhang, C.; Li, W.K.; Zha, J.W. Effect of trap level density on breakdown strength and space charge distribution of polypropylene/low-density polyethylene composites. Polym. Compos. 2020, 41, 780–787. [Google Scholar] [CrossRef]
- Liao, R.; Li, X.; Bai, G.; Yang, L.; Gu, J. Influence of montmorillonite on electrical treeing and breakdown characteristics of low-density polyethylene. J. Reinf. Plast. Compos. 2014, 33, 2117–2128. [Google Scholar] [CrossRef]
- Du, B.X.; Han, T.; Su, J.G. Tree characteristics in silicone rubber/SiO2 nanocomposites under low temperature. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 503–510. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Wu, K.; Peng, Z.; Cheng, Y.; Tu, D. Space charge measurement in LPDE films under temperature gradient and DC stress. IEEE Trans. Dielectr. Electr. Insul. 2010, 1, 1796–1805. [Google Scholar] [CrossRef]
- Takada, T.; Hayase, Y.; Tanaka, Y.; Okamoto, T. Space charge trapping in electrical potential well caused by permanent and induced dipoles for LDPE/MgO nanocomposite. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 152–160. [Google Scholar] [CrossRef]
- Lei, Q.Q.; Wang, X.; He, L.J.; Zhang, D.; Han, B.; Song, W.; Sun, Z. Review, Thinking and Countermeasure About Engineering Dielectric Theory. High Volt. Eng. 2007, 33, 1–4. [Google Scholar]
- Zha, J.-W.; Zhu, Y.-H.; Li, W.-K.; Bai, J.; Dang, Z.-M. Low dielectric permittivity and high thermal conductivity silicone rubber composites with micro-nano-sized particles. Appl. Phys. Lett. 2012, 101, 062905. [Google Scholar] [CrossRef]
- Khaled, U.; Alzahrani, S.; Khan, Y. Dielectric properties improvement of LDPE based on nano fillers. In Proceedings of the 2016 IEEE International Conference on High Voltage Engineering and Application, Chengdu, China, 19–22 September 2016; pp. 1–4. [Google Scholar]
- Virtanen, S.; Vaughan, A.S.; Yang, L.; Saiz, F.; Quirke, N. Dielectric Breakdown Strength and Electrical Conductivity of Low Density Polyethylene Octylnanosilica Composite. In Proceedings of the 2016 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Toronto, ON, Canada, 16–19 October 2016; pp. 58–61. [Google Scholar]
- Zhang, L.; Zhou, Y.; Cui, X.; Sha, Y.; Le, T.H.; Ye, Q.; Tian, J. Effect of nanparticle surface modificantion on breakdown and space charge behavior of XLPE/SiO2 nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1554–1564. [Google Scholar] [CrossRef]
- Yan, H.; Song, S.; Zhao, H.; Yao, Z. Dielectric Properties of Polystyrene Graft Copolymer/Low Density Polyethylene. High Volt. Eng. 2018, 44, 3840–3847. [Google Scholar]
- Chen, P.; Wang, S.; Chen, Z.; Li, J. Effect of nano-TiO2 on Dielectric Properties of XLPE for HVDC Cable. High Volt. Eng. 2018, 44, 3848–3856. [Google Scholar]
- Zhang, J.; Zuo, J.; Ai, W.; Liu, S.; Zhu, D.; Zhang, J.; Wei, C. Preparation of a new high-efficiency resin deodorant from coal gasification fine slag and its application in the removal of volatile organic compounds in polypropylene composites. J. Hazard. Mater. 2020, 384, 121347. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C. Polymer Structure Analysis; Science Press: Beijing, China, 2010; pp. 157–169. [Google Scholar]
- Zheng, J.; Zhou, X.; Xie, X. Non-isothermal crystallization kinetics of polypropylene containing silica hybrid particles as fillers. Acta Mater. Compos. Sin. 2013, 30, 18–23. [Google Scholar]
- He, J.; Peng, S.; Zhou, Y.; Yang, Y.; Hu, J. Interface properties of polymer nanocomposites. Proc. CSEE 2016, 36, 6596–6605. [Google Scholar]
- Peng, S.; He, J.; Hu, J.; Huang, X.; Jiang, P. Influence of functionalized MgO nanoparticles on electrical properties of polyethylene nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 1512–1519. [Google Scholar] [CrossRef]
- Tu, D.M.; Wang, X.; Lü, Z.P.; Wu, K.; Peng, Z.R. Formation and inhibition mechanisms of space charges in direct current polyethylene insulation explained by energy band theory. Acta Phys. Sin. 2012, 61, 017104. [Google Scholar] [CrossRef]
- Dang, B.; He, J.; Hu, J.; Zhou, Y. Large improvement in trap level and space charge distribution of polypropylene by enhancing the crystalline amorphous interface effect in blends. Polym. Int. 2016, 65, 371–379. [Google Scholar] [CrossRef]
- Xie, D.; Min, D.; Liu, W.; Li, S.; Min, C. Correlation between dielectric breakdown and interface traps characteristics. High Volt. Eng. 2018, 44, 432–439. [Google Scholar]




| Name | Parameter/Model | Manufacturer |
|---|---|---|
| LDPE | Density: 0.910–0.925 mg/cm3 | Daqing Petrochemical (Daqing, China) |
| ZnO | Particle sizes: 30 nm, 1 µm | Beijing Deke Daojin Science and Technology Co., Ltd. (Beijing, China) |
| Electronic balance | YP202N | Shanghai Precision & Scientific Instrument Corporation (Shanghai, China) |
| Vacuum drying oven | DZF-6020MBE | Shanghai Boxun Industry & Commerce Co., Ltd. (Shanghai, China) |
| Ultrasonic cleaner | KQ5200DE | Kunshan Ultrasonic Instruments Co., Ltd. (Kunshan, China) |
| Torque rheometer | Rheocord 300 | Thermo Scientific Co., Ltd. (Harbin, China) |
| Scanning electron microscope | JSM-F100 | JEOL Co., Ltd. (Tokyo, Japan) |
| Dielectric spectrum analyzer | DMS-2000 | Partulan Co., Ltd. (Wuhan, China) |
| Plate vulcanizing machine | XLB25-D | HaimenJinma Rubber & Plastics Machinery Technology Co., Ltd. (Haimen, China) |
| Differential scanning calorimeter | DSC-1 | Mettler Toledo (Zurich, Switzerland) |
| Sample | Tc (°C) | Tcon (°C) | ΔTc (°C) | Tm (°C) | Xc (%) |
|---|---|---|---|---|---|
| LDPE | 93.67 | 102.31 | 8.64 | 109.75 | 34.90 |
| N3 | 94.40 | 102.54 | 8.16 | 110.25 | 37.23 |
| M3 | 95.12 | 101.14 | 6.02 | 110.60 | 35.64 |
| N3M2 | 95.89 | 102.97 | 7.08 | 110.46 | 37.79 |
| Sample | Breakdown Field Strength Eb/(kV·mm−1) | Numbers | Shape Parameter β |
|---|---|---|---|
| LDPE | 120.7 | 30 | 10.40 |
| N3 | 139.8 | 30 | 11.74 |
| M3 | 96.63 | 30 | 10.86 |
| N3M2 | 124.6 | 30 | 13.84 |
| Sample | Breakdown Field Strength Eb/(kV·mm−1) | Numbers | Shape Parameter β |
|---|---|---|---|
| LDPE | 297.4 | 30 | 9.372 |
| N3 | 330.2 | 30 | 10.35 |
| M3 | 262.7 | 30 | 11.12 |
| N3M2 | 311.4 | 30 | 12.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.; Cheng, Y. Effects of Inorganic ZnO Particle Doping on Crystalline Polymer Morphology and Space Charge Behavior. Coatings 2020, 10, 932. https://doi.org/10.3390/coatings10100932
Yu G, Cheng Y. Effects of Inorganic ZnO Particle Doping on Crystalline Polymer Morphology and Space Charge Behavior. Coatings. 2020; 10(10):932. https://doi.org/10.3390/coatings10100932
Chicago/Turabian StyleYu, Guang, and Yujia Cheng. 2020. "Effects of Inorganic ZnO Particle Doping on Crystalline Polymer Morphology and Space Charge Behavior" Coatings 10, no. 10: 932. https://doi.org/10.3390/coatings10100932
APA StyleYu, G., & Cheng, Y. (2020). Effects of Inorganic ZnO Particle Doping on Crystalline Polymer Morphology and Space Charge Behavior. Coatings, 10(10), 932. https://doi.org/10.3390/coatings10100932





