A Review of Green Scale Inhibitors: Process, Types, Mechanism and Properties
Abstract
:1. Introduction
2. Scaling Process
2.1. Initiation
2.2. Transport
2.3. Deposition
2.4. Removal
2.5. Aging
3. Types of Scales
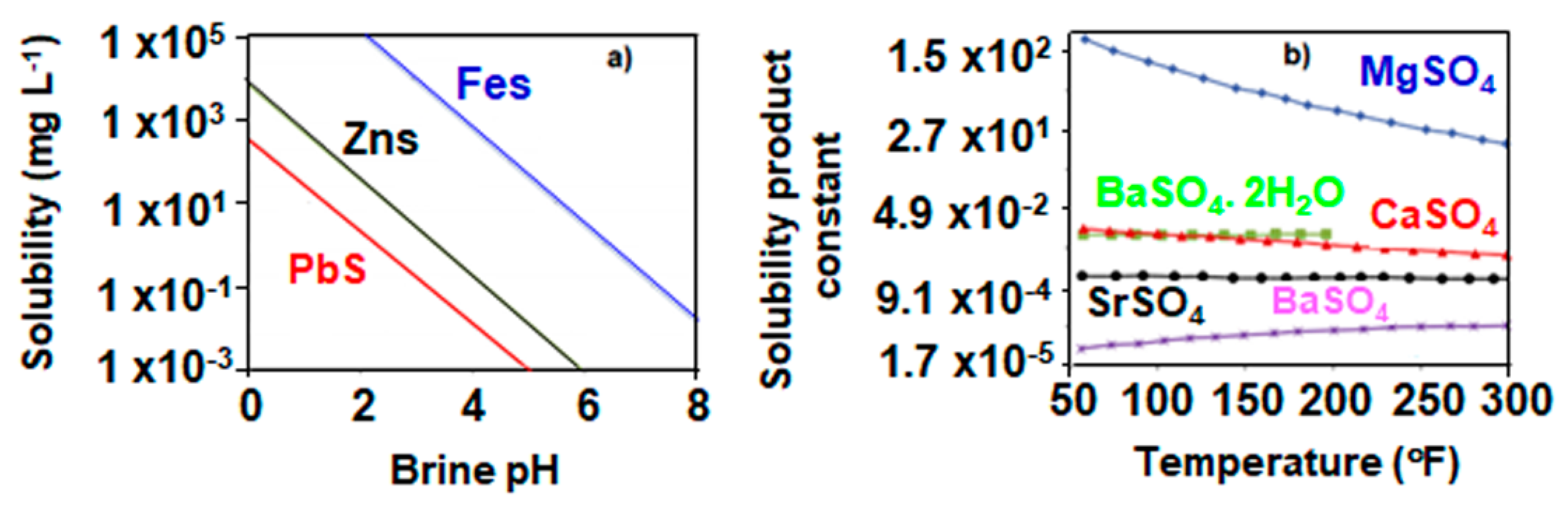
3.1. Sulfide Scales
3.1.1. Iron Sulfide
3.1.2. Other Sulfide Scales
3.2. Sulfate Scales
3.2.1. Barium Sulfate
3.2.2. Calcium Sulfate
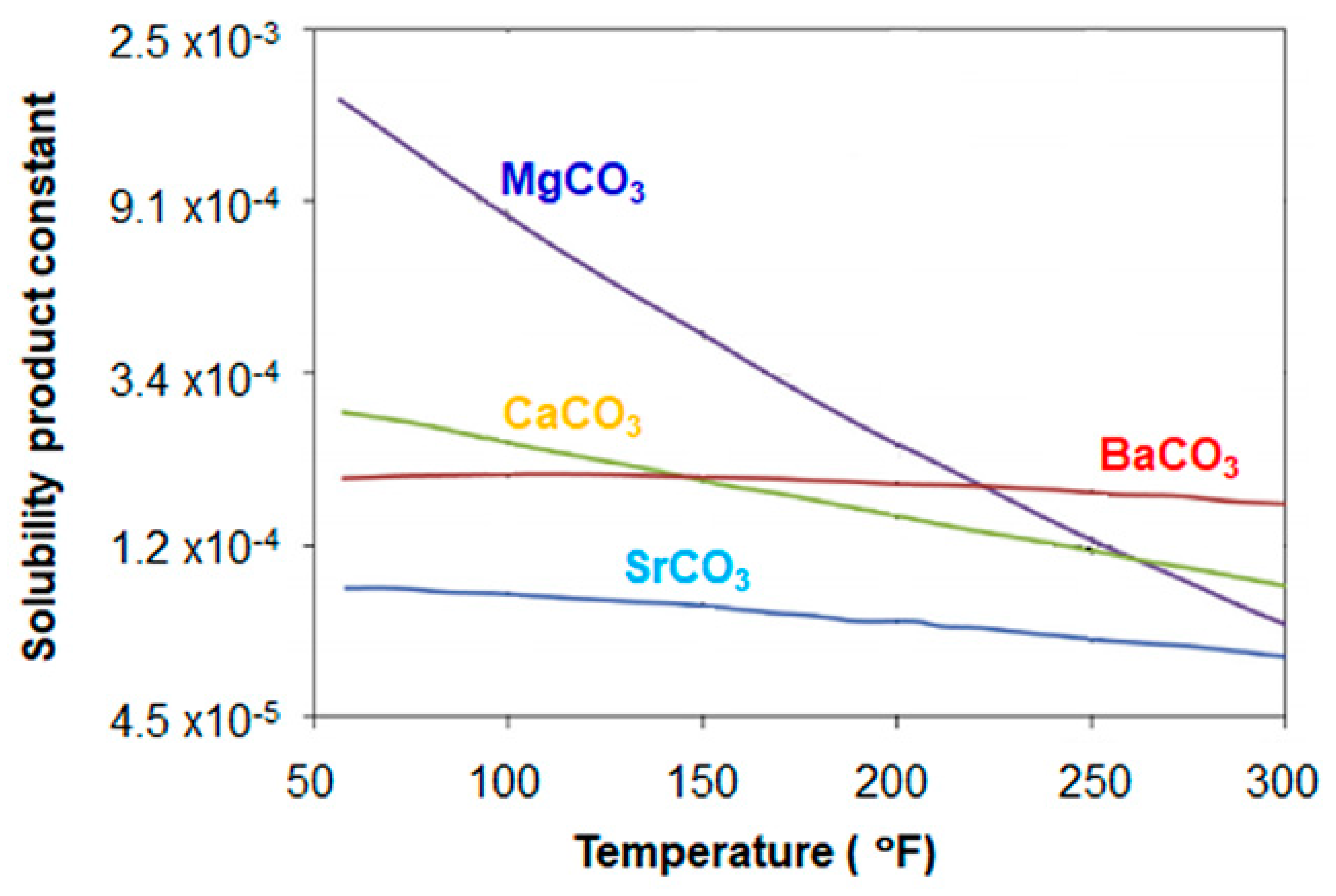
3.3. Carbonate Scales
4. Mechanism of Scale Inhibitions
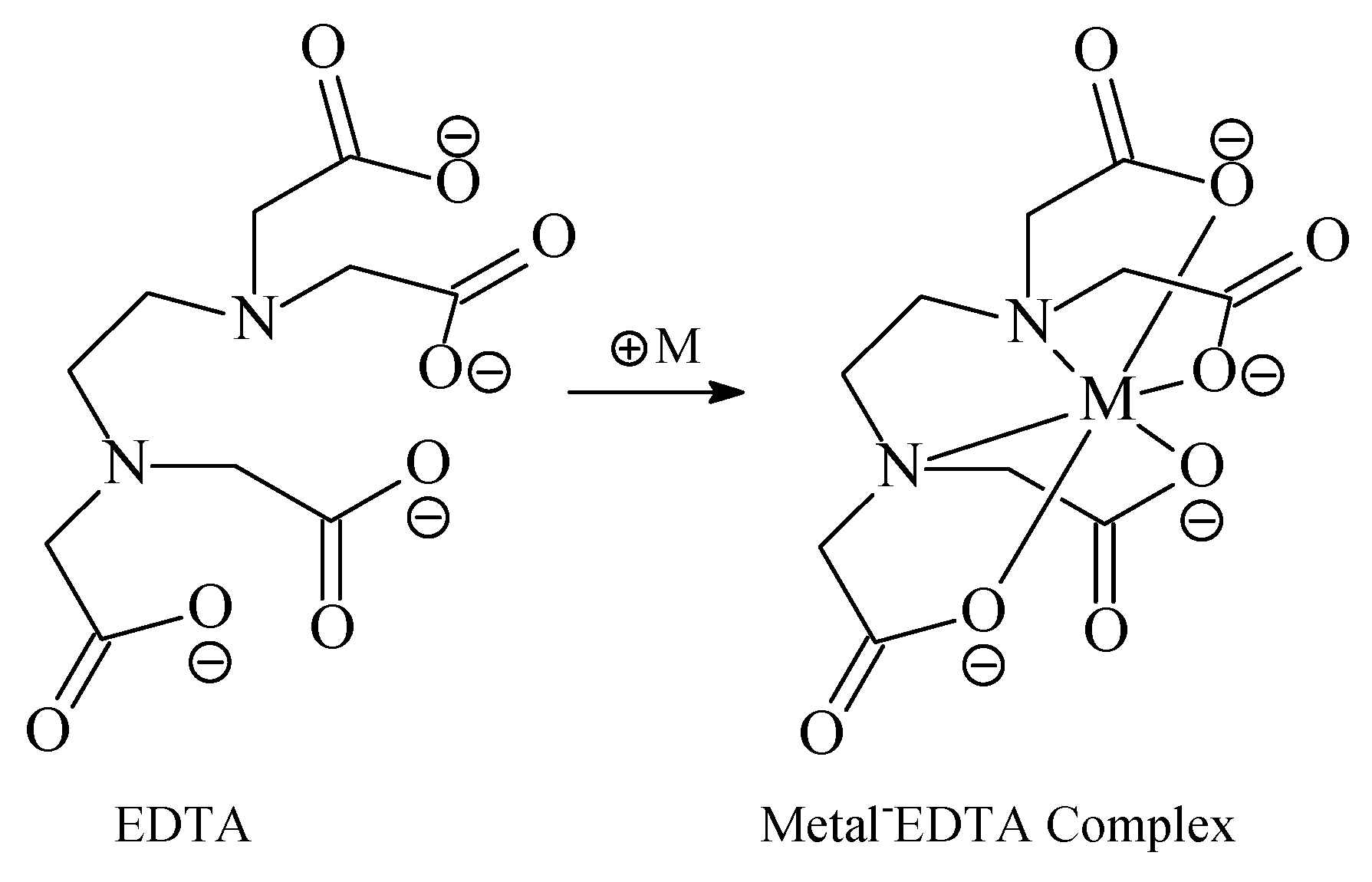
4.1. Chelants (Sequestrants)
4.2. Threshold Scale Inhibitors
4.3. Fluorescent-Tagged Scale Inhibitors
5. Scale Inhibitors
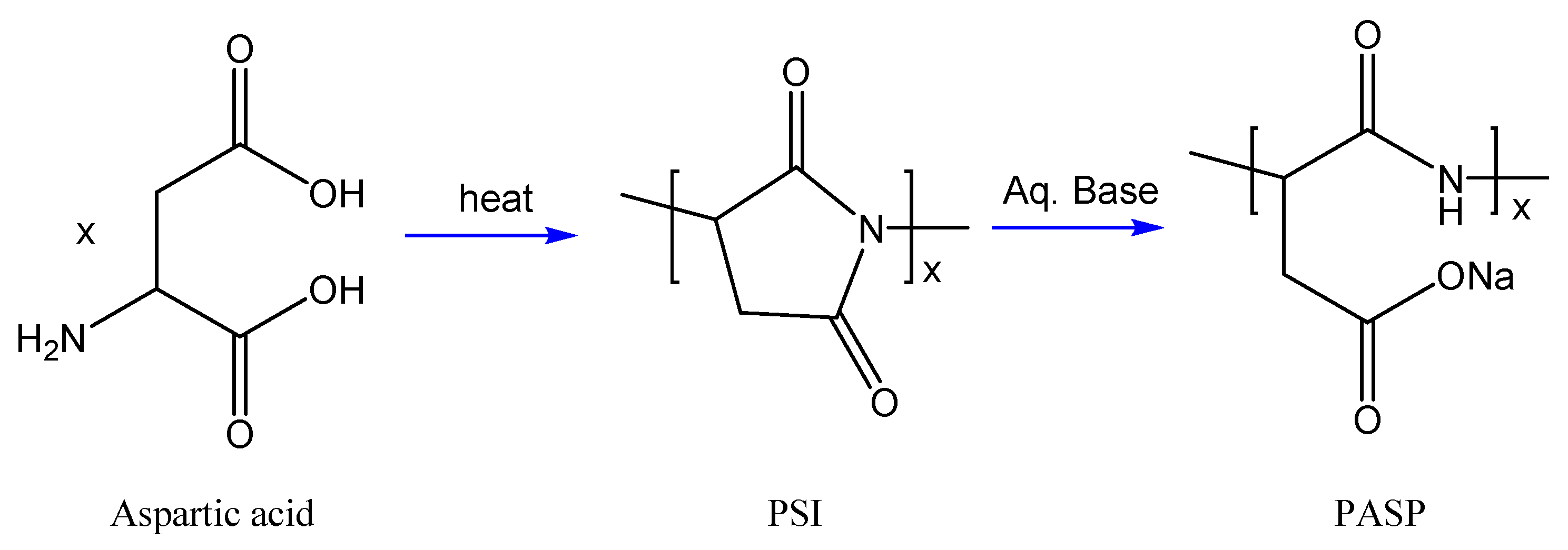
5.1. Natural Organic Molecule
5.2. Biodegradable Polymers
5.3. Modified Natural Polymers
5.3.1. Chitosan and Substituted/Modified Chitosan
5.3.2. Pectate
5.4. Scale Inhibitors Extracted from Natural Sources
5.5. Other Scale Inhibitors
5.5.1. Calcium Carbonate
5.5.2. Calcium Sulfate
5.5.3. Calcium Phosphate
- (i)
- During pretreatment, the concentrations of orthophosphate, Ca, Al, Fe, and fluoride should be reduced.
- (ii)
- Dispersants can be used in the source when Ca3(PO4)3 appears in the form of nanoparticles, and
- (iii)
- The reverse osmosis (RO) feed should be maintained at low pH.
6. Comparative Performance Evaluation of Traditional and Green Scale Inhibitors
7. Significance of Green Scale Inhibitors
- Optimal dosing of antiscalants is essential; otherwise, they can be a foulant as concentrations are high.
- Scale inhibitors are shown to increase the biofouling potential in RO systems. A few scale inhibitors can enhance the biological growth by up to 10 times.
- Pretreatment chemicals’ carryover may react with scale inhibitors and form foulants or oppose the inhibition efficiencies. Using cationic flocculants for pretreatment can individually respond with few types of scale inhibitors forming sticky foulants.
- Polyacrylate shows a membrane type of foulant in the presence of Fe and other metal ions. Similarly, HEDP loses its efficiency of scale inhibition at high alkalinities and in the presence of chlorine.
- Observing the presence of scale inhibitors in the system is complicated, compared to evaluating the doses of acid by changing the pH.
8. Conclusions and Future Aspects
- Concept of scales;
- Different types of scales encountered in oil field reservoirs;
- Development of the scaling process on the surface;
- General mechanism of scale inhibitors;
- Environmentally benign green scale inhibitors (synthetic and natural source);
- Significance of scale inhibitors with a particular focus on green scale inhibitors.
Funding
Conflicts of Interest
References
- Bin Merdhah, A. Inhibition of calcium sulfate and strontium sulfate scale in waterflood. SPE Prod. Oper. 2010, 25, 545–552. [Google Scholar] [CrossRef]
- Lu, H.; Kan, A.T.; Zhang, P.; Yu, J.; Fan, C.; Tomson, M.B. Phase stability and solubility of calcium sulfate in the system NaCl/monoethylene glycol/water. In Proceedings of the SPE International Conference on Oilfield Scale (SPE-130697-MS), Aberdeen, UK, 26–27 May 2010; pp. 1–26. [Google Scholar]
- Zhang, Z.-J.; Lu, M.-L.; Liu, J.; Chen, H.-L.; Chen, Q.-L.; Wang, B. Fluorescent-tagged hyper-branched polyester for inhibition of CaSO4 scale and the scale inhibition mechanism. Mater. Today Commun. 2020, 25, 101359. [Google Scholar] [CrossRef]
- Jordan, M.M.; Williams, H.; Samaniego, S.L.; Frigo, D.M. New insights on the impact of high temperature conditions (176 °C) on carbonate and sulphate scale dissolver performance. In Proceedings of the SPE International Oilfield Scale Conference and Exhibition (SPE-169785-MS), Aberdeen, UK, 14–15 May 2014; pp. 1–17. [Google Scholar]
- Olajire, A.A. A review of oilfield scale management technology for oil and gas production. J. Pet. Sci. Eng. 2015, 135, 723–737. [Google Scholar] [CrossRef]
- Kan, A.; Tomson, M. Scale prediction for oil and gas production. SPE J. 2012, 17, 362–378. [Google Scholar] [CrossRef]
- Yuan, X.; Dong, S.; Zheng, Q.; Yang, W.; Huang, T. Novel and efficient curcumin based fluorescent polymer for scale and corrosion inhibition. Chem. Eng. J. 2020, 389, 124296. [Google Scholar] [CrossRef]
- Abd-El-Khalek, D.; Abd-El-Nabey, B. Evaluation of sodium hexametaphosphate as scale and corrosion inhibitor in cooling water using electrochemical techniques. Desalination 2013, 311, 227–233. [Google Scholar] [CrossRef]
- Kamal, M.S.; Hussein, I.; Mahmoud, M.; Sultan, A.S.; Saad, M.A.S. Oil-field scale formation and chemical removal: A review. J. Pet. Sci. Eng. 2018, 171, 127–139. [Google Scholar] [CrossRef]
- Qiang, X.; Sheng, Z.; Zhang, H. Study on scale inhibition performances and interaction mechanism of modified collagen. Desalination 2013, 309, 237–242. [Google Scholar] [CrossRef]
- Yap, J.; Fuller, M.J.; Schafer, L.; Kelkar, S.K. Removing iron sulfide scale: A novel approach. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference (SPE-138520-MS), Abu Dhabi, UAE, 1–4 November 2010. [Google Scholar]
- Lakshmi, D.S.; Senthilmurugan, B.; Drioli, E.; Figoli, A. Application of ionic liquid polymeric microsphere in oilfield scale control process. J. Petrol. Sci. Eng. 2013, 112, 69–77. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Ghosh, B.; Sanker, S. High performance maleic acid based oil well scale inhibitors—Development and comparative evaluation. J. Ind. Eng. Chem. 2011, 17, 415–420. [Google Scholar] [CrossRef]
- Antony, N.; Sherine, H.B.; Rajendran, S. Investigation of the inhibiting effect carboxymethylcellulose-Zn2+ system on the corrosion of carbon steel in neutral chloride solution. Arab. J. Sci. Eng. 2010, 35, 41–53. [Google Scholar]
- Li, J.; Tang, M.-J.; Ye, Z.; Chen, L.; Zhou, Y.; Cheng, L. Scale formation and control in oil and gas fields: A review. J. Dispers. Sci. Technol. 2016, 38, 661–670. [Google Scholar] [CrossRef]
- Kelland, M.A. Production Chemicals for the Oil and Gas Industry; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Bader, M. Sulfate removal technologies for oilfields seawater injection operations. J. Petrol. Sci. Eng. 2007, 55, 93–110. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Z.; Wang, B.; He, J. Scale inhibition performance of sodium carboxymethyl cellulose on heat transfer surface at various temperatures: Experiments and molecular dynamics simulation. Int. J. Heat Mass Transf. 2019, 141, 457–463. [Google Scholar] [CrossRef]
- Baugh, T.D.; Lee, J.; Winters, K.; Waters, J.; Wilcher, J. A fast and information-rich test method for scale inhibitor performance. In Proceedings of the Offshore Technology Conference (OTC 23150), Houston, TX, USA, 30 April–3 May 2012; pp. 1–10. [Google Scholar]
- Huang, H.; Yao, Q.; Jiao, Q.; Liu, B.; Chen, H. Polyepoxysuccinic acid with hyper-branched structure as an environmentally friendly scale inhibitor and its scale inhibition mechanism. J. Saudi Chem. Soc. 2019, 23, 61–74. [Google Scholar] [CrossRef]
- Ntourou, K.; DeFranco, E.O.; Conture, E.G.; Walden, T.A.; Mushtaq, N. A parent-report scale of behavioral inhibition: Validation and application to preschool-age children who do and do not stutter. J. Fluen. Disord. 2020, 63, 105748. [Google Scholar] [CrossRef]
- Yu, W.; Wang, Y.; Li, A.; Yang, H. Evaluation of the structural morphology of starch-graft-poly (acrylic acid) on its scale-inhibition efficiency. Water Res. 2018, 141, 86–95. [Google Scholar] [CrossRef]
- Yang, Y.; Khan, F.; Thodi, P.; Abbassi, R. Corrosion induced failure analysis of subsea pipelines. Reliab. Eng. Syst. Saf. 2017, 159, 214–222. [Google Scholar] [CrossRef]
- Li, X.; Chen, G.; Zhu, H. Quantitative risk analysis on leakage failure of submarine oil and gas pipelines using Bayesian network. Process. Saf. Environ. Prot. 2016, 103, 163–173. [Google Scholar] [CrossRef]
- Alaoui, K.; Ouakki, M.; Abousalem, A.; Serrar, H.; Galai, M.; Derbali, S.; Nouneh, K.; Boukhris, S.; Touhami, M.E.; El Kacimi, Y. Molecular dynamics, Monte-Carlo simulations and atomic force microscopy to study the interfacial adsorption behavior of some triazepine carboxylate compounds as corrosion inhibitors in acid medium. J. Bio Tribo Corros. 2019, 5, 11–16. [Google Scholar] [CrossRef]
- Alaoui, K.; Touir, R.; Galai, M.; Serrar, H.; Ouakki, M.; Kaya, S.; Tuzun, B.; Boukhris, S.; Touhami, M.E.; El Kacimi, Y. Electrochemical and computational studies of some triazepine carboxylate compounds as acid corrosion inhibitors for mild steel. J. Bio Tribo Corros. 2018, 4, 1–18. [Google Scholar] [CrossRef]
- Majeed, N. Polymeric materials for scale inhibition in cooling water systems. Tikritj. Eng. Sci. 2011, 18, 1–11. [Google Scholar]
- Popov, K.; Oshchepkov, M.; Afanas’Eva, E.; Koltinova, E.; Dikareva, Y.; Rönkkömäki, H. A new insight into the mechanism of the scale inhibition: DLS study of gypsum nucleation in presence of phosphonates using nanosilver dispersion as an internal light scattering intensity reference. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 122–129. [Google Scholar] [CrossRef]
- Amjad, Z.; Zuhl, R.W. The use of polymers to improve control of calcium phosphonate and calcium carbonate in high stressed cooling water systems. Analysts 2011, 13, 1–4. [Google Scholar]
- Amjad, Z.; Landgraf, R.; Penn, J. Calcium sulfate dihydrate (gypsum) scale inhibition by PAA, PAPEMP, and PAA/PAPEMP blend. Int. J. Corros. Scale Inhib. 2014, 3, 35–47. [Google Scholar] [CrossRef]
- Wang, C.; Li, S.-P.; Li, T.-D. Calcium carbonate inhibition by a phosphonate-terminated poly(maleic-co-sulfonate) polymeric inhibitor. Desalination 2009, 249, 1–4. [Google Scholar] [CrossRef]
- Ansari, S.Z.; Pandit, A.B. Inhibition of Gypsum Scales on MS metal surface using hydrodynamic forces. Chem. Eng. Process. Process. Intensif. 2020, 147, 107706. [Google Scholar] [CrossRef]
- Sanni, O.S.; Bukuaghangin, O.; Charpentier, T.V.; Neville, A. Evaluation of laboratory techniques for assessing scale inhibition efficiency. J. Pet. Sci. Eng. 2019, 182, 106347. [Google Scholar] [CrossRef]
- Zhang, S.; Qu, H.; Yang, Z.; Fu, C.-E.; Tian, Z.; Yang, W. Scale inhibition performance and mechanism of sulfamic/amino acids modified polyaspartic acid against calcium sulfate. Desalination 2017, 419, 152–159. [Google Scholar] [CrossRef]
- Fu, L.; Lv, J.; Zhou, L.; Li, Z.; Tang, M.; Li, J. Study on corrosion and scale inhibition mechanism of polyaspartic acid grafted β-cyclodextrin. Mater. Lett. 2020, 264, 127276. [Google Scholar] [CrossRef]
- Miles, A.F.; Bourne, H.M.; Smith, R.G.; Collins, I.R. Development of a novel water in oil microemulsion based scale inhibitor delivery system. In Proceedings of the SPE International Symposium on Oilfield Scale (SPE 80390), Aberdeen, UK, 29–30 January 2003. [Google Scholar]
- Zhang, D.-Q.; An, Z.-X.; Pan, Q.-Y.; Gao, L.-X.; Zhou, G.-D. Comparative study of bis-piperidiniummethyl-urea and mono-piperidiniummethyl-urea as volatile corrosion inhibitors for mild steel. Corros. Sci. 2006, 48, 1437–1448. [Google Scholar] [CrossRef]
- Jordan, M.M.; Kramer, T.E.; Barbin, D.; Linares-Samaniego, S.; Arnette, W. Scale inhibitor squeeze treatments selection, deployed and monitoring in a deepwater Gulf of Mexico oilfield. In Proceedings of the SPE Annual Technical Conference and Exhibition (SPE 146400), Denver, CO, USA, 30 October–2 November 2011. [Google Scholar]
- Vazquez, O.; Mackay, E.J.; Al Shuaili, K.H.; Sorbie, K.; Jordan, M.M. Modelling a surfactant preflush with non-aqueous and aqueous scale inhibitor squeeze treatments. In Proceedings of the EUROPEC/EAGE Conference and Exhibition (SPE 113212), Rome, Italy, 9–12 June 2008. [Google Scholar]
- Jain, T.; Sanchez, E.; Owens-Bennett, E.; Trussell, R.; Walker, S.L.; Liu, H. Impacts of antiscalants on the formation of calcium solids: Implication on scaling potential of desalination concentrate. Environ. Sci. Water Res. Technol. 2019, 5, 1285–1294. [Google Scholar] [CrossRef]
- Gan, T.; Zhang, Y.; Yang, M.; Hu, H.; Huang, Z.; Feng, Z.; Chen, D.; Chen, C.; Liang, J. Synthesis, characterization and application of a multifunctional cellulose derivative as an environmentally friendly corrosion and scale inhibitor in simulated cooling water systems. Ind. Eng. Chem. Res. 2018, 57, 10786–10797. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, X.; Cheng, S.; Fu, C.; Tian, Z.; Yang, Z.; Yang, W.; Weiben, Y. Enhanced scale inhibition against Ca3(PO4)2 and Fe2O3 in water using multi-functional fluorescently-tagged antibacterial scale inhibitors. Environ. Sci. Water Res. Technol. 2020, 6, 951–962. [Google Scholar] [CrossRef]
- Peng, R.; Chen, G.; Zhou, F.; Man, R.; Huang, J. Catalyst-free synthesis of triazine-based porous organic polymers for Hg2+ adsorptive removal from aqueous solution. Chem. Eng. J. 2019, 371, 260–266. [Google Scholar] [CrossRef]
- Malcolm, K. Effect of various cations on the formation of calcium carbonate and barium sulfate scale with and without scale inhibitors. Ind. Eng. Chem. Res. 2011, 50, 5852–5861. [Google Scholar]
- Li, J.; Zhou, Y.; Yao, Q.; Wang, T.; Zhang, A.; Chen, Y.; Wu, W.; Sun, W. Preparation and evaluation of a polyether-based polycarboxylate as a kind of inhibitor for water systems. Ind. Eng. Chem. Res. 2017, 56, 2624–2633. [Google Scholar] [CrossRef]
- Liu, G.; Xue, M.; Yang, H. Polyether copolymer as an environmentally friendly scale and corrosion inhibitor in seawater. Desalination 2017, 419, 133–140. [Google Scholar] [CrossRef]
- Hoang, T.A.; Ang, H.M.; Rohl, A.L. Effects of temperature on the scaling of calcium sulphate in pipes. Powder Technol. 2007, 179, 31–37. [Google Scholar] [CrossRef]
- Dayarathne, H.; Jeong, S.; Jang, A. Chemical-free scale inhibition method for seawater reverse osmosis membrane process: Air micro-nano bubbles. Desalination 2019, 461, 1–9. [Google Scholar] [CrossRef]
- Herz, A.; Malayeri, M.; Müller-Steinhagen, H. Fouling of roughened stainless steel surfaces during convective heat transfer to aqueous solutions. Energy Convers. Manag. 2008, 49, 3381–3386. [Google Scholar] [CrossRef]
- Hoang, T.A. Mechanisms of scale formation and inhibition. In Mineral Scales and Deposits, 1st ed.; Amjad, Z., Demadis, K.D., Eds.; Elsevier: Cambridge, MA, USA, 2015; pp. 47–83. [Google Scholar]
- Berry, S.L.; Boles, J.L.; Singh, A.K.; Hashim, I. Enhancing production by removing zinc sulfide scale from an offshore well: A case history. SPE Prod. Oper. 2012, 27, 318–326. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Rahsepar, M. The use of green Bistorta Officinalis extract for effective inhibition of corrosion and scale formation problems in cooling water system. J. Alloys Compd. 2019, 770, 669–678. [Google Scholar] [CrossRef]
- Tan, H.; Skinner, W.M.; Addai-Mensah, J. pH-mediated interfacial chemistry and particle interactions in aqueous chlorite dispersions. Chem. Eng. Res. Des. 2013, 91, 448–456. [Google Scholar] [CrossRef]
- Zeino, A.; Albakri, M.; Khaled, M.; Zarzour, M. Comparative study of the synergistic effect of ATMP and DTPMPA on CaSO 4 scale inhibition and evaluation of induction time effect. J. Water Process. Eng. 2018, 21, 1–8. [Google Scholar] [CrossRef]
- Yang, L.; Yang, W.; Xu, B.; Yin, X.; Chen, Y.; Liu, Y.; Ji, Y.; Huan, Y. Synthesis and scale inhibition performance of a novel environmental-friendly and hydrophilic terpolymer inhibitor. Desalination 2017, 416, 166–174. [Google Scholar] [CrossRef]
- Ma, J.; Fuss, T.; Shi, J. Iron sulfide scale deposition in deep sour reservoirs. In Proceedings of the SPE International Conference and Exhibition on Formation Damage Control (SPE), Louisiana, LA, USA, 24–26 February 2016. [Google Scholar]
- Mahmoud, M. Effect of chlorite clay mineral dissolution on the improved oil recovery from sandstone rocks during DTPA chelating agent flooding. SPE J. 2018, 23, 1–19. [Google Scholar] [CrossRef]
- Bageri, B.S.; Mahmoud, M.; Abdulraheem, A.; Al-Mutairi, S.; Elkatatny, S.; Shawabkeh, R.A. Single stage filter cake removal of barite weighted water based drilling fluid. J. Pet. Sci. Eng. 2017, 149, 476–484. [Google Scholar] [CrossRef]
- Al-Harbi, B.G.; Graham, A.J.; Sorbie, K. Zinc and lead interactions in combined sulphide scales. In Proceedings of the SPE International Conference on Oilfield Chemistry (SPE-184509-MS), Montgomery, TX, USA, 3–5 April 2017. [Google Scholar]
- Baraka-Lokmane, S.; Hurtevent, C.; Rossiter, M.; Bryce, F.; Lepoivre, F.; Marais, A.; Tillement, O.; Simpson, C.; Graham, G.M. Design and performance of novel sulphide nanoparticle scale inhibitors for North Sea HP/Hatfields. In Proceedings of the SPE International Oilfield Scale Conference and Exhibition (SPE-179866-MS), Aberdeen, UK, 11–12 May 2016. [Google Scholar]
- Lopez, T.H.; Yuan, M.; Williamson, D.A.; Przybylinski, J.L. Comparing efficacy of scale inhibitors for inhibition of zinc sulfide and lead sulfide scales. In Proceedings of the SPE International Symposium on Oilfield Scale (SPE-95097-MS), Aberdeen, UK, 11–12 May 2005. [Google Scholar]
- Collins, I.; Jordán, M. Occurrence, prediction, and prevention of zinc sulfide scale within Gulf Coast and North Sea high-temperature and high-salinity fields. SPE Prod. Facil. 2003, 18, 200–209. [Google Scholar] [CrossRef]
- Chilingar, G.V.; Mourhatch, R.; Al-Qahtani, G.D. The Fundamentals of Corrosion and Scaling for Petroleum and Environmental Engineers; Elsevier Science: Houston, TX, USA, 2013; ISBN 780127999913. [Google Scholar]
- Li, Y.H.; Crane, S.D.; Coleman, J.R. A novel approach to predict the co-precipitation of BaSO4 and SrSO. In Proceedings of the SPE Production Operations Symposium (SPE-29489-MS), Oklahoma, OK, USA, 2–4 April 1995. [Google Scholar]
- Kuwahara, Y. In Situ atomic force microscopy study of dissolution of the barite (001) surface in water at 30 °C. Geochim. Cosmochim. Acta 2011, 75, 41–51. [Google Scholar] [CrossRef]
- Bageri, B.; Mahmoud, M.; Shawabkeh, R.; Al-Mutairi, S.; Abdulraheem, A. Toward a complete removal of barite (barium sulfate BaSO4) scale using chelating agents and catalysts. Arab. J. Sci. Eng. 2017, 42, 1667–1674. [Google Scholar] [CrossRef]
- Al-Khaldi, M.H.; Aljuhani, A.; Al-Mutairi, S.H.; Gurmen, M.N. New insights into the removal of calcium sulfate scale. In Proceedings of the SPE European Formation Damage Conference (SPE-144158-MS), Noordwijk, The Netherlands, 7–10 June 2011. [Google Scholar]
- Mahmoud, M. Effect of elemental-sulfur deposition on the rock petrophysical properties in sour-gas reservoirs. SPE J. 2014, 19, 703–715. [Google Scholar] [CrossRef]
- Oddo, J.; Smith, J.; Tomson, M. Analysis of and solutions to the CaCO3 and CaSO4 scaling problems encountered in wells offshore Indonesia. In Proceedings of the SPE Annual Technical Conference and Exhibition (SPE-22782-MS), Dallas, TX, USA, 6–9 October 1991. [Google Scholar]
- Moghadasi, J.; Müller-Steinhagen, H.; Jamialahmadi, M.; Sharif, A. Model study on the kinetics of oilfield formation damage due to salt precipitation from injection. J. Petrol. Sci. Eng. 2004, 43, 201–217. [Google Scholar] [CrossRef]
- DeLorey, J.; Allen, S.; McMaster, L. Precipitation of calcium sulphate during carbonate acidizing: Minimizing the risk. In Proceedings of the Annual Technical Meeting (Petsoc-96–84), Calgary, AB, Canada, 10–12 June 1996. [Google Scholar] [CrossRef]
- Amiri, M.; Moghadasi, J.; Jamialahmadi, M. Prediction of iron carbonate scale formation in Iranian oilfields at different mixing ratio of injection water with formation water. Energy Sources Part A Recover. Util. Environ. Eff. 2013, 35, 1256–1265. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Z.; Yang, F.; Zhen, Y. Ionic Liquid: A Promising Material for Petroleum Production and Processing. Curr. Org. Chem. 2020, in press. [Google Scholar] [CrossRef]
- Hamid, S.; De Jesus, O.; Jacinto, C.; Izetti, R.; Pinto, H.; Droguett, E.L.; Edwards, C.; Cassidy, J.; Zhang, H.; Dagenais, P.; et al. A practical method of predicting calcium carbonate scale formation in well completions. SPE Prod. Oper. 2016, 31, 1–11. [Google Scholar] [CrossRef]
- Ramstad, K.; Tydal, T.; Askvik, K.M.; Fotland, P. Predicting carbonate scale in oil producers from high temperature reservoirs. SPE J. 2005, 10, 363–373. [Google Scholar] [CrossRef]
- Wang, W.; Wei, W. Silica and silicate scale formation and control: Scale modeling, lab testing, scale characterization and field observation. In Proceedings of the SPE International Oilfield Scale Conference and Exhibition (SPE–179897–MS), Aberdeen, UK, 11–12 May 2016. [Google Scholar]
- Kumar, S.; Naiya, T.K.; Kumar, T. Developments in oilfield scale handling towards green technology-A review. J. Pet. Sci. Eng. 2018, 169, 428–444. [Google Scholar] [CrossRef]
- Abdel-Aal, N.; Sawada, K. Inhibition of adhesion and precipitation of CaCO3 by aminopolyphosphonate. J. Cryst. Growth 2003, 256, 188–200. [Google Scholar] [CrossRef]
- Issabayev, Y.A.; Boiko, G.I.; Lyubchenko, N.P.; Shaikhutdinov, Y.M.; Muhr, H.; Colombeau, L. Synthesis of unexplored amino phosphonic acid and evaluation as scale inhibitor for industrial water applications. J. Water Process. Eng. 2018, 22, 192–202. [Google Scholar] [CrossRef]
- Sorbie, K.; Mackay, E.J. Mixing of injected, connate and aquifer brines in waterflooding and its relevance to oilfield scaling. J. Pet. Sci. Eng. 2000, 27, 85–106. [Google Scholar] [CrossRef]
- Tomson, M.; Fu, G.; Watson, M.; Kan, A. Mechanisms of mineral scale inhibition. SPE Prod. Facil. 2003, 18, 192–199. [Google Scholar] [CrossRef]
- Zhang, P.; Shen, D.; Kan, A.T.; Tomson, M.B. Phosphino-polycarboxylic acid-modified inhibitor nanomaterial for oilfield scale control: Transport and inhibitor return information media. RSC Adv. 2016, 6, 59195–59205. [Google Scholar] [CrossRef]
- Popov, K.I.; Oshchepkov, M.S.; Shabanova, N.A.; Dikareva, Y.M.; Larchenko, V.E.; Koltinova, E.Y. DLS study of a phosphonate induced gypsum scale inhibition mechanism using indifferent nanodispersions as the standards for light scattering intensity comparison. Int. J. Corros. Scale Inhib. 2018, 7, 9–24. [Google Scholar]
- Oshchepkov, M.S.; Kamagurov, S.; Tkachenko, S.; Ryabova, A.; Popov, K. Insight into the mechanisms of scale inhibition: A case study of a task-specific fluorescent-tagged scale inhibitor location on Gypsum crystals. ChemNanoMat 2019, 5, 586–592. [Google Scholar] [CrossRef]
- Oshchepkov, M.; Golovesov, V.; Ryabova, A.; Redchuk, A.; Tkachenko, S.; Pervov, A.G.; Popov, K. Gypsum crystallization during reverse osmosis desalination of water with high sulfate content in presence of a novel fluorescent-tagged polyacrylate. Crystals 2020, 10, 309. [Google Scholar] [CrossRef] [Green Version]
- Mbogoro, M.M.; Peruffo, M.; Adobes-Vidal, M.; Field, E.L.; O’Connell, M.A.; Unwin, P.R. Quantitative 3D visualization of the growth of individual Gypsum microcrystals: Effect of Ca2+:SO42– ratio on kinetics and crystal morphology. J. Phys. Chem. C 2017, 121, 12726–12734. [Google Scholar] [CrossRef]
- Dobberschütz, S.; Nielsen, M.R.; Sand, K.K.; Civioc, R.; Bovet, N.; Stipp, S.L.S.; Andersson, M.P. The mechanisms of crystal growth inhibition by organic and inorganic inhibitors. Nat. Commun. 2018, 9, 1578. [Google Scholar] [CrossRef] [Green Version]
- Oshchepkov, M.; Golovesov, V.; Ryabova, A.; Tkachenko, S.; Redchuk, A.; Rönkkömäki, H.; Rudakova, G.; Pervov, A.; Popov, K. Visualization of a novel fluorescent-tagged bisphosphonate behavior during reverse osmosis desalination of water with high sulfate content. Sep. Purif. Technol. 2020, 117382. [Google Scholar] [CrossRef]
- Oshchepkov, M.; Popov, K.; Ryabova, A.; Redchuk, A.; Tkachenko, S.; Dikareva, J.; Koltinova, E. Barite crystallization in presence of novel fluorescent-tagged antiscalants. Int. J. Corros. Scale Inhib. 2019, 8, 998–1021. [Google Scholar]
- Sorbie, K.S.; Laing, N. How scale inhibitors work: Mechanisms of selected barium sulphate scale inhibitors across a wide temperature range. In Proceedings of the SPE International Symposium on Oilfield Scale (SPE-87470-MS), Aberdeen, UK, 26–27 May 2004. [Google Scholar]
- Abdel-Gaber, A.M.; Abd-El-Nabey, B.A.; Khamis, E.; Abd-El-Khalek, D.E. Investigation of fig leaf extract as a novel environmentally friendly antiscalent for CaCO3 calcareous deposits. Desalination 2008, 230, 314–328. [Google Scholar] [CrossRef]
- Abdel-Gaber, A.; Abd-El-Nabey, B.; Khamis, E.; Abd-El-Khalek, D. A natural extract as scale and corrosion inhibitor for steel surface in brine solution. Desalination 2011, 278, 337–342. [Google Scholar] [CrossRef]
- Abdel-Gaber, A.M.; Abd-El-Nabey, B.A.; Khamis, E.; Abd-El-Khalek, D.E.; Aglan, H.; Ludwick, A. Green antiscalant for cooling water systems. Int. J. Electrochem. Sci. 2012, 7, 11930–11940. [Google Scholar]
- Al-Sabagh, A.; El Basiony, N.; Sadeek, S.; Migahed, M. Scale and corrosion inhibition performance of the newly synthesized anionic surfactant in desalination plants: Experimental, and theoretical investigations. Desalination 2018, 437, 45–58. [Google Scholar] [CrossRef]
- Yang, X.; Xu, G. The influence of xanthan on the crystallization of calcium carbonate. J. Cryst. Growth 2011, 314, 231–238. [Google Scholar] [CrossRef]
- Wada, N.; Kanamura, K.; Umegaki, T. Effects of carboxylic acids on the crystallization of calcium carbonate. J. Colloid Interface Sci. 2001, 233, 65–72. [Google Scholar] [CrossRef]
- Reddy, M.M.; Hoch, A.R. Calcite crystal growth rate inhibition by polycarboxylic acids. J. Colloid Interface Sci. 2001, 235, 365–370. [Google Scholar] [CrossRef] [Green Version]
- Malkaj, P.; Kanakis, J.; Dalas, E.; Kanakis, I. The effect of leucine on the crystal growth of calcium carbonate. J. Cryst. Growth 2004, 266, 533–538. [Google Scholar] [CrossRef]
- Dalas, E.; Chalias, A.; Gatos, D.; Barlos, K. The inhibition of calcium carbonate crystal growth by the cysteine-rich Mdm2 peptide. J. Colloid Interface Sci. 2006, 300, 536–542. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, L.; Sun, W.; Wang, M.; Tian, J.; Yang, Z.; Wang, S.; Xia, L.; He, S.; Zhou, Y.; et al. The role of corrosion inhibition in the mitigation of CaCO3 scaling on steel surface. Corros. Sci. 2018, 140, 182–195. [Google Scholar] [CrossRef]
- Popov, K.; Kovaleva, N.E.; Rudakova, G.Y.; Kombarova, S.P.; Larchenko, V.E. Recent state-of-the-art of biodegradable scale inhibitors for cooling-water treatment applications (Review). Therm. Eng. 2016, 63, 122–129. [Google Scholar] [CrossRef]
- Davies, M.C.; Dawkins, J.V.; Hourston, D. Radical copolymerization of maleic anhydride and substituted styrenes by reversible addition-fragmentation chain transfer (RAFT) polymerization. Polymer 2005, 46, 1739–1753. [Google Scholar] [CrossRef]
- Sugama, T. Oxidized potato-starch films as primer coatings of aluminum. J. Mater. Sci. 1997, 3, 3995–4003. [Google Scholar] [CrossRef]
- Nasirtabrizi, M.H.; Ziaei, Z.M.; Jadid, A.P.; Fatin, L.Z. Synthesis and chemical modification of maleic anhydride copolymers with phthalimide groups. Int. J. Ind. Chem. 2013, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Von Bonin, W. Maleic Acid Anhydride Copolymers and Their Preparation. U.S. Patent 4,390,672, 1983. [Google Scholar]
- Fukumoto, Y.; Moriyama, M. Production of Polymaleic Acid. U.S. Patent 4,709,091, 24 November 1987. [Google Scholar]
- Jing, G.; Tang, S. The summary of the scale and the methods to inhibit and remove scale formation in the oil well and the gathering line. Recent Patents Chem. Eng. 2011, 4, 291–296. [Google Scholar] [CrossRef]
- Ajikumar, P.K.; Low, B.J.M.; Valiyaveettil, S. Role of soluble polymers on the preparation of functional thin films of calcium carbonate. Surf. Coat. Technol. 2005, 198, 227–230. [Google Scholar] [CrossRef]
- Migahed, M.; Rashwan, S.; Kamel, M.M.; Habib, R. Synthesis, characterization of polyaspartic acid-glycine adduct and evaluation of their performance as scale and corrosion inhibitor in desalination water plants. J. Mol. Liq. 2016, 224, 849–858. [Google Scholar] [CrossRef]
- Quan, Z.; Chen, Y.; Wang, X.; Shi, C.; Liu, Y.; Ma, C. Experimental study on scale inhibition performance of a green scale inhibitor polyaspartic acid. Sci. China Ser. B Chem. 2008, 51, 695–699. [Google Scholar] [CrossRef]
- Thombre, S.M.; Sarwade, B.D. Synthesis and biodegradability of polyaspartic acid: A critical review. J. Macromol. Sci. Part A 2005, 42, 1299–1315. [Google Scholar] [CrossRef]
- Bennett, G.D. A green polymerization of aspartic acid for the undergraduate organic laboratory. J. Chem. Educ. 2005, 82, 1380. [Google Scholar] [CrossRef]
- Gao, Y.; Fan, L.; Ward, L.P.; Liu, Z. Synthesis of polyaspartic acid derivative and evaluation of its corrosion and scale inhibition performance in seawater utilization. Desalination 2015, 365, 220–226. [Google Scholar] [CrossRef]
- Liu, D.; Dong, W.; Li, F.; Hui, F.; Lédion, J. Comparative performance of polyepoxysuccinic acid and polyaspartic acid on scaling inhibition by static and rapid controlled precipitation methods. Desalination 2012, 304, 1–10. [Google Scholar] [CrossRef]
- Euvrard, M.; Martinod, A.; Neville, A. Effects of carboxylic polyelectrolytes on the growth of calcium carbonate. J. Cryst. Growth 2011, 317, 70–78. [Google Scholar] [CrossRef]
- Gabriel, J.D.S.; Tiera, M.J.; Tiera, V.A.D.O. Synthesis, characterization and antifungal activities of amphiphilic derivatives of Diethylaminoethyl Chitosan against Aspergillus flavus. J. Agric. Food Chem. 2015, 63, 5725–5731. [Google Scholar] [CrossRef]
- Feng, Y.; Lin, X.; Li, H.; Akkihebbal, K.S.; Sridhar, T.; Suresh, A.K.; Bellare, J.; Wang, H. Synthesis and characterization of chitosan-grafted BPPO ultrafiltration composite membranes with enhanced antifouling and antibacterial properties. Ind. Eng. Chem. Res. 2014, 53, 14974–14981. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Yeh, J.-Y.; Tsai, C.-F. Antibacterial characteristics and activity of water-soluble Chitosan derivatives prepared by the Maillard Reaction. Molecules 2011, 16, 8504–8514. [Google Scholar] [CrossRef] [Green Version]
- Aziz, M.A.; Cabral, J.D.; Brooks, H.J.L.; Moratti, S.C.; Hanton, L.R. Antimicrobial properties of a Chitosan dextran-based hydrogel for surgical use. Antimicrob. Agents Chemother. 2011, 56, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Chen, S.; Liu, T.; Chang, X.; Yin, Y. Carboxymethyl chitosan-Cu mixture as an inhibitor used for mild steel in 1.0 M HCl. Electrochim. Acta 2007, 52, 5932–5938. [Google Scholar] [CrossRef]
- Sakai, T.; Sakamoto, T.; Hallaert, J.; Vandamme, E.J. Pectin, pectinase, and protopectinase: Production, properties and applications. Adv. Appl. Microbiol. 1993, 39, 213–294. [Google Scholar] [CrossRef]
- Schweiger, R.G. Acetyl pectates and their reactivity with polyvalent metal ions1. J. Org. Chem. 1964, 29, 2973–2975. [Google Scholar] [CrossRef]
- Hromádková, Z.; Malovikova, A.; Mozes, S.; Srokova, I.E.; Ibringerova, A. Hydrophobically modified pectates as novel functional polymers in food and non-food applications. BioResources 2008, 3, 71–78. [Google Scholar]
- Zaafarany, I. Inhibition of acidic corrosion of iron by some Carrageenan compounds. Curr. World Environ. 2006, 1, 101–108. [Google Scholar] [CrossRef]
- Abd-El-Khalek, D.E.; Abd-El-Nabey, B.A.; Abdel-Kawi, M.A.; Ebrahim, S.; Ramadan, S.R. The inhibition of crystal growth of gypsum and barite scales in industrial water systems using green antiscalant. Water Supply 2019, 19, 2140–2146. [Google Scholar] [CrossRef]
- Castillo, L.; Torin, E.; García, J.A.; Carrasquero, M.; Navas, M.; Viloria, A. New product for inhibition of calcium carbonate scale in natural gas and oil facilities based on aloe vera: Application in Venezuelan oilfields. In Proceedings of the Latin American and Caribbean Petroleum Engineering Conference (SPE 123007), Cartagena, Columbia, 31 May–3 June 2009. [Google Scholar] [CrossRef]
- Belarbi, Z.; Gamby, J.; Makhloufi, L.; Sotta, B.; Tribollet, B. Inhibition of calcium carbonate precipitation by aqueous extract of Paronychia argentea. J. Cryst. Growth 2014, 386, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Miksic, B.A.; Kharshan, M.A.; Furman, A.Y. Vapor corrosion and scale inhibitors formulated from biodegradable and renewable raw materials. In Proceedings of the European Symposium on Corrosion Inhibitors, Ferrara, Italy, 29 August–2 September 2005. [Google Scholar]
- Chaussemier, M.; Pourmohtasham, E.; Gelus, D.; Pécoul, N.; Perrot, H.; Lédion, J.; Cheap-Charpentier, H.; Horner, O. State of art of natural inhibitors of calcium carbonate scaling. A review article. Desalination 2015, 356, 47–55. [Google Scholar] [CrossRef]
- Bonoli, M.; Bendini, A.; Cerretani, L.; Lercker, G.; Toschi, T.G. Qualitative and semiquantitative analysis of phenolic compounds in extra virgin olive oils as a function of the ripening degree of olive fruits by different analytical techniques. J. Agric. Food Chem. 2004, 52, 7026–7032. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, G.; Zierkiewicz, W.; Adach, A.; Kopacz, M.; Zapala, I.; Bulik, I.; Cies’lak-Golonka, M.; Grabowski, T.; Wietrzyk, J. A typical calcium coordination number: Physico-chemical study, cytotoxicity, DFT calculations and silicopharmacokinetic characteristics of calcium caffeates. J. Inorg. Biochem. 2009, 103, 1189–1195. [Google Scholar] [CrossRef]
- Lee, O.-H.; Lee, B.-Y.; Lee, J.; Lee, H.-B.; Son, J.-Y.; Park, C.-S.; Shetty, K.; Kim, Y.-C. Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Bioresour. Technol. 2009, 100, 6107–6113. [Google Scholar] [CrossRef]
- Mehdipour, M.; Ramezanzadeh, B.; Arman, S.Y. Electrochemical noise investigation of aloe plant extract as green inhibitor on the corrosion of stainless steel in 1 M H2SO4. J. Ind. Eng. Chem. 2015, 21, 318–327. [Google Scholar] [CrossRef]
- Hassan, K.H.; Khadom, A.A.; Kurshed, N.H. Citrus aurantium leaves extracts as a sustainable corrosion inhibitor of mild steel in sulfuric acid. S. Afr. J. Chem. Eng. 2016, 22, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhang, X.; Lei, J.; He, J.; Zhang, S.; Pan, F. Adsorption and corrosion inhibition of Osmanthus fragrance leaves extract on carbon steel. Corros. Sci. 2012, 63, 82–90. [Google Scholar] [CrossRef]
- Viloria, A.; Castillo, L.; Garcia, J.A.; Carrasquero Ordaz, M.A.; Torin, E.V. Process Using Aloe for Inhibiting Scale. U.S. Patent 8,039,421 B2, 18 October 2011. [Google Scholar]
- Lash, J.E.; Burns, G. Heats of crystallization of CaSO4·2H2O. Bull. Soc. Chim. Belg. 1984, 93, 271–279. [Google Scholar]
- Christoffersen, M.; Christoffersen, J.; Weijnen, M.; Van Rosmalen, G. Crystal growth of calcium sulphate dihydrate at low supersaturation. J. Cryst. Growth 1982, 58, 585–595. [Google Scholar] [CrossRef]
- Chesters, S.P. Innovations in the inhibition and cleaning of reverse osmosis membrane scaling and fouling. Desalination 2009, 238, 22–29. [Google Scholar] [CrossRef]
- Greenberg, G.; Hasson, D.; Semiat, R. Limits of RO recovery imposed by calcium phosphate precipitation. Desalination 2005, 183, 273–288. [Google Scholar] [CrossRef]
- Zach-Maor, A.; Semiat, R.; Rahardianto, A.; Cohen, Y.; Wilson, S.; Gray, S. Diagnostic analysis of RO desalting treated wastewater. Desalination 2008, 230, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Kubo, S.; Takahashi, T.; Morinaga, H.; Ueki, H. Inhibition of calcium phosphate scale on heat exchanger: The relation between laboratory test results and tests on heat transfer surfaces. Corrosion 1979, 79, 15. [Google Scholar]
- Qin, J.J.; Wai, M.N.; Oo, M.H.; Kekre, K.A.; Seah, H. Impact of anti-scalant on fouling of reverse osmosis membranes in reclamation of secondary effluent. Water Sci. Technol. 2009, 60, 2767–2774. [Google Scholar] [CrossRef]
- Znini, M.; Majidi, L.; Bouyanzer, A.; Paolini, J.; Desjobert, J.-M.; Costa, J.; Hammouti, B. Essential oil of salvia aucheri mesatlantica as a green inhibitor for the corrosion of steel in 0.5M H2SO4. Arab. J. Chem. 2012, 5, 467–474. [Google Scholar] [CrossRef]
- Al-Shammiri, M.; Safar, M.; Al-Dawas, M. Evaluation of two different antiscalants in real operation at the Doha research plant. Desalination 2000, 128, 1–16. [Google Scholar] [CrossRef]
- Turek, M.; Mitko, K.; Piotrowski, K.; Dydo, P.; Laskowska, E.; Jakóbik-Kolon, A. Agata prospects for high water recovery membrane desalination. Desalination 2017, 401, 180–189. [Google Scholar] [CrossRef]
- Shemer, H.; Hasson, D. Characterization of the inhibitory effectiveness of environmentally friendly anti-scalants. Desalin. Water Treat. 2014, 55, 3478–3484. [Google Scholar] [CrossRef]
- Hasson, D.; Shemer, H.; Sher, A. State of the art of friendly “green” scale control inhibitors: A review article. Ind. Eng. Chem. Res. 2011, 50, 7601–7607. [Google Scholar] [CrossRef]
- Lattemann, S.; Höpner, T. Environmental impact and impact assessment of seawater desalination. Desalination 2008, 220, 1–15. [Google Scholar] [CrossRef]
- Kroiss, H.; Rechberger, H.; Egle, L. Phosphorus in water quality and waste management. Integr. Waste Manag. 2011, II. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhou, Y.; Yao, Q.; Huang, J.; Liu, G.; Wang, H.; Cao, K.; Chen, Y.; Bu, Y.; Wu, W.; et al. Double-hydrophilic polyether antiscalant used as a crystal growth modifier of calcium scales in cooling-water systems. J. Appl. Polym. Sci. 2013, 131, 39792. [Google Scholar] [CrossRef]
- Fu, C.; Zhou, Y.; Liu, G.; Huang, J.; Sun, W.; Wu, W. Inhibition of Ca3(PO4)2, CaCO3, and CaSO4 precipitation for industrial recycling water. Ind. Eng. Chem. Res. 2011, 50, 10393–10399. [Google Scholar]
- Ling, L.; Zhou, Y.; Huang, J.; Yao, Q.; Liu, G.; Zhang, P.; Sun, W.; Wu, W. Carboxylate-terminated double-hydrophilic block copolymer as an effective and environmental inhibitor in cooling water systems. Desalination 2012, 304, 33–40. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, Z.; Jin, X.; Sun, D.; Wang, D.; Yang, T.; Zhang, J.; Han, X. Preparation of modified oligochitosan and evaluation of its scale inhibition and fluorescence properties. J. Appl. Polym. Sci. 2015, 132, 42518. [Google Scholar] [CrossRef]
- Zeng, D.; Chen, T.; Zhou, S. Synthesis of polyaspartic acid/chitosan graft copolymer and evaluation of its scale inhibition and corrosion inhibition performance. Int. J. Electrochem. Sci. 2015, 10, 9513–9527. [Google Scholar]
- Cao, K.; Huang, J.; Zhou, Y.; Liu, G.; Wang, H.; Yao, Q.; Liu, Y.; Sun, W.; Wu, W. A multicarboxyl antiscalant for calcium phosphate and calcium carbonate deposits in cooling water systems. Desalin. Water Treat. 2013, 52, 7258–7264. [Google Scholar] [CrossRef]
- Abd-El-Khalek, D.; Abd-El-Nabey, B.; Abdel-Kawi, M.A.; Ramadan, S. Investigation of a novel environmentally friendly inhibitor for calcium carbonate scaling in cooling water. Desalin. Water Treat. 2014, 57, 1–7. [Google Scholar] [CrossRef]
- Maher, Y.A.; Ali, M.E.; Salama, H.E.; Sabaa, M.W. Preparation, characterization and evaluation of chitosan biguanidine hydrochloride as a novel antiscalant during membrane desalination process. Arab. J. Chem. 2020, 13, 2964–2981. [Google Scholar] [CrossRef]
- Harris, A.; Marshall, A. The evaluation of scale control additives. In Proceedings of the Conference on Progress in the Prevention of Fouling in Industrial Plant, University of Nottingham, Nottingham, UK, 1–3 April 1981. [Google Scholar]
- Manoli, F.; Dalas, E. The effect of sodium alginate on the crystal growth of calcium carbonate. J. Mater. Sci. Mater. Electron. 2002, 13, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jia, L.; Liu, K.; Gao, P.; Ge, H.; Fu, L. Inhibition of calcium sulfate scale by poly (citric acid). Desalination 2016, 392, 1–7. [Google Scholar] [CrossRef]
- Xue, X.; Fu, C.; Li, N.; Zheng, F.; Yang, W.; Yang, X. Performance of a non-phosphorus antiscalant on inhibition of calcium-sulfate precipitation. Water Sci. Technol. 2012, 66, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liang, Y.; Liu, Y.; Zhang, X.; Hu, X.; Tu, S.; Wu, A.; Zhang, C.; Zhong, J.; Zhao, S.; et al. Isolation of broad-specificity domain antibody from phage library for development of pyrethroid immunoassay. Anal. Biochem. 2016, 502, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Ma, W.; Wang, L.; Liu, R.; Wei, L.-S.; Wang, Q. Inhibition of calcium and magnesium-containing scale by a new antiscalant polymer in laboratory tests and a field trial. Desalination 2006, 196, 237–247. [Google Scholar] [CrossRef]
- El-Etre, A. Khillah extract as inhibitor for acid corrosion of SX 316 steel. Appl. Surf. Sci. 2006, 252, 8521–8525. [Google Scholar] [CrossRef]
- Raja, P.B.; Fadaeinasab, M.; Qureshi, A.K.; Rahim, A.A.; Osman, H.; Litaudon, M.; Awang, K. Evaluation of green corrosion inhibition by alkaloid extracts of ochrosia oppositifolia and isoreserpiline against Mild Steel in 1 M HCl Medium. Ind. Eng. Chem. Res. 2013, 52, 10582–10593. [Google Scholar] [CrossRef]
- Mpelwa, M.; Tang, S.-F. State of the art of synthetic threshold scale inhibitors for mineral scaling in the petroleum industry: A review. Pet. Sci. 2019, 16, 830–849. [Google Scholar] [CrossRef] [Green Version]
| Scale Type | Scale |
|---|---|
| Sulfides | |
| Pyrrhotite | Fe7S8 |
| Troilite | FeS |
| Mackinawite | Fe9S8 |
| Pyrite | FeS2 |
| Marcasite | FeS2 |
| Greigite | Fe3S4 |
| Sphalerite | ZnS |
| Galena | PbS |
| Sulfates | |
| Gypsum | CaSO4·2H2O |
| Anhydrate | CaSO4 |
| Barite | BaSO4 |
| Hemihydrate | CaSO4·5H2O |
| Celestite | SrSO4 |
| Carbonates | |
| Calcite | CaCO3 |
| Vaterite | CaCO3 |
| Aragonite | CaCO3 |
| Siderite | FeCO3 |
| Dolomite | CaMg(CO3)2 |
| Iron scales | |
| Ferrous Hydroxide | Fe(OH)2 |
| Ferrous Hydroxide | Fe(OH)3 |
| Hematite | Fe2O3 |
| Magnetite | Fe3O4 |
| Akaganeite | α-FeOOH |
| Goethite | β-FeOOH |
| Lepidocrocite | γ-FeOOH |
| Hibbingite | Fe2(OH)3Cl |
| Antiscalants | Schematic Structure | Dosages (mg L−1) | Performance (%IE) | Scalant | Ref. |
|---|---|---|---|---|---|
| Olive leaf extract (Biopheols) | 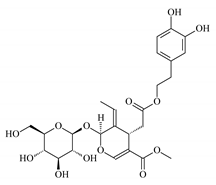 | 50 | 83 | CaCO3 | [15] |
| Copolymer modified with the palygorskite | 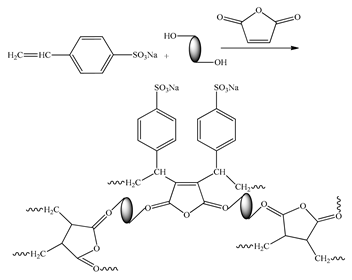 | 50 | 99 | CaCO3 | [15] |
| Heteropolysa-ccharide sulfonate (PS–NAEP) | 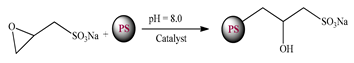 | 100 | 95 55 | CaSO4 Ca3(PO4)2 | [15] |
| Modification of collagen (P-MACs) | 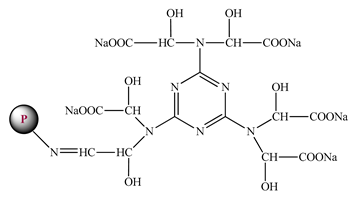 | 7 | 100 | CaSO4 | [94] |
| Antiscalants | Structure of Active Constituent | Dosages (mg L−1) | Performance (% IE) | Scalant | Ref. |
|---|---|---|---|---|---|
| Ficus carica L. (Fig) leaf extract | 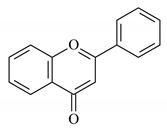 Flavonoid | 150 | 86 | CaCO3 | [91] |
| Olea europaea L. (Olive) leaf extract |  Caffeic acid | 50 | 83 | CaCO3 | [92] |
| Punica granutum leaf extract | 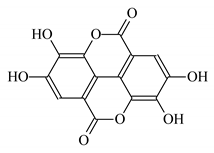 Ellagic acid | 100 | 60 | CaCO3 | [93] |
| Punica granutum hull extract | 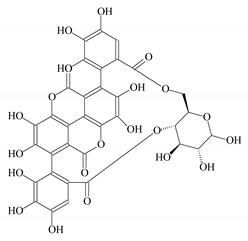 Punicalin | 100 | 88 | CaCO3 | [93] |
| Helianthus annus seed extract | 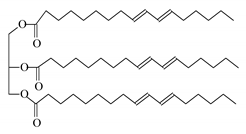 Sunflower oil | 50 | 100 | CaSO4 | [125] |
| 50 | 84 | BaSO4 | [125] | ||
| Aloe vera extract gel | 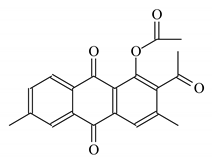 Anthraquinone 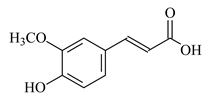 Ferulic acid | 15 | 80 | CaCO3 | [126] |
| Paronychia argentea lam extract | 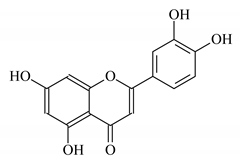 Luteolin | 70 | 100 | CaCO3 | [127] |
| Soybean-based polymer | 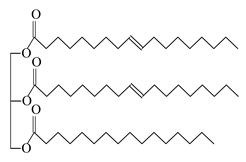 Soybean oil methyl ester | 4250 3100 | 93 17 | CaSO4 CaCO3 | [128] |
| Polysaccharide from seaweed |  K-carrageenan | 4200 3100 | 90 17 | CaSO4 CaCO3 | [128] |
| Scale Inhibitors | Dosages (mg L−1) | Performance (% IE) | Scalant | Ref. |
|---|---|---|---|---|
| PBTC | 12 | 91 | CaCO3 | [151] |
| HEDP | 12 | 64 | - | [151] |
| PEG8DMA/AA | 12 | 89 | - | [151] |
| PESA | 12 | 90 | - | [114] |
| PASP | 12 | 78 | - | [114] |
| HPMA | 20 | 37 | - | [152] |
| PAA | 20 | 29 | - | [152] |
| AA-APEC | 20 | 69 | - | [152] |
| MA-APES | 20 | 26 | - | [152] |
| AA-APEL-PA | 8 | 99 | - | [153] |
| CM-QAOC | 10 | 70 | - | [154] |
| Cs-PASP | 8 | 92 | - | [155] |
| AA-APEC | 8 | 96 | - | [156] |
| Palm leaves extract | 75 | 90 | - | [157] |
| CG | 15 | 91 | - | [158] |
| Poly(carboxylic acid) | 1 | 80 | - | [97] |
| Poly(maleic acid) | 10 | 56 | - | [159] |
| Sodium alginate | 0.02 | 94 | - | [160] |
| PAA/APEG-PG-COOH | 14 | 82 | - | [153] |
| PBTC | 4 | 60 | CaSO4 | [151] |
| HEDP | 4 | 90 | - | [151] |
| PEG8DMA/AA | 4 | 99 | - | [151] |
| HPMA | 2 | 79 | - | [152] |
| PAA | 2 | 38 | - | [152] |
| AA-APEC | 2 | 39 | - | [152] |
| AA-APES | 2 | 2 | - | [152] |
| PESA | 10 | 90 | - | [161] |
| PASP | 10 | 98 | - | [161] |
| PAPEMP | 3 | 79 | - | [162] |
| Poly(citric acid) | 25 | 99 | - | [163] |
| AA-APEC | 2 | 84 | - | [162] |
| CG | 10 | 95 | - | [158] |
| PAP1 | 9 | 97 | - | [164] |
| PAA/APEG-PG-COOH | 3 | 100 | - | [153] |
| PBTC | 12 | 43 | Ca3(PO4)2 | [152] |
| HEDP | 12 | 32 | - | [152] |
| PESA | 12 | 34 | - | [152] |
| HPMA | 12 | 43 | - | [152] |
| PAA | 12 | 90 | - | [152] |
| AA-APEC | 12 | 98 | - | [152] |
| MA-APES | 12 | 88 | - | [152] |
| PAA/APEG-PG-COOH | 6 | 100 | - | [153] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafar Mazumder, M.A. A Review of Green Scale Inhibitors: Process, Types, Mechanism and Properties. Coatings 2020, 10, 928. https://doi.org/10.3390/coatings10100928
Jafar Mazumder MA. A Review of Green Scale Inhibitors: Process, Types, Mechanism and Properties. Coatings. 2020; 10(10):928. https://doi.org/10.3390/coatings10100928
Chicago/Turabian StyleJafar Mazumder, Mohammad A. 2020. "A Review of Green Scale Inhibitors: Process, Types, Mechanism and Properties" Coatings 10, no. 10: 928. https://doi.org/10.3390/coatings10100928
APA StyleJafar Mazumder, M. A. (2020). A Review of Green Scale Inhibitors: Process, Types, Mechanism and Properties. Coatings, 10(10), 928. https://doi.org/10.3390/coatings10100928





