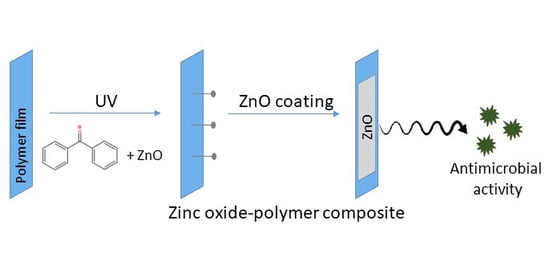
Biodegradation and Antimicrobial Properties of Zinc Oxide–Polymer Composite Materials for Urinary Stent Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PLA and PP Films
2.2. Synthesis of ZnO Microparticles
2.3. Deposition of ZnO Coating on Polymeric Substrates
- For method 1, the coated PLA was thermally treated at 50 °C for 30 min and 60 °C for 15 min while coated PP films were treated at 70 °C for 15 min.
- In method 2, ZnO paste was UV grafted onto the PLA substrate using the protocol developed by Shin et al. [37] with adaptations. For this, 20 mL of 10 wt.% acrylic acid aqueous solution was added to 20 mL of benzophenone 0.2 M and stirred under dark conditions. Then, ZnO paste containing 1 g of ZnO was added to the previous solution and stirred for 30 min in the absence of light. The final solution was poured in a glass petri dish containing the PLA films and exposed to UV light for five minutes at 40 W. After curing, samples were neutralized by immersion in a sodium bicarbonate (Sigma Aldrich) 0.1 M solution for 10 min, rinsed with distilled water and dried overnight at 40 °C.
- In method 3, 1 g of ZnO powder was dispersed in 2 mL of ethanol and sonicated for 15 min. Different amounts of benzophenone 0.2 M ethanolic solution (0, 0.25 and 0.50 mL) were then added to this solution and stirred until the solution became homogeneous. Then, PP and PLA were coated with ZnO + BP solution and exposed to UV light (0, 5 and 10 min), dried for 1 h at 37 °C and rinsed with water for removing excess reagents. All samples were dried overnight at 37 °C prior to analysis. For this method, PP was previously treated with a 0.1 M sodium hydroxide solution for 18 h for surface activation while PLA did not have any previous treatment.
2.4. Characterization Setup
2.4.1. Physicochemical Characterization
2.4.2. Biodegradation Essays
2.4.3. Antibacterial Tests
2.4.4. Statistical Analysis
3. Results and Discussion
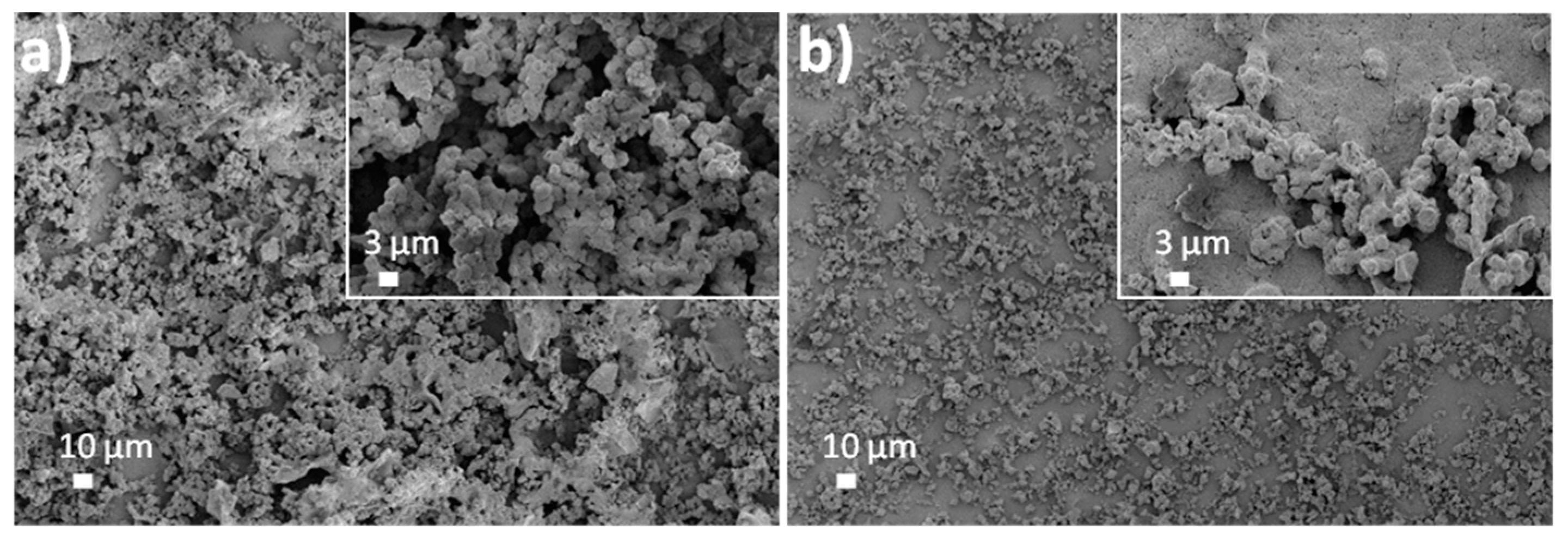
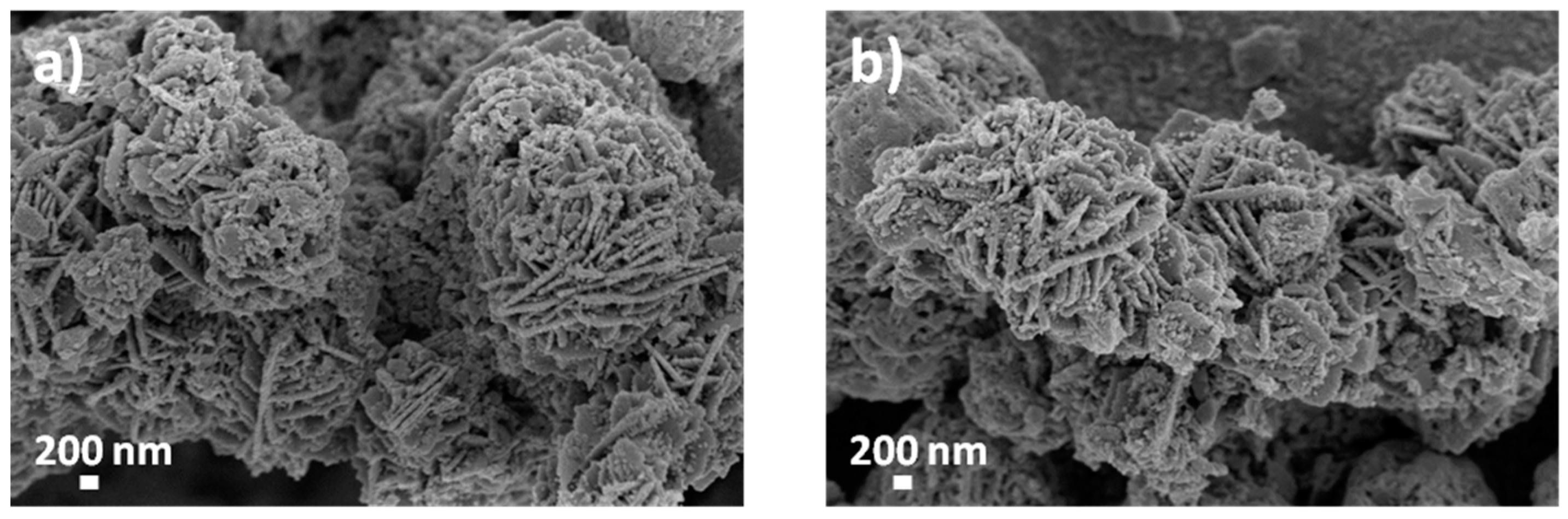
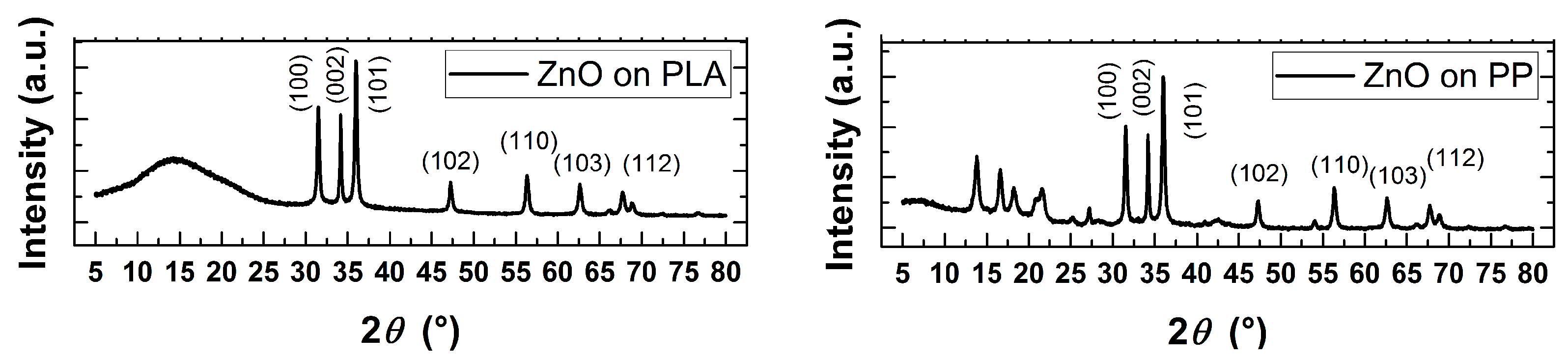
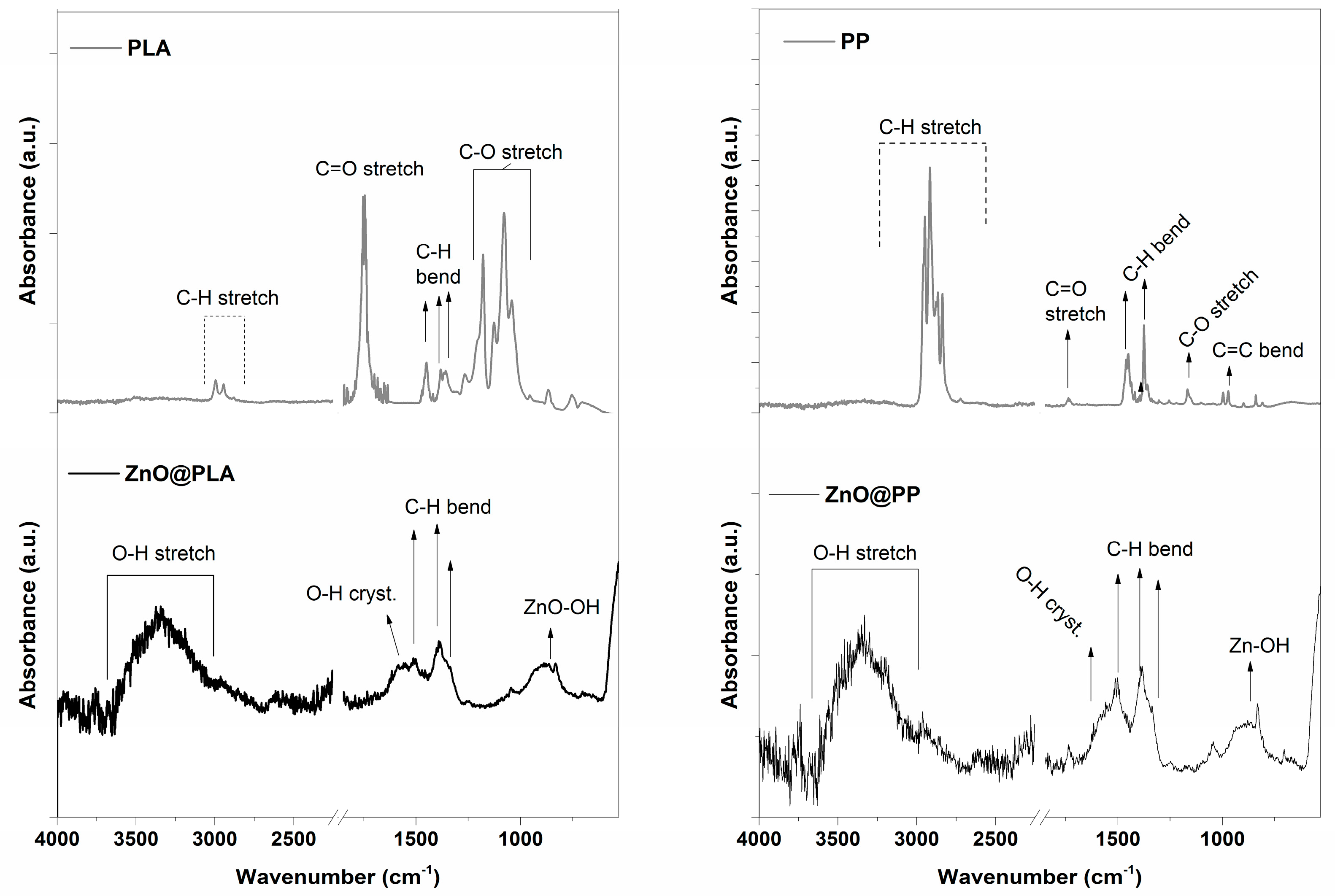
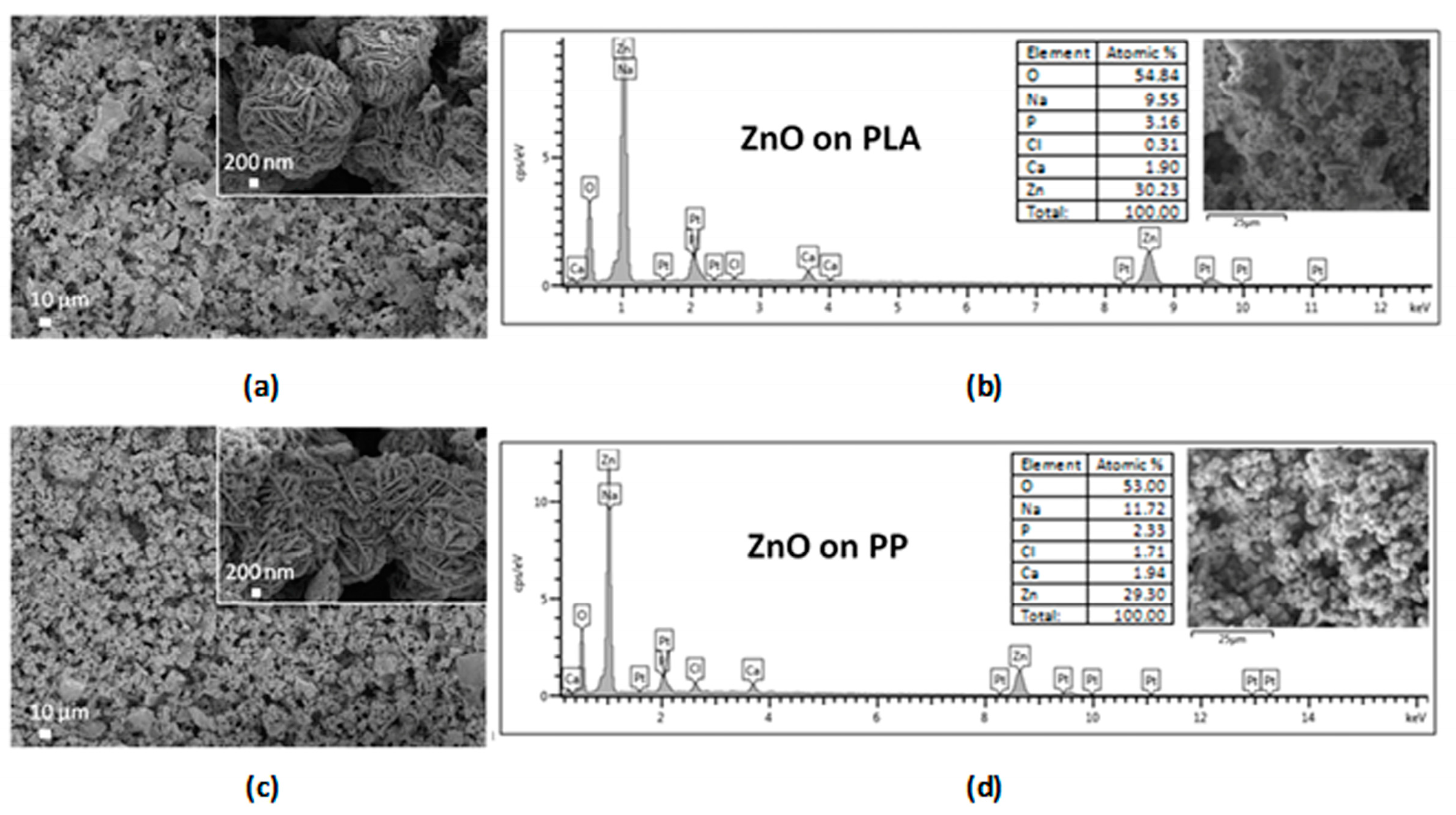
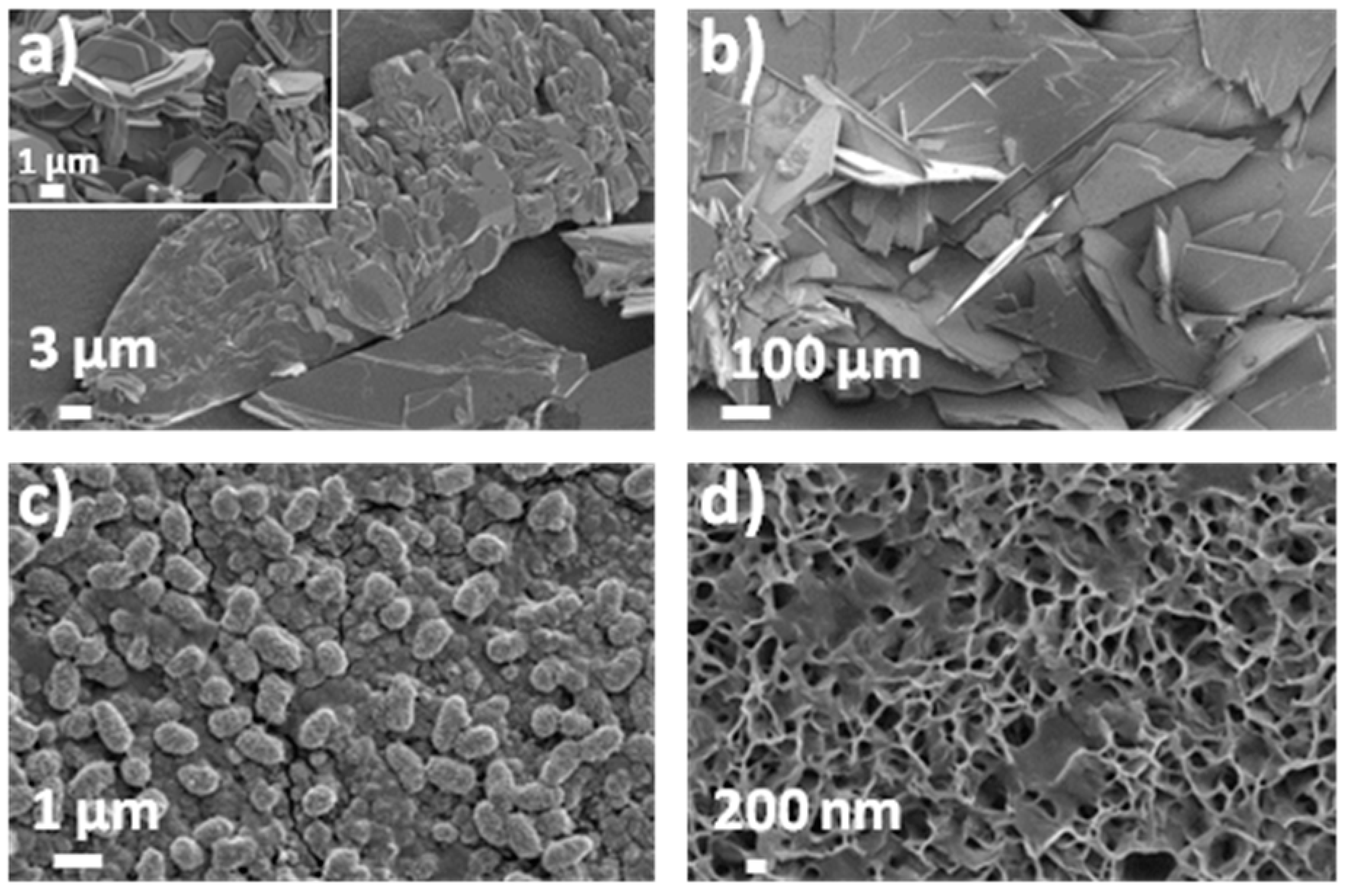
3.1. Morphological, Structural and Chemical Characterization
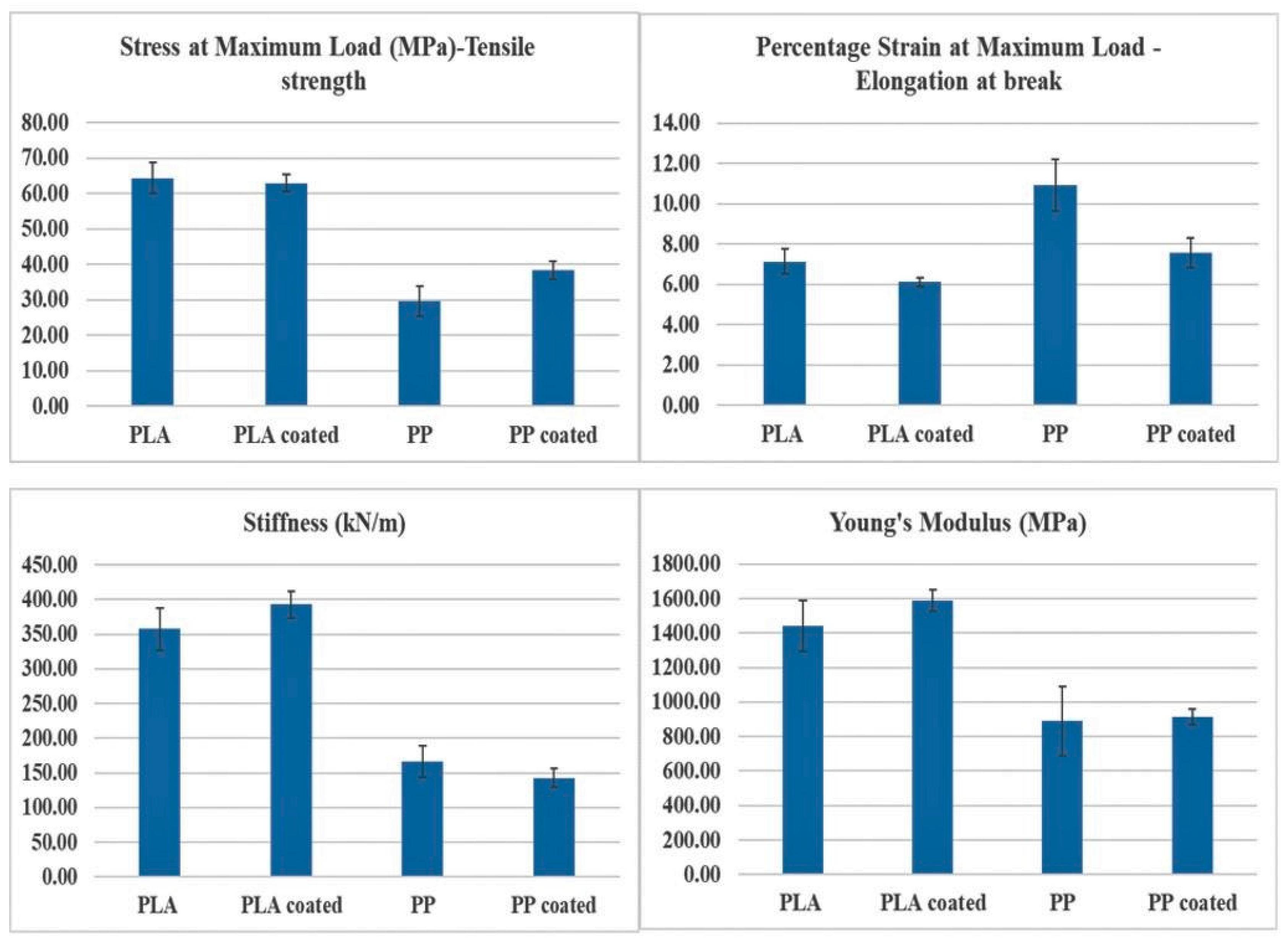
3.2. Tensile Testing
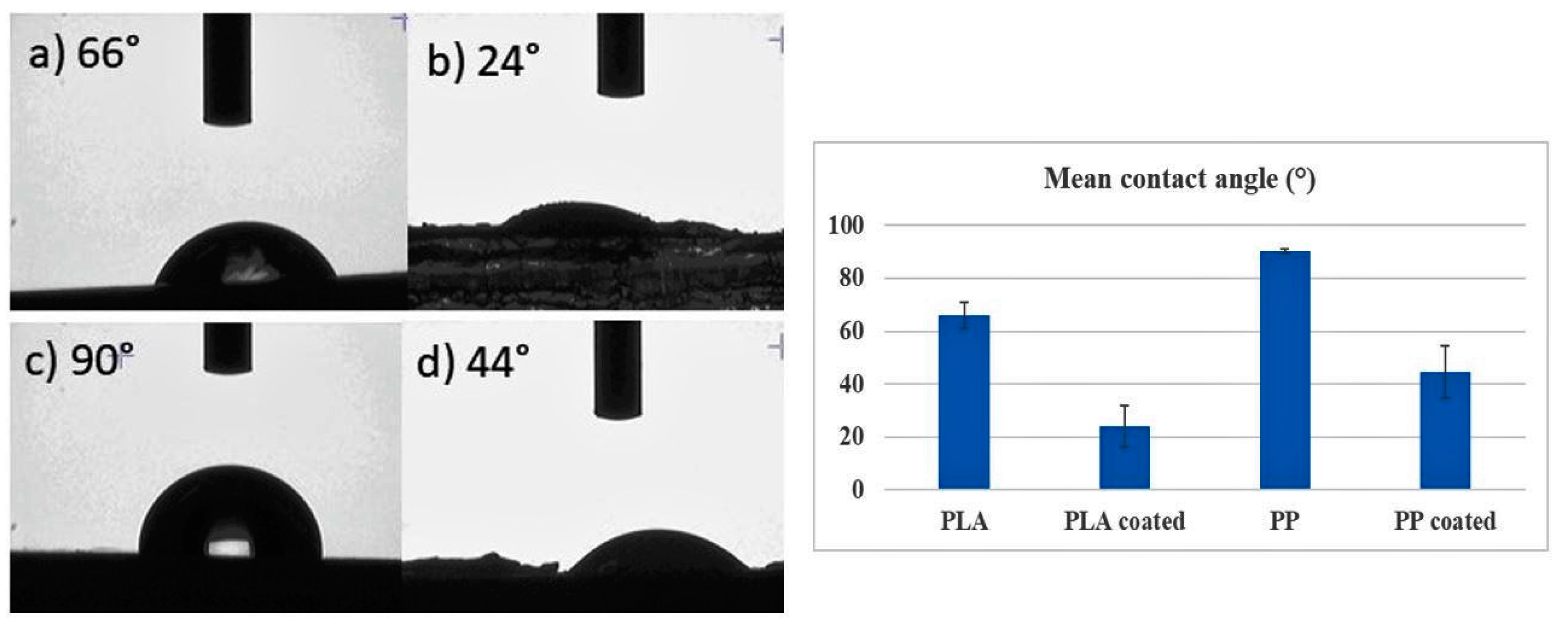
3.3. Surface Wettability
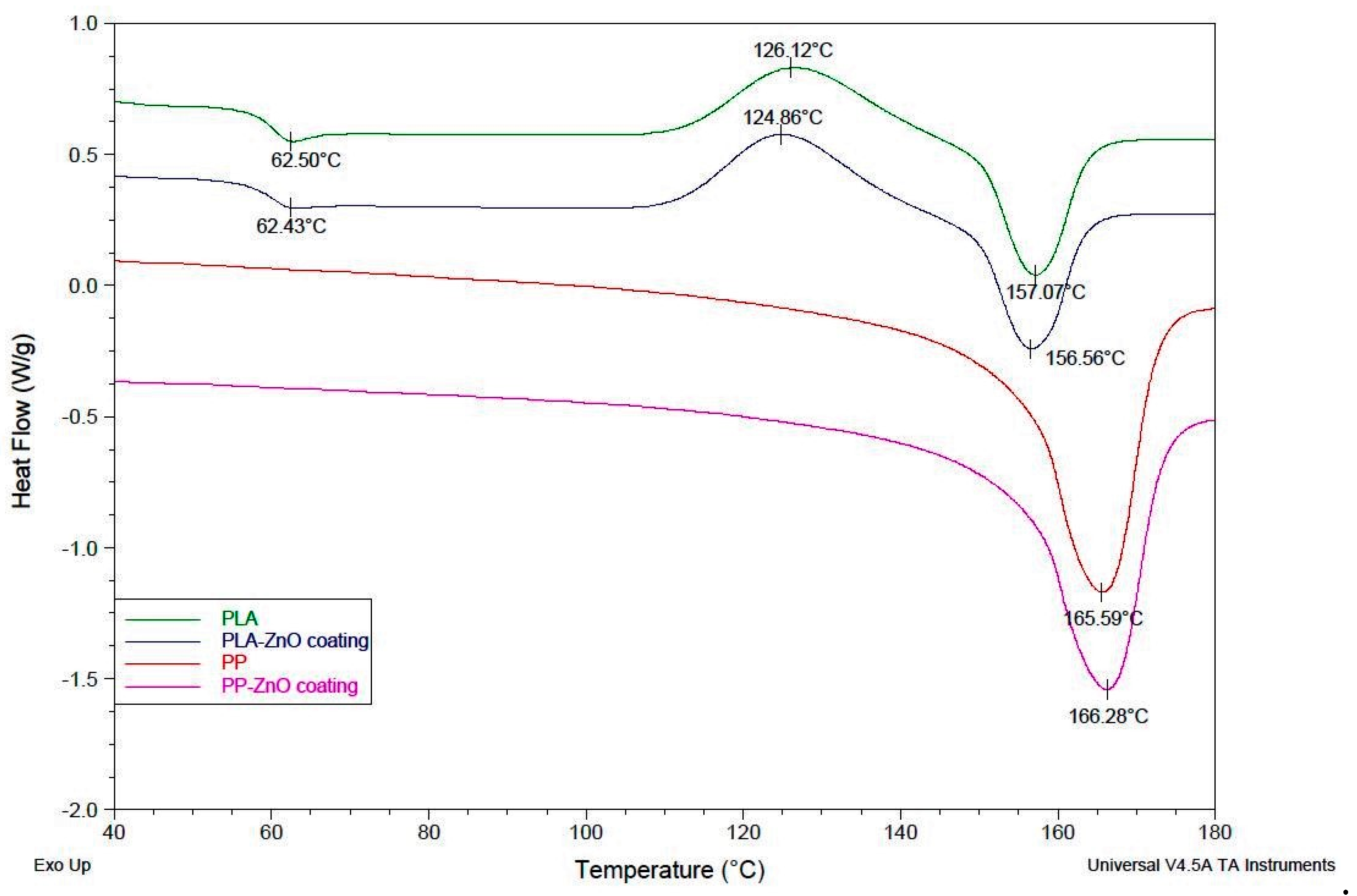
3.4. Differential Scanning Calorimetry (DSC)
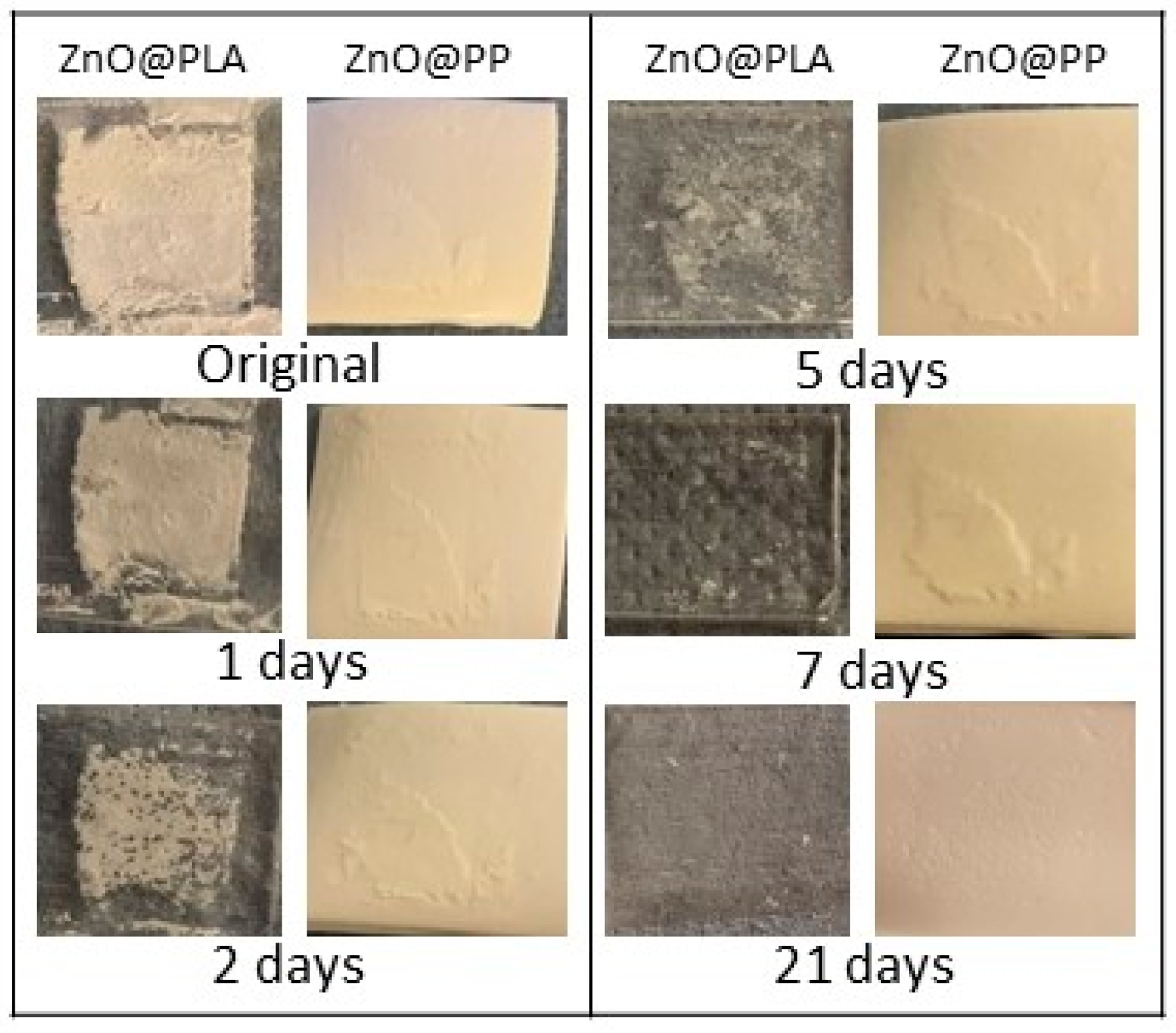
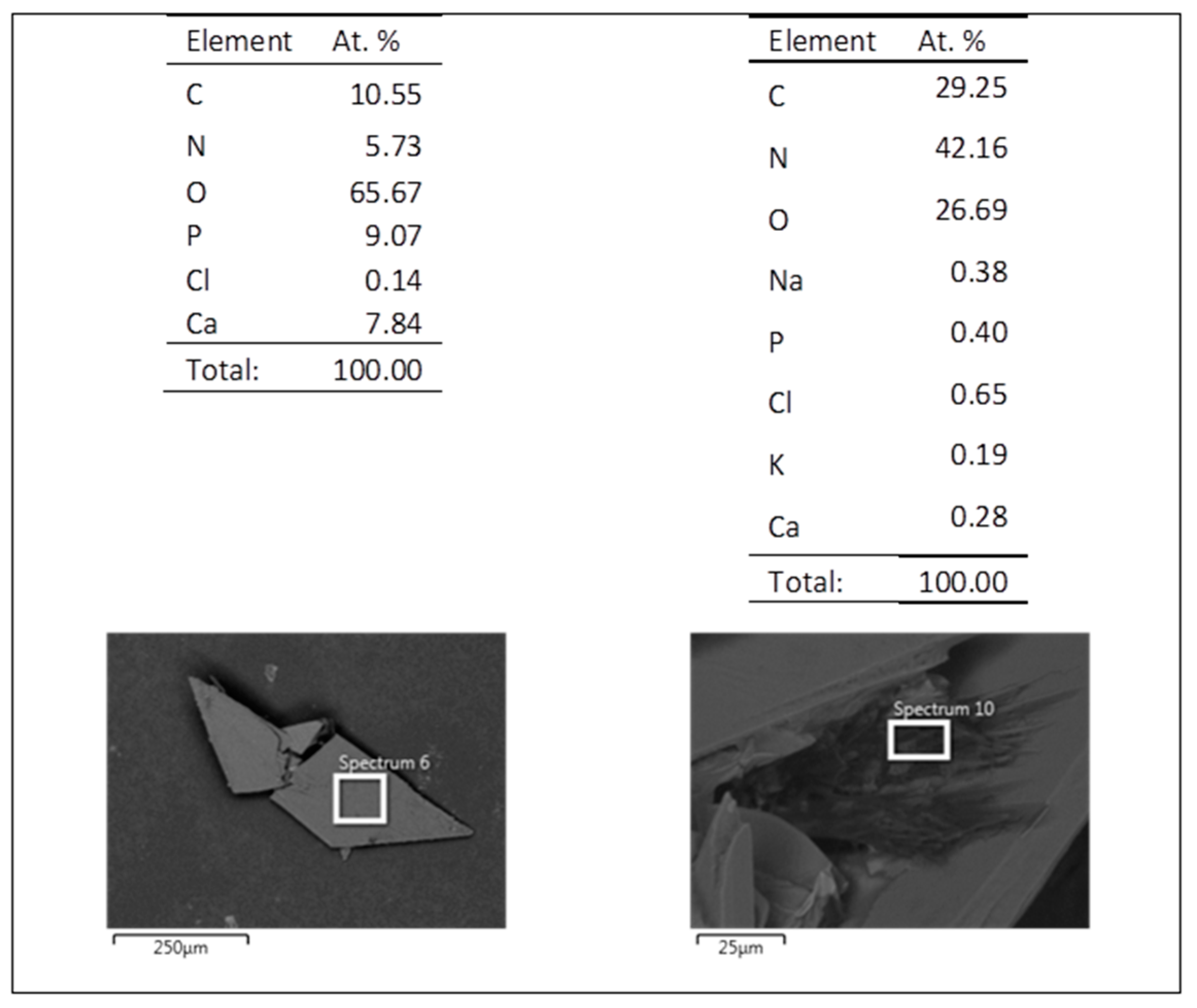
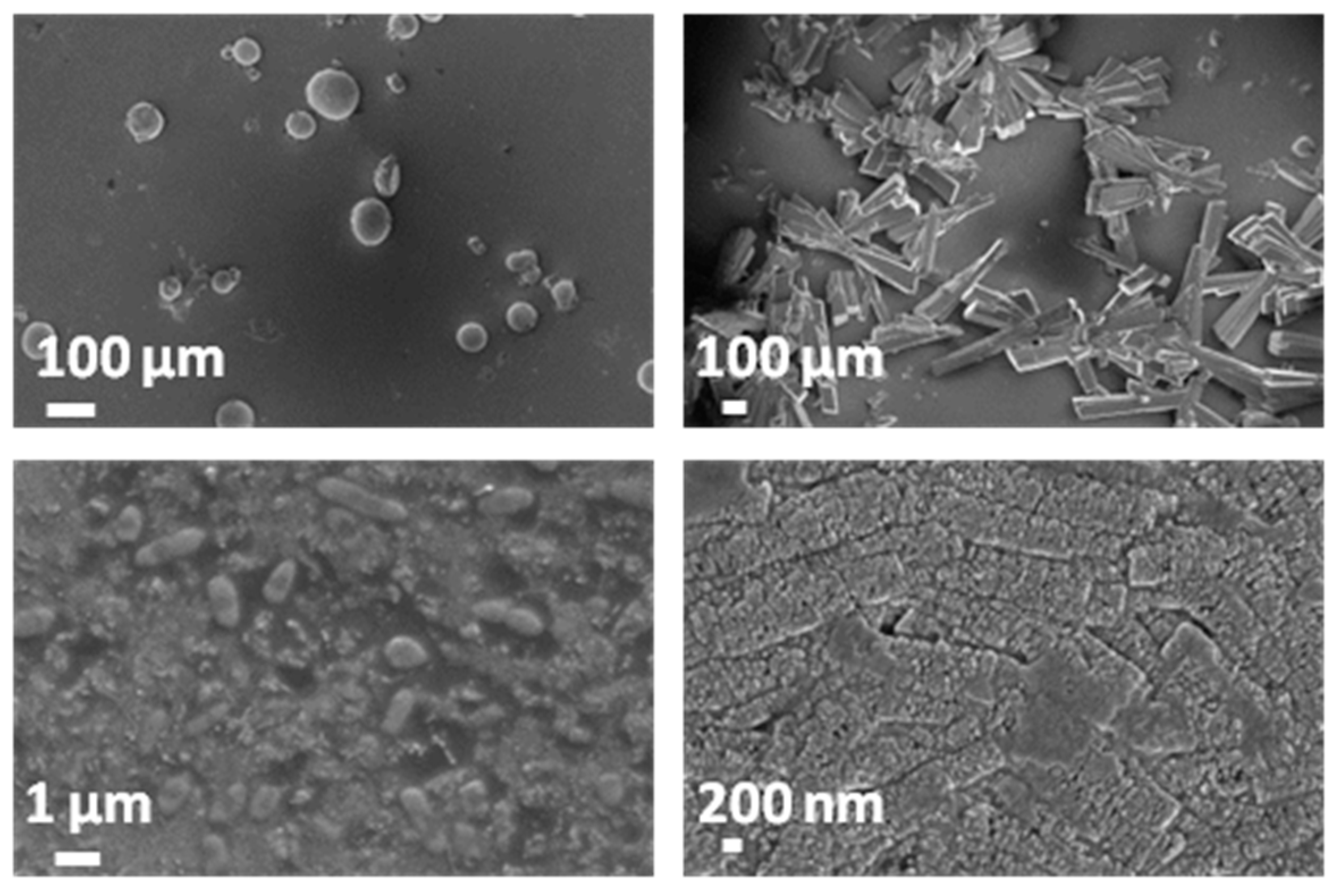
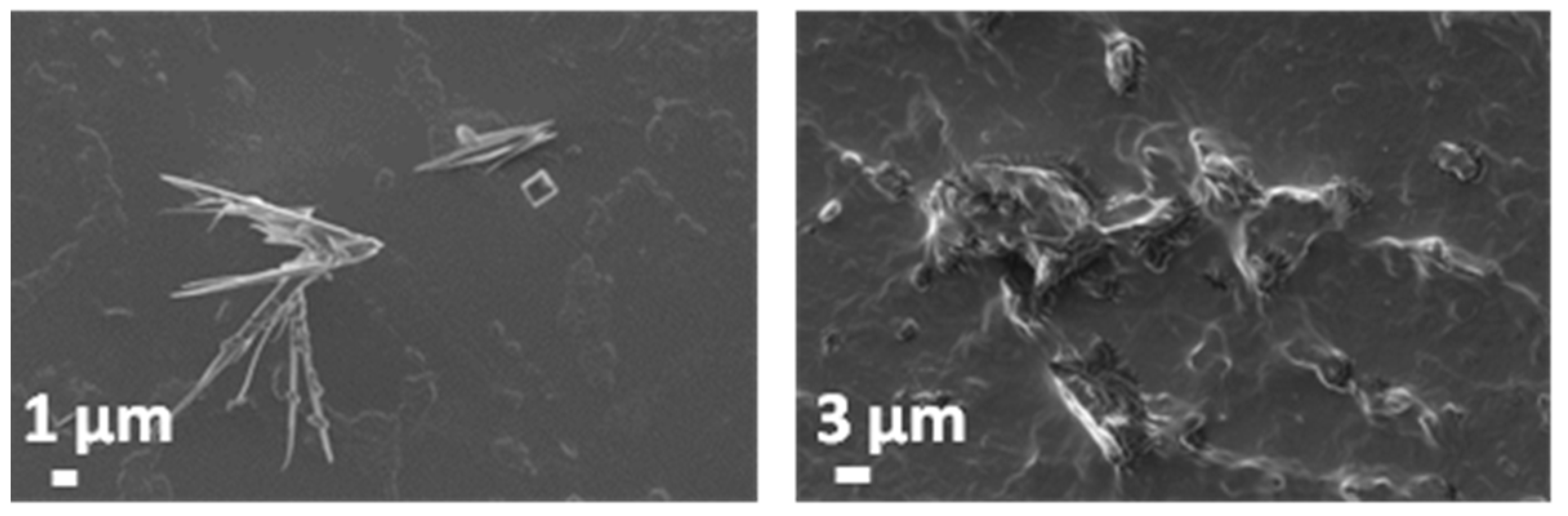
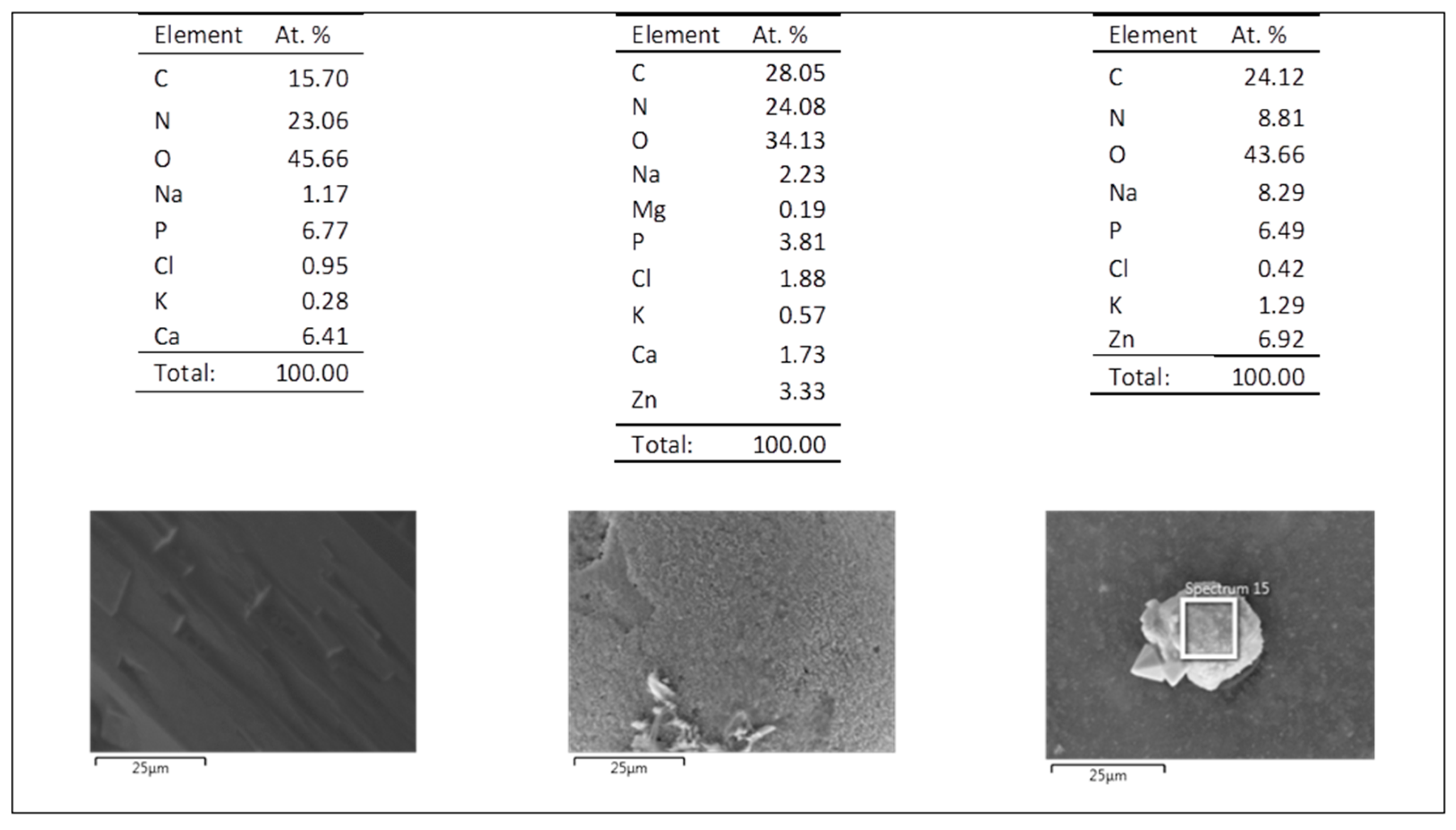
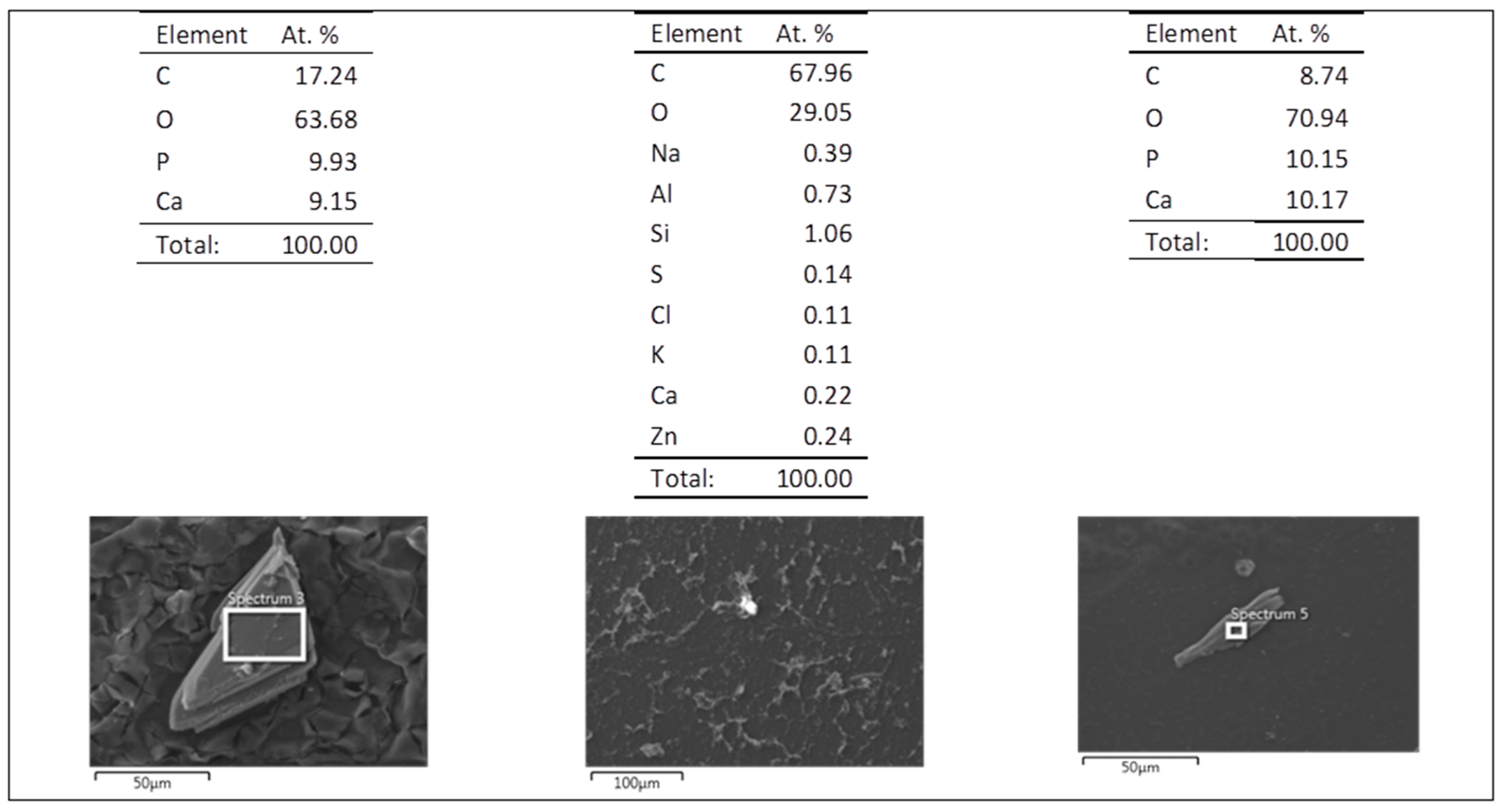
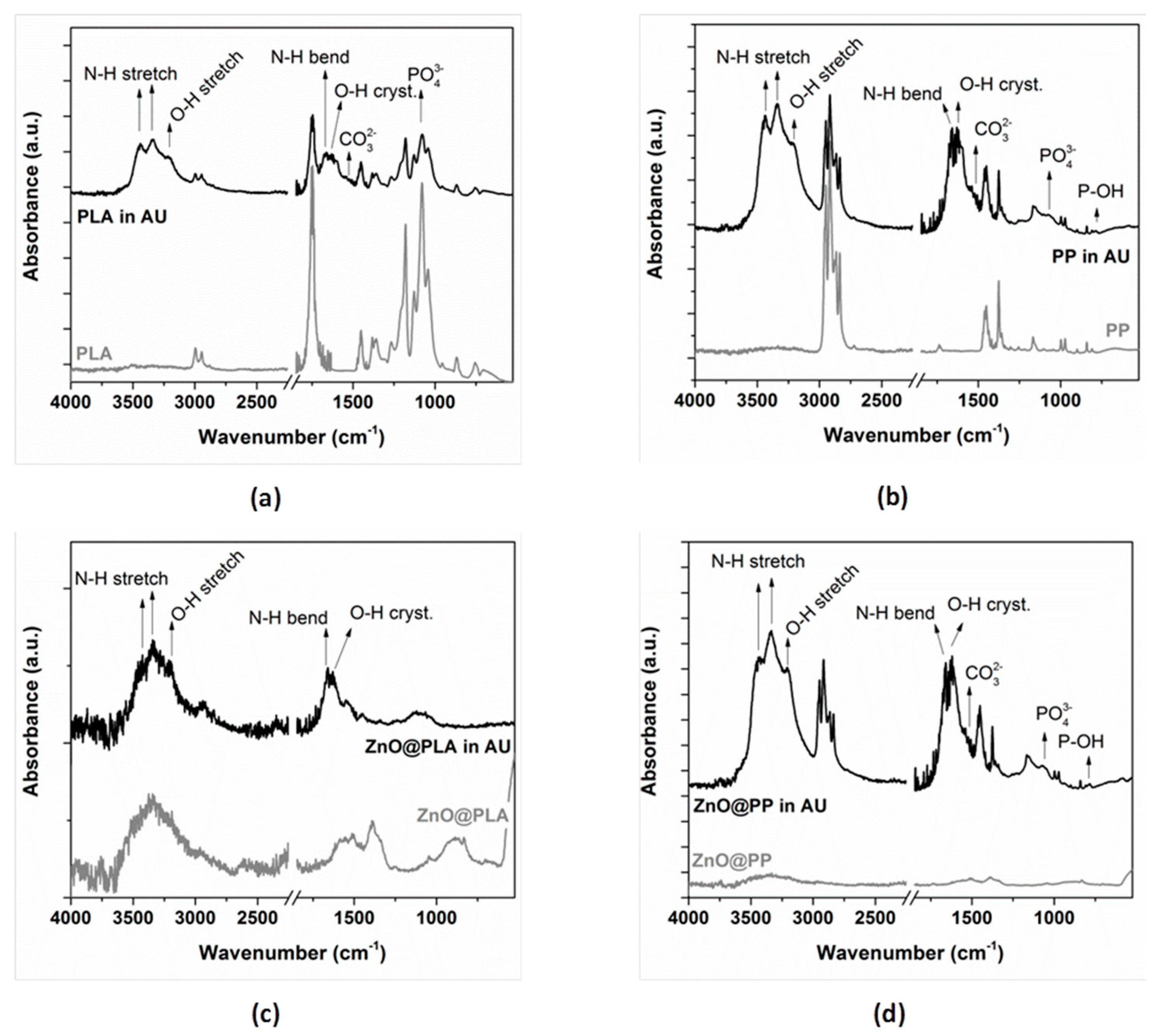
3.5. Evaluation of the Biodegradation Properties in Biological Fluids
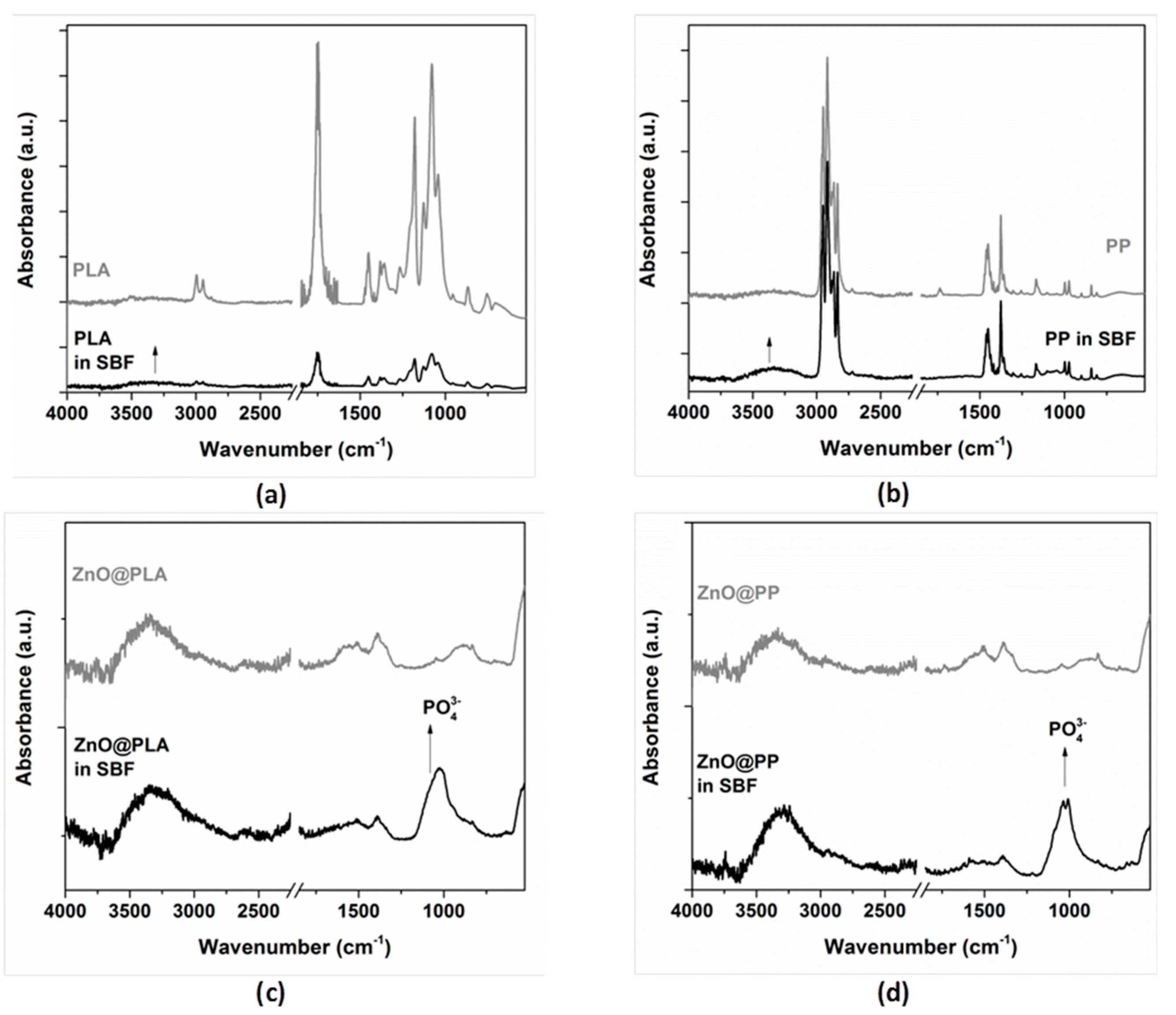
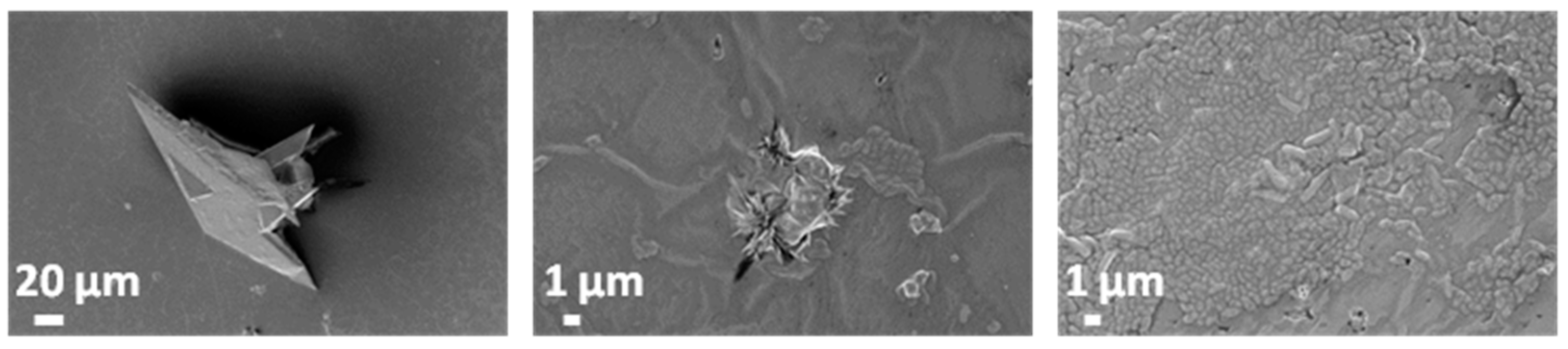
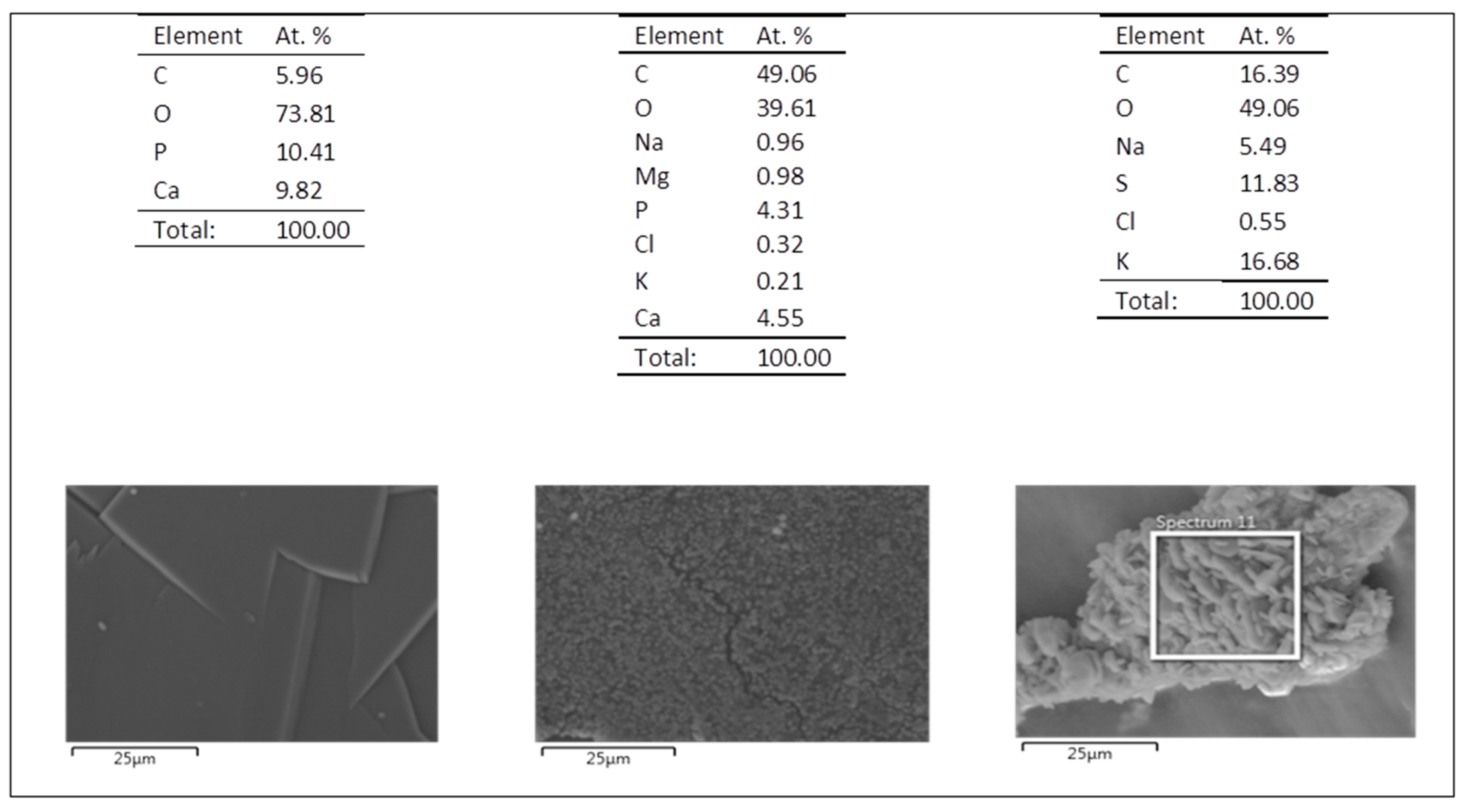
3.5.1. Simulated Body Fluid (SBF)
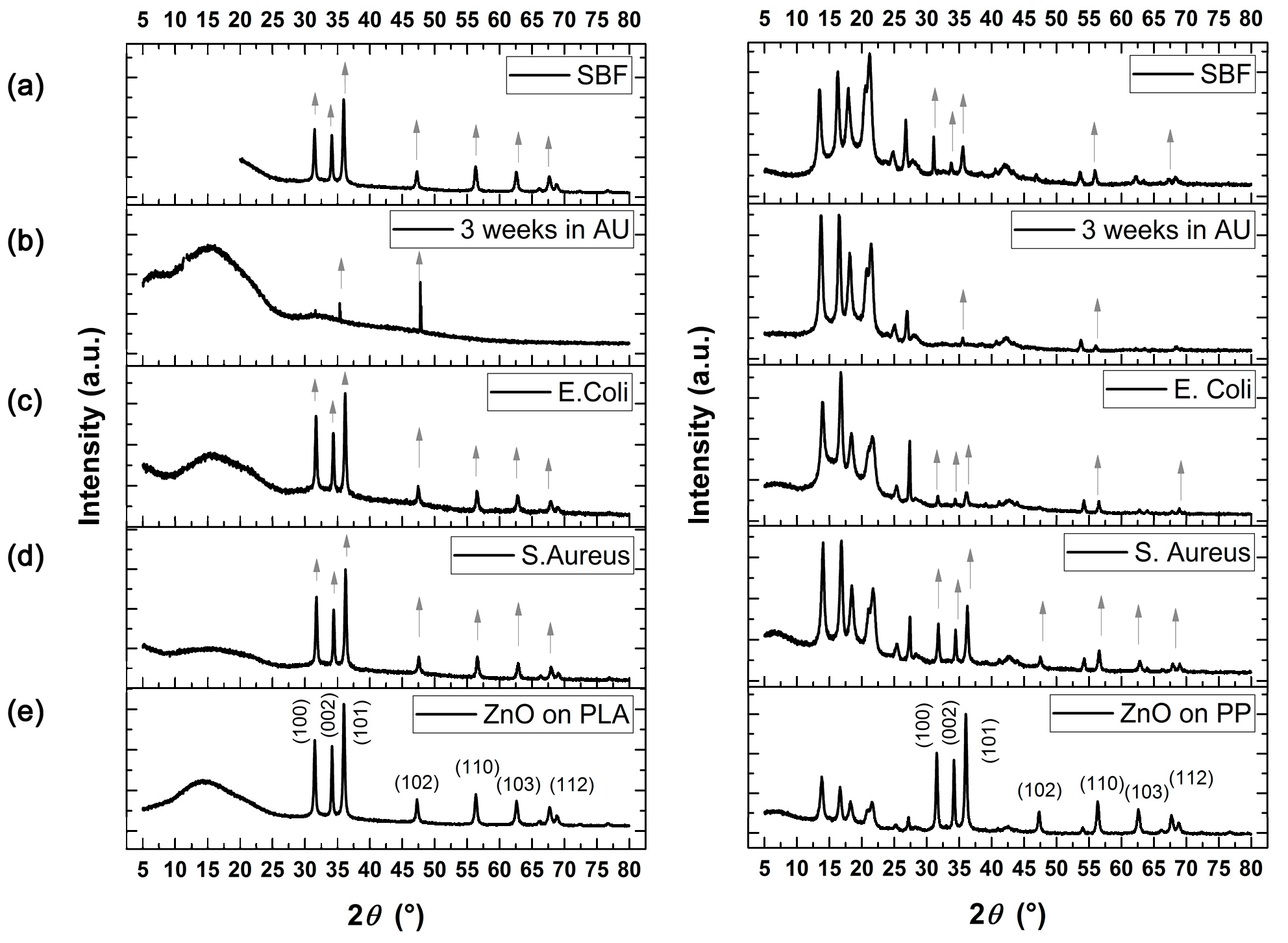
3.5.2. Artificial Urine
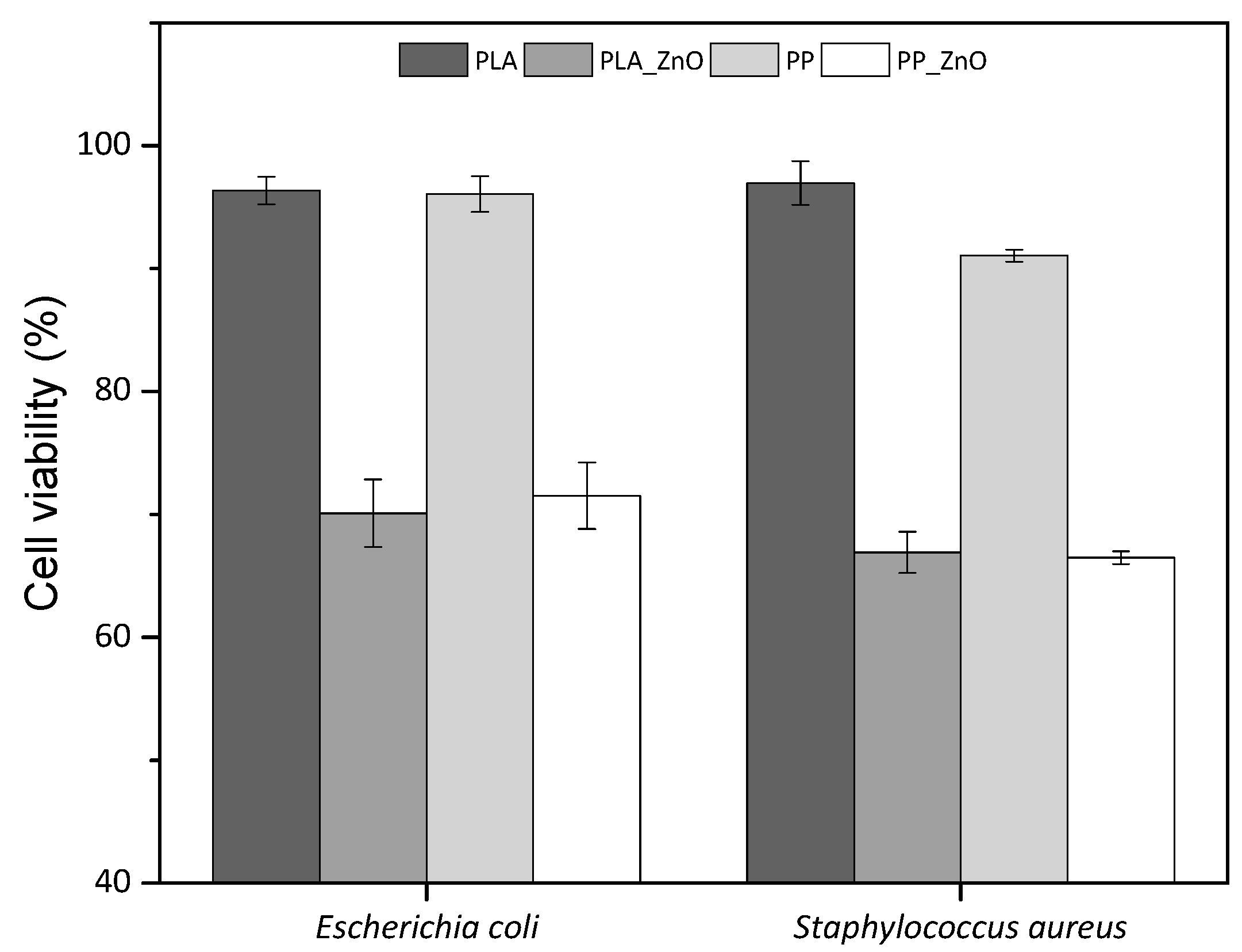
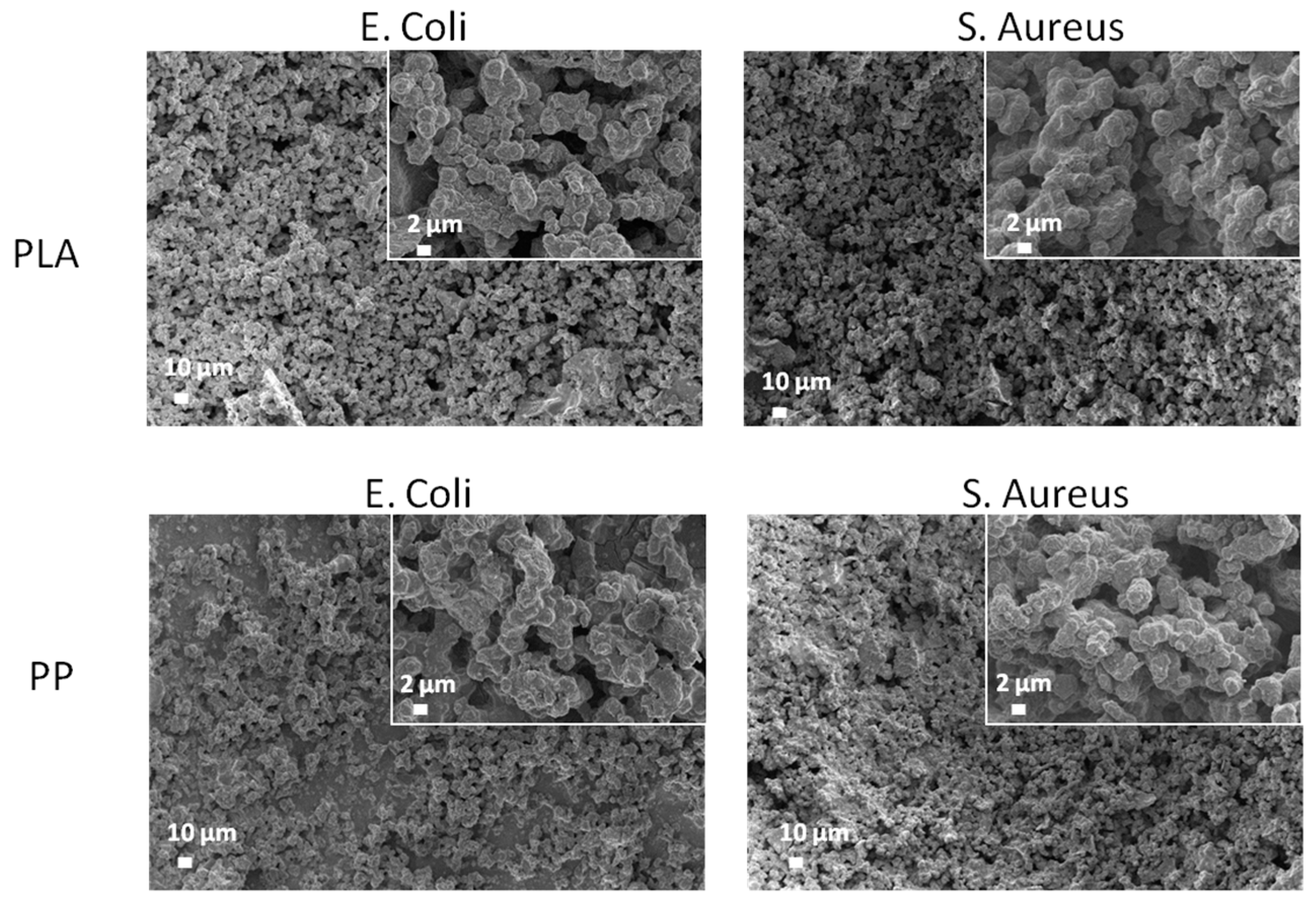
3.6. Antimicrobial Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lam, J.S.; Gupta, M. Update on ureteral stents. Urology 2004, 64, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Whiteside, S.; Cadieux, P.A.; Denstedt, J.D. Ureteral stent technology: Drug-eluting stents and stent coatings. Asian J. Urol. 2015, 2, 194–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-H.; Kim, K.-W.; Choi, Y.H.; Lee, S.B.; Baba, Y. Numerical analysis of urine flow with multiple sizes of double-j stents. Appl. Sci. 2020, 10, 4291. [Google Scholar] [CrossRef]
- Abdelaziz, A.Y.; Fouda, W.B.; Mosharafa, A.A.; Abelrasoul, M.A.; Fayyad, A.; Fawzi, K. Forgotten ureteral stents: Risk factors, complications and management. Afr. J. Urol. 2018, 24, 28–33. [Google Scholar] [CrossRef]
- Liu, S.; Luo, G.; Sun, B.; Lu, J.; Zu, Q.; Yang, S.; Zhang, X.; Dong, J. Early removal of double-j stents decreases urinary tract infections in living donor renal transplantation: A prospective, randomized clinical trial. Transplant. Proc. 2017, 49, 297–302. [Google Scholar] [CrossRef]
- Ahallal, Y.; Khallouk, A.; Fassi, M.J.E.; Farih, M.H. Risk factor analysis and management of ureteral double-j stent complications. Rev. Urol. 2010, 12, e147–e151. [Google Scholar]
- Forbes, C.; Scotland, K.B.; Lange, D.; Chew, B.H. Innovations in ureteral stent technology. Urol. Clin. 2019, 46, 245–255. [Google Scholar] [CrossRef]
- Singha, P.; Locklin, J.; Handa, H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2017, 50, 20–40. [Google Scholar] [CrossRef] [Green Version]
- Cauda, F.; Cauda, V.; Fiori, C.; Onida, B.; Garrone, E. Heparin coating on ureteral double j stents prevents encrustations: An in vivo case study. J. Endourol. 2008, 22, 465–472. [Google Scholar] [CrossRef]
- Mosayyebi, A.; Vijayakumar, A.; Yue, Q.Y.; Bres-Niewada, E.; Manes, C.; Carugo, D.; Somani, B.K. Engineering solutions to ureteral stents: Material, coating and design. Cent. Eur. J. Urol. 2017, 70, 270–274. [Google Scholar]
- Cauda, F.; Cauda, V.; Fiori, C. Coated ureteral stent. In Biomaterials and Tissue Engineering in Urology; Denstedt, J., Atala, A., Eds.; Woodhead Publishing Ltd.: London, UK, 2009; pp. 134–156. [Google Scholar]
- Laurenti, M.; Grochowicz, M.; Dragoni, E.; Carofiglio, M.; Limongi, T.; Cauda, V. Biodegradable and drug-eluting inorganic composites based on mesoporous zinc oxide for urinary stent applications. Materials 2020, 13, 3821. [Google Scholar] [CrossRef] [PubMed]
- Dave, R.N.; Joshi, H.M.; Venugopalan, V.P. Novel biocatalytic polymer-based antimicrobial coatings as potential ureteral biomaterial: Preparation and in vitro performance evaluation. Antimicrob. Agents Chemother. 2011, 55, 845–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesh, C.; Clear, O.; Major, I.; Lyons, J.G.; Devine, D.M. Faster release of lumen-loaded drugs than matrix-loaded equivalent in polylactic acid/halloysite nanotube. Materials 2019, 12, 1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turalija, M.; Bischof, S.; Budimir, A.; Gaan, S. Antimicrobial PLA films from environment friendly additives. Compos. Part B Eng. 2016, 102, 94–99. [Google Scholar] [CrossRef]
- Zhang, H.; Hortal, M.; Jorda Beneyto, M.; Rosa, E.; Lara, M.; Lorente, I. ZnO-PLA nanocomposite coated paper for antimicrobial packaging application. LWT-Food Sci. Technol. 2016, 78, 250–257. [Google Scholar] [CrossRef]
- De Silva, R.T.; Pasbakhsh, P.; Lee, S.M.; Kit, A.Y. Zno deposited/encapsulated halloysite–poly(lactic acid) (PLA) nanocomposites for high performance packaging films with improved mechanical and antimicrobial properties. Appl. Clay Sci. 2015, 111, 10–20. [Google Scholar] [CrossRef]
- Pantani, R.; Gorrasi, G.; Vigliotta, G.; Murariu, M.; Dubois, P. PLA-ZnO nanocomposite films: Water vapor barrier properties and specific end-use characteristics. Eur. Polym. J. 2013, 49, 3471–3482. [Google Scholar] [CrossRef]
- Marra, A.; Silvestre, C.; Duraccio, D.; Cimmino, S. Polylactic acid/zinc oxide biocomposite films for food packaging application. Int. J. Biol. Macromol. 2016, 88, 254–262. [Google Scholar] [CrossRef]
- Anžlovar, A.; Kržan, A.; Žagar, E. Degradation of PLA/ZnO and PHBV/ZnO composites prepared by melt processing. Arab. J. Chem. 2018, 11, 343–352. [Google Scholar] [CrossRef]
- Maddah, H.A. Polypropylene as a promising plastic: A review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar]
- Okuyucu, S.; Gorur, H.; Oksuz, H.; Akoglu, E. Endoscopic dacryocystorhinostomy with silicone, polypropylene, and t-tube stents; randomized controlled trial of efficacy and safety. Am. J. Rhinol. Allergy 2015, 29, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Viswanatha, B.; Vijayashree, M.S. Silicone stenting and polypropylene stenting in endoscopic dacryocystorhinostomy: A prospective comparative study. Res. Otolaryngol. 2015, 4, 49–53. [Google Scholar]
- Mitsuoka, M.; Hayashi, A.; Takamori, S.; Tayama, K.; Shirouzu, K. Experimental study of the histocompatibility of covered expandable metallic stents in the trachea. Chest 1998, 114, 110–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, T.; Zheng, R.; Yu, J.; Edmonds, L.; Wu, W.; Cao, J.; Gao, F.; Zhu, Y.; Cheng, Y.; Cui, W. Fabrication and evaluation of polymer-based esophageal stents for benign esophagus stricture insertion. RSC Adv. 2016, 6, 16891–16898. [Google Scholar] [CrossRef]
- Zhao, H.; Li, R.K.Y. A study on the photo-degradation of zinc oxide (ZnO) filled polypropylene nanocomposites. Polymer 2006, 47, 3207–3217. [Google Scholar] [CrossRef]
- Jakubiak, S.; Tomaszewska, J.; Jackiewicz, A.; Michalski, J.; Kurzydłowski, K.J. Polypropylene–zinc oxide nanorod hybrid material for applications in separation processes. Chem. Process Eng. 2016, 37, 393–403. [Google Scholar] [CrossRef] [Green Version]
- Bojarska, M.; Nowak, B.; Skowroński, J.; Piątkiewicz, W.; Gradoń, L. Growth of ZnO nanowires on polypropylene membrane surface—characterization and reactivity. Appl. Surf. Sci. 2017, 391, 457–467. [Google Scholar] [CrossRef]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Garino, N.; Sanvitale, P.; Dumontel, B.; Laurenti, M.; Colilla, M.; Izquierdo-Barba, I.; Cauda, V.; Vallet-Regì, M. Zinc oxide nanocrystals as a nanoantibiotic and osteoinductive agent. RSC Adv. 2019, 9, 11312–11321. [Google Scholar] [CrossRef]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.-A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Dimapilis, E.A.S.; Hsu, C.-S.; Mendoza, R.M.O.; Lu, M.-C. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Racca, L.; Canta, M.; Dumontel, B.; Ancona, A.; Limongi, T.; Garino, N.; Laurenti, M.; Canavese, G.; Cauda, V. Zinc oxide nanostructures in biomedicine. In Smart Nanoparticles for Biomedicine; Ciofani, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 171–187. [Google Scholar]
- Pugliese, D.; Bella, F.; Cauda, V.; Lamberti, A.; Sacco, A.; Tresso, E.; Bianco, S. A chemometric approach for the sensitization procedure of ZnO flowerlike microstructures for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2013, 5, 11288–11295. [Google Scholar] [CrossRef] [PubMed]
- Cauda, V.; Stassi, S.; Lamberti, A.; Morello, M.; Fabrizio Pirri, C.; Canavese, G. Leveraging ZnO morphologies in piezoelectric composites for mechanical energy harvesting. Nano Energy 2015, 18, 212–221. [Google Scholar] [CrossRef]
- Shin, J.; Liu, X.; Chikthimmah, N.; Lee, Y.S. Polymer surface modification using uv treatment for attachment of natamycin and the potential applications for conventional food cling wrap (ldpe). Appl. Surf. Sci. 2016, 386, 276–284. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Chichester, UK, 2004. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Sarangapani, S.; Cavedon, K.; Gage, D. An improved model for bacterial encrustation studies. J. Biomed. Mater. Res. 1995, 29, 1185–1191. [Google Scholar] [CrossRef]
- Kruenate, J.; Tongpool, R.; Panyathanmaporn, T.; Kongrat, P. Optical and mechanical properties of polypropylene modified by metal oxides. Surf. Interface Anal. 2004, 36, 1044–1047. [Google Scholar] [CrossRef]
- Jayaramudu, J.; Das, K.; Sonakshi, M.; Siva Mohan Reddy, G.; Aderibigbe, B.; Sadiku, R.; Sinha Ray, S. Structure and properties of highly toughened biodegradable polylactide/zno biocomposite films. Int. J. Biol. Macromol. 2014, 64, 428–434. [Google Scholar] [CrossRef]
- Tang, Z.; Fan, F.; Chu, Z.; Fan, C.; Qin, Y. Barrier properties and characterizations of poly(lactic acid)/ZnO nanocomposites. Molecules 2020, 25, 1310. [Google Scholar] [CrossRef] [Green Version]
- Laurenti, M.; Lamberti, A.; Genchi, G.G.; Roppolo, I.; Canavese, G.; Vitale-Brovarone, C.; Ciofani, G.; Cauda, V. Graphene oxide finely tunes the bioactivity and drug delivery of mesoporous ZnO scaffolds. ACS Appl. Mater. Interfaces 2019, 11, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, M.; Cauda, V. Gentamicin-releasing mesoporous ZnO structures. Materials (Basel) 2018, 11, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salma, K.; Borodajenko, N.; Plata, A.; Berzina-Cimdina, L.; Stunda, A. Fourier transform infrared spectra of technologically modified calcium phosphates. In Proceedings of the 14th Nordic-Baltic Conference on Biomedical Engineering and Medical Physics, Riga, Latvia, 16–20 June 2008. [Google Scholar]
- Richardson, J.J.; Lange, F.F. Controlling low temperature aqueous synthesis of ZnO. 1. Thermodynamic analysis. Cryst. Growth Des. 2009, 9, 2570–2575. [Google Scholar] [CrossRef]
- Sedłak, A.; Janusz, W. Specific adsorption of carbonate ions at the zinc oxide/electrolyte solution interface. Physicochem. Probl. Miner. Process. 2008, 42, 57–66. [Google Scholar]
- Haleblian, G.; Kijvikai, K.; Rosette, J.d.l.; Preminger, G. Ureteral stenting and urinary stone management: A systematic review. J. Urol. 2008, 179, 424–430. [Google Scholar] [CrossRef]
- Mizielińska, M.; Kowalska, U.; Jarosz, M.; Sumińska, P.; Landercy, N.; Duquesne, E. The effect of UV aging on antimicrobial and mechanical properties of PLA films with incorporated zinc oxide nanoparticles. Int. J. Environ. Res. Public Health 2018, 15, 794. [Google Scholar] [CrossRef] [Green Version]
- Mania, S.; Cieślik, M.; Konzorski, M.; Święcikowski, P.; Nelson, A.; Banach, A.; Tylingo, R.T. The synergistic microbiological effects of industrial produced packaging polyethylene films incorporated with zinc nanoparticles. Polymers 2020, 12, 1198. [Google Scholar] [CrossRef]
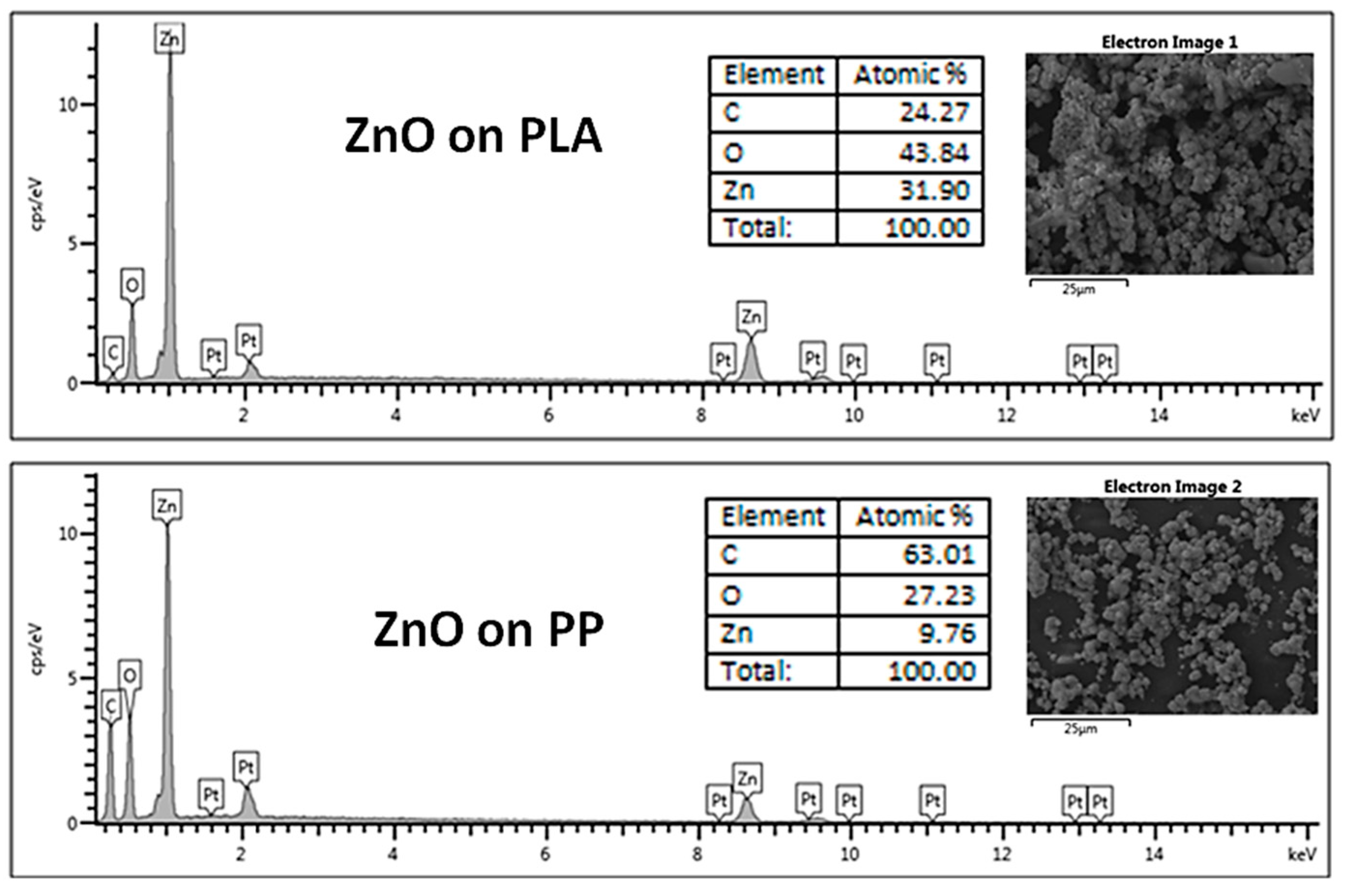

| Element | E. coli | S. aureus | ||
|---|---|---|---|---|
| PLA/ZnO, at.% | PP/ZnO, at.% | PLA/ZnO, at.% | PP/ZnO, at.% | |
| C | 28.89 | 51.08 | 35.93 | 29.94 |
| N | 4.33 | – | – | 4.70 |
| O | 38.17 | 30.23 | 37.49 | 38.85 |
| Na | 7.69 | 4.97 | 7.21 | 7.36 |
| P | 3.17 | 2.12 | 2.86 | 2.75 |
| Cl | 1.14 | 0.69 | 1.26 | 0.79 |
| K | 0.92 | 0.91 | 0.92 | 0.74 |
| S | – | 0.13 | – | – |
| Ca | – | 0.35 | – | – |
| Zn | 15.69 | 9.52 | 14.33 | 14.87 |
| Total. | 100.00 | 100.00 | 100.00 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesh, C.; Laurenti, M.; Bandeira, M.; Lanzagorta, E.; Lucherini, L.; Cauda, V.; Devine, D.M. Biodegradation and Antimicrobial Properties of Zinc Oxide–Polymer Composite Materials for Urinary Stent Applications. Coatings 2020, 10, 1002. https://doi.org/10.3390/coatings10101002
Venkatesh C, Laurenti M, Bandeira M, Lanzagorta E, Lucherini L, Cauda V, Devine DM. Biodegradation and Antimicrobial Properties of Zinc Oxide–Polymer Composite Materials for Urinary Stent Applications. Coatings. 2020; 10(10):1002. https://doi.org/10.3390/coatings10101002
Chicago/Turabian StyleVenkatesh, Chaitra, Marco Laurenti, Marina Bandeira, Eduardo Lanzagorta, Lorenzo Lucherini, Valentina Cauda, and Declan M. Devine. 2020. "Biodegradation and Antimicrobial Properties of Zinc Oxide–Polymer Composite Materials for Urinary Stent Applications" Coatings 10, no. 10: 1002. https://doi.org/10.3390/coatings10101002
APA StyleVenkatesh, C., Laurenti, M., Bandeira, M., Lanzagorta, E., Lucherini, L., Cauda, V., & Devine, D. M. (2020). Biodegradation and Antimicrobial Properties of Zinc Oxide–Polymer Composite Materials for Urinary Stent Applications. Coatings, 10(10), 1002. https://doi.org/10.3390/coatings10101002