Corrosion and Wear Resistance of Fe-Based Amorphous Coatings
Abstract
:1. Introduction
2. Experimental Materials and Methods
3. Experimental Results and Analysis
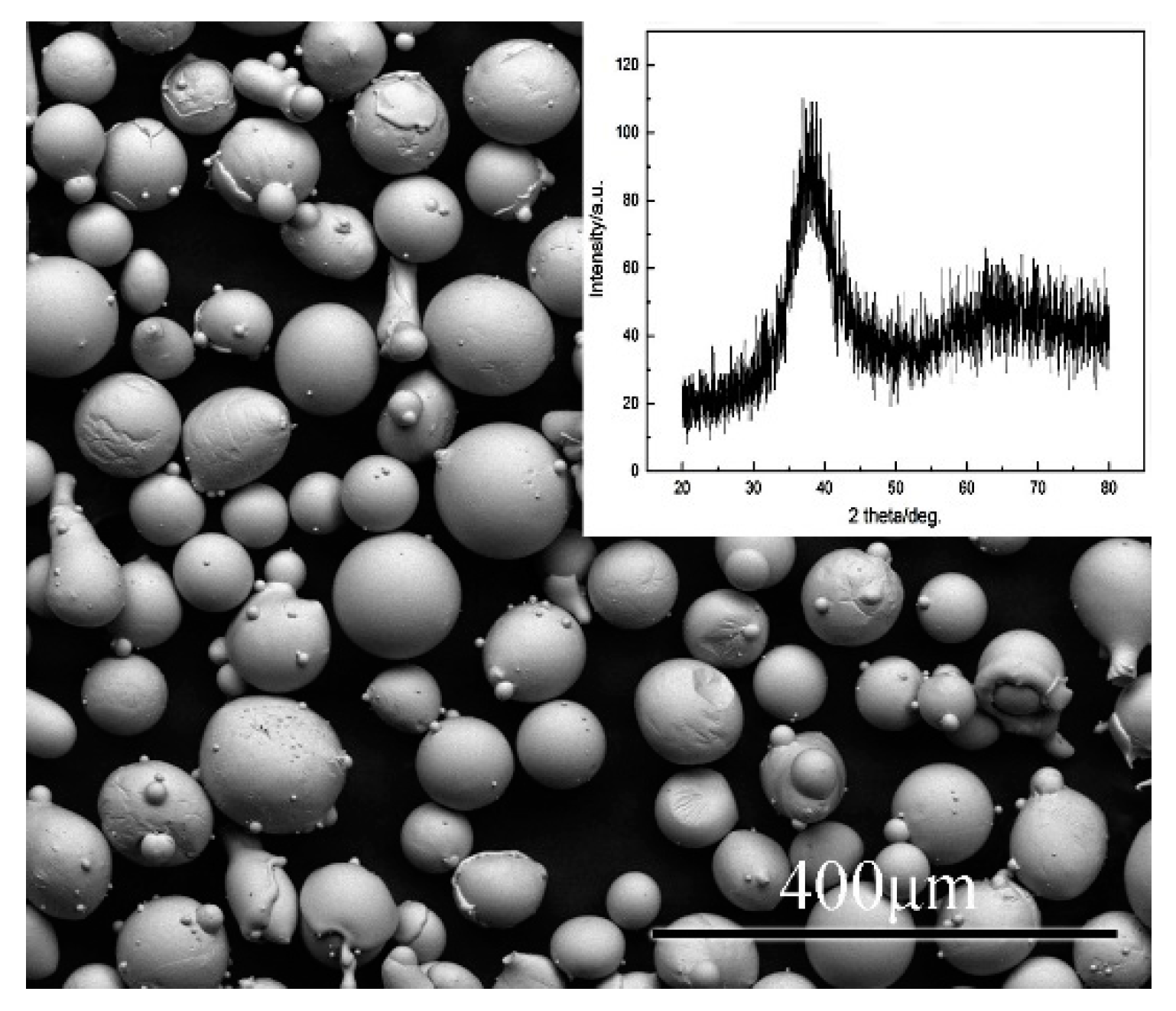
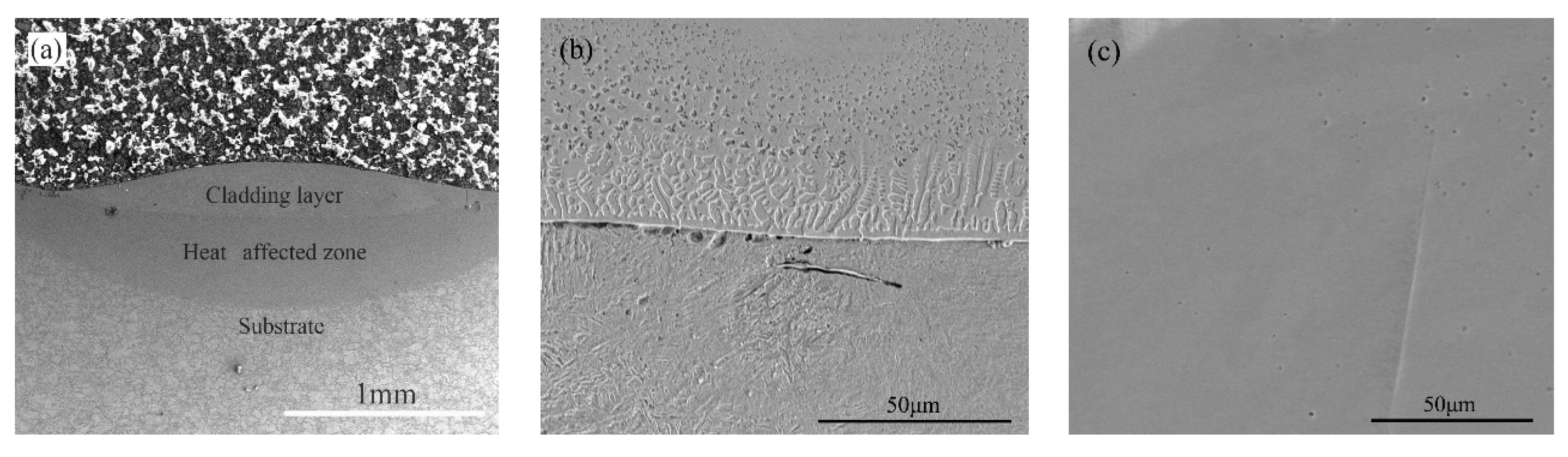
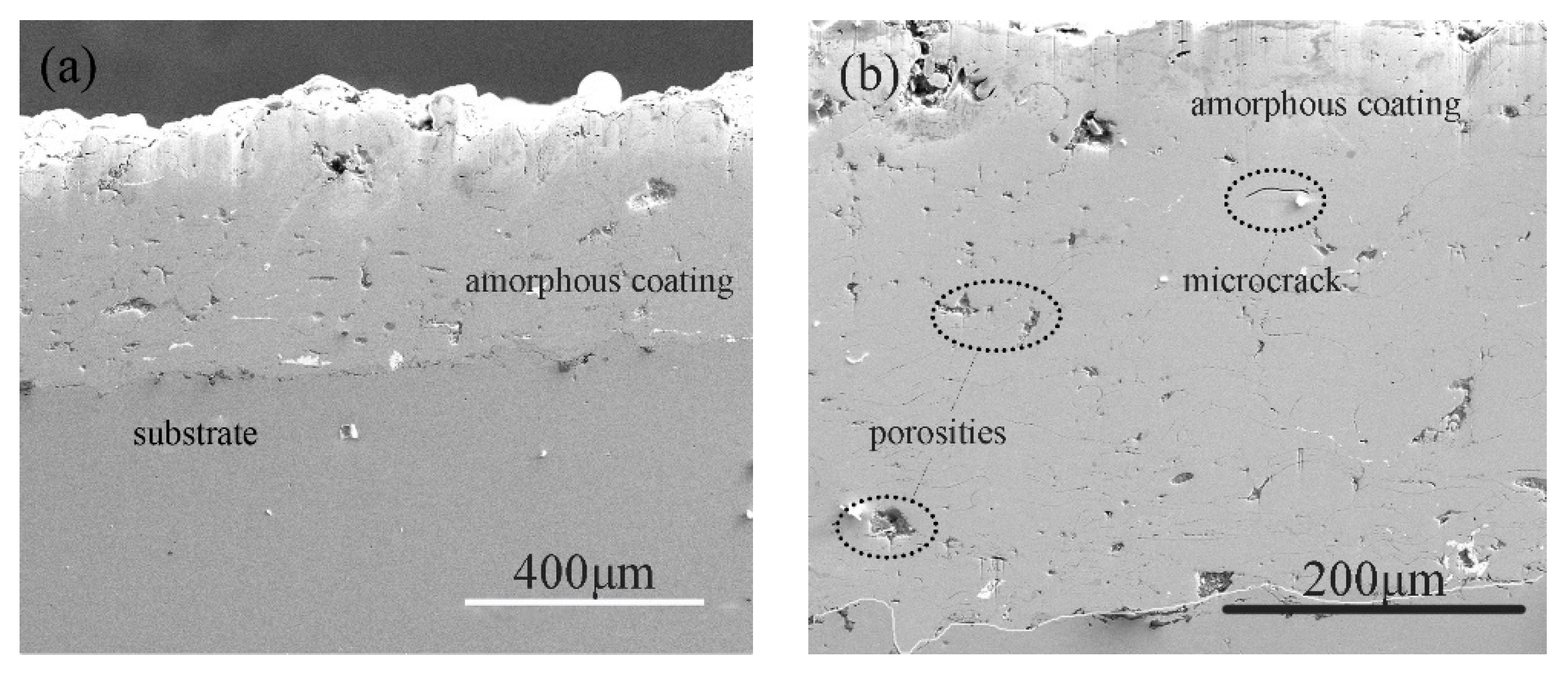
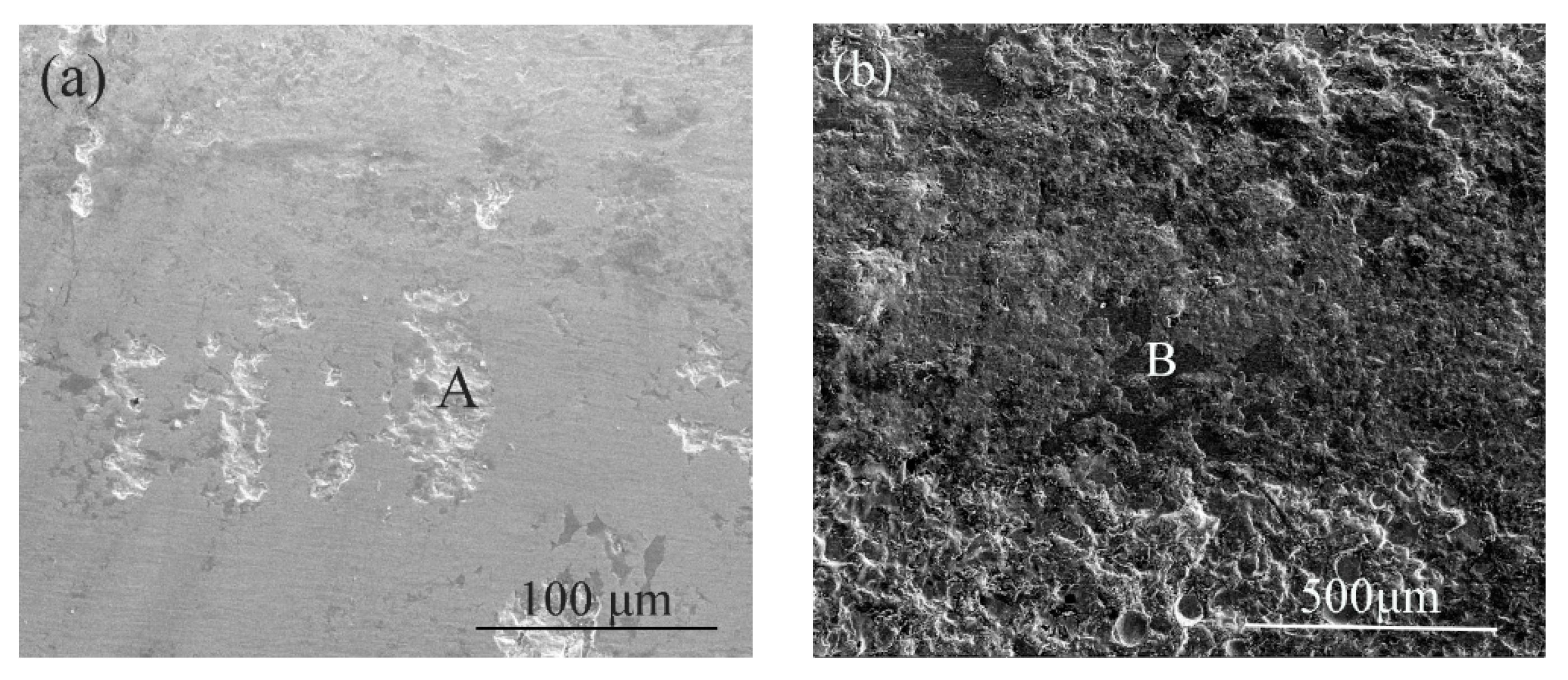
3.1. Structural Characterization of the Coatings
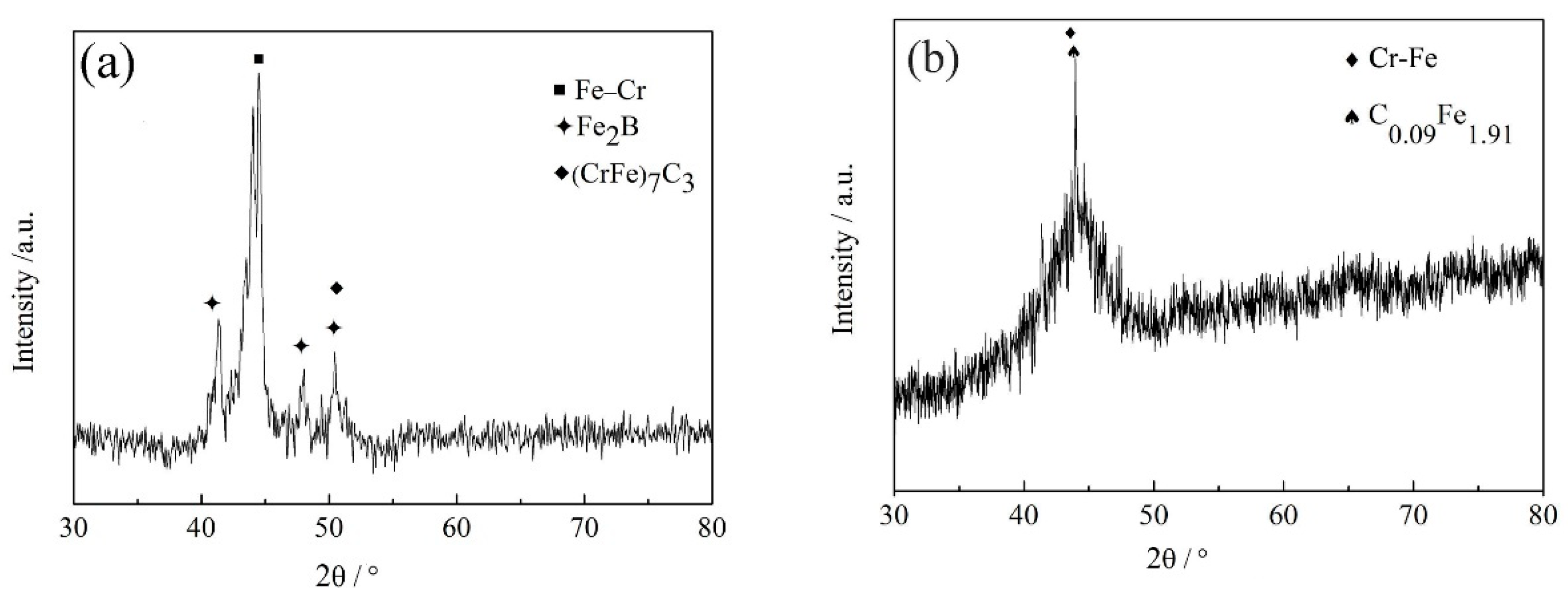
3.2. Phasse of the Coatings
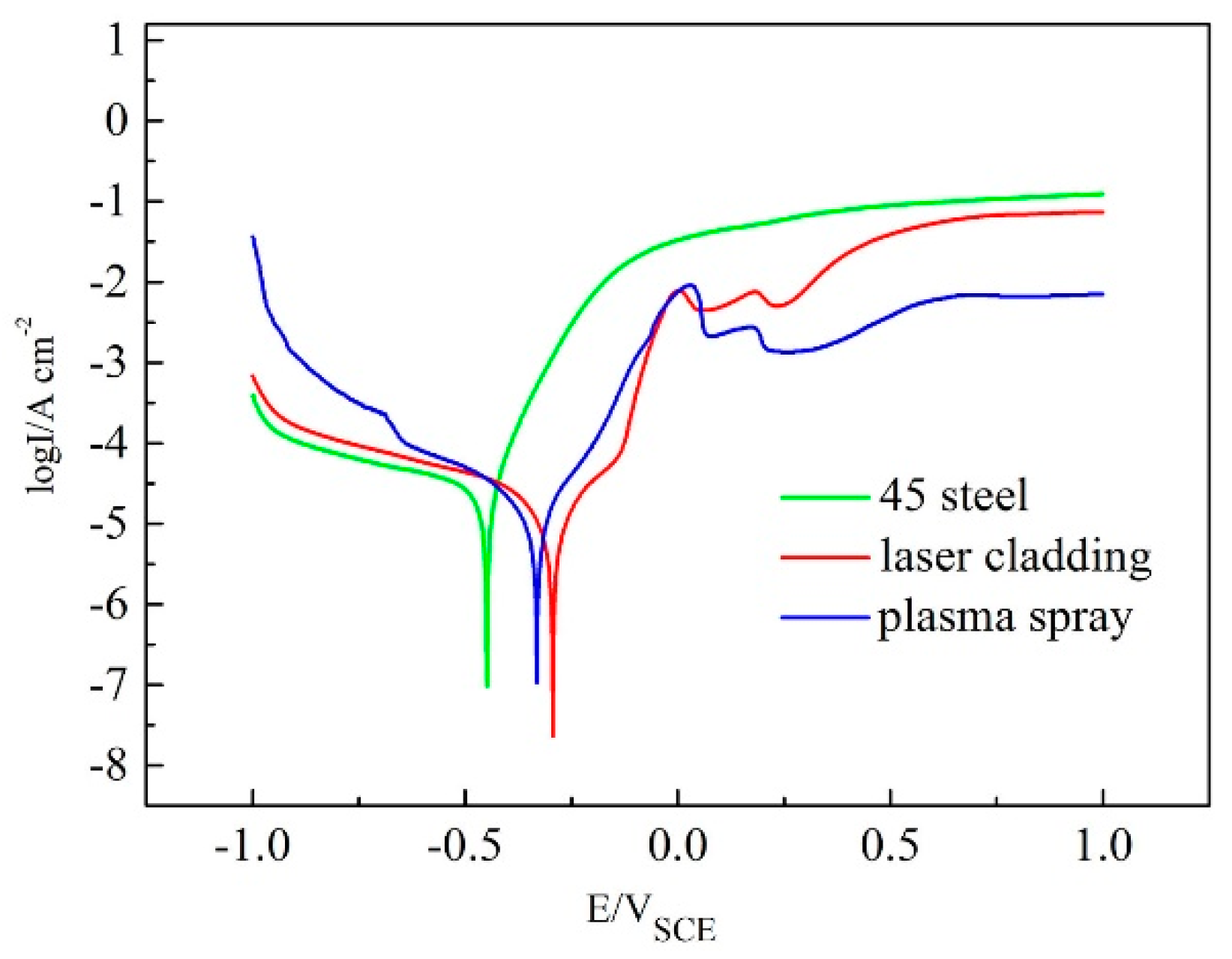
3.3. Corrosion Resistance of the Coatings
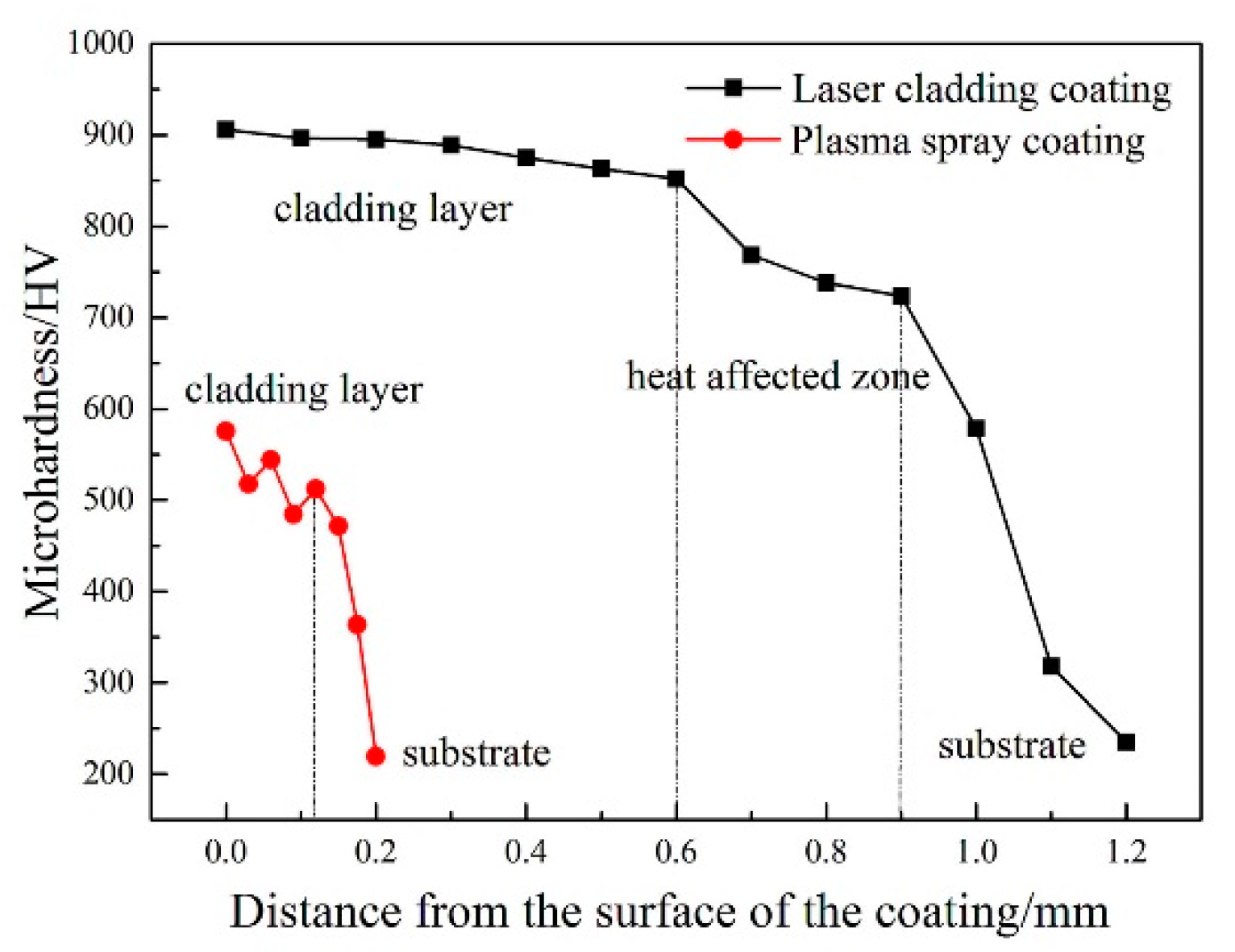
3.4. Microhardness of the Coatings
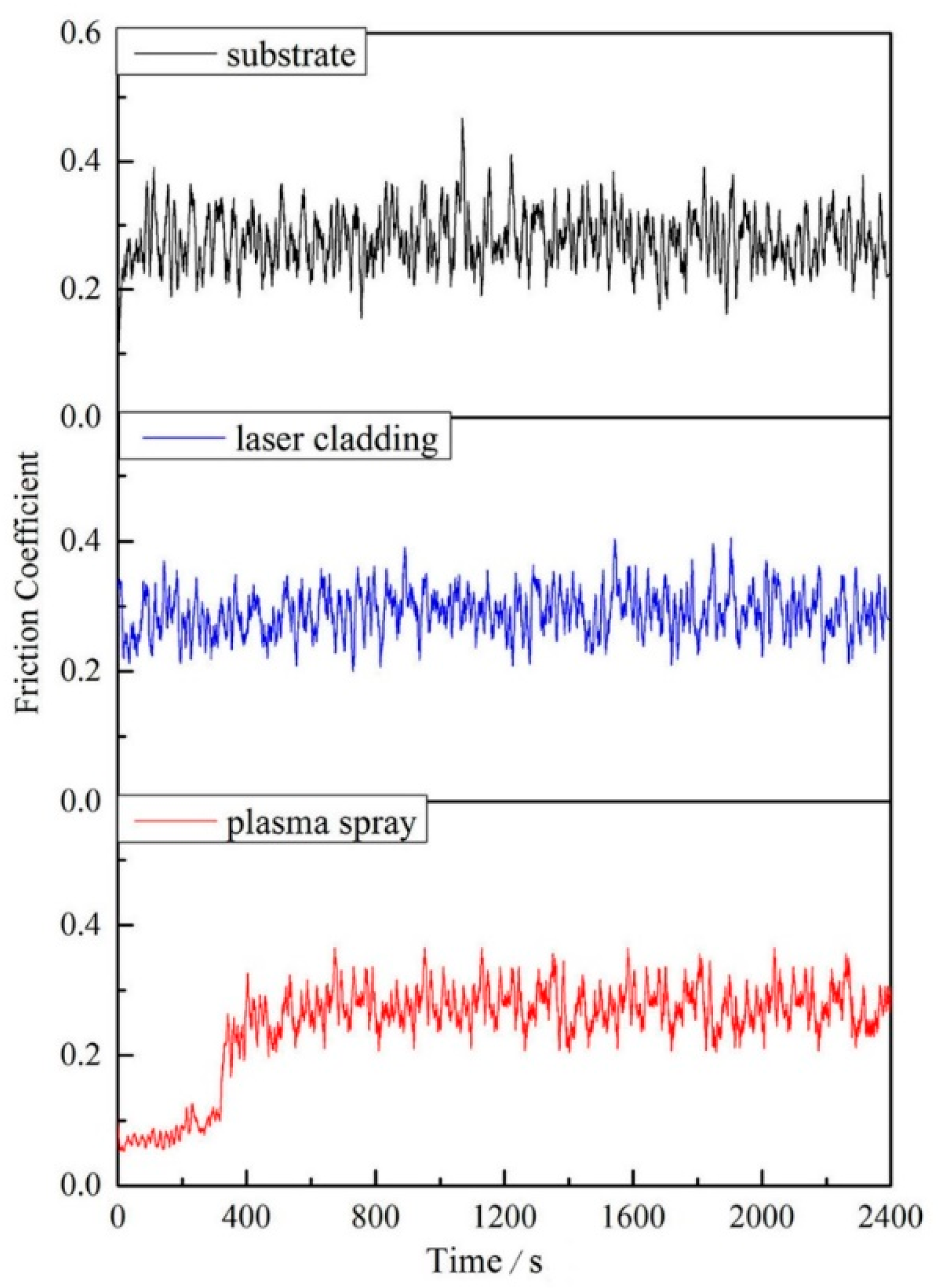
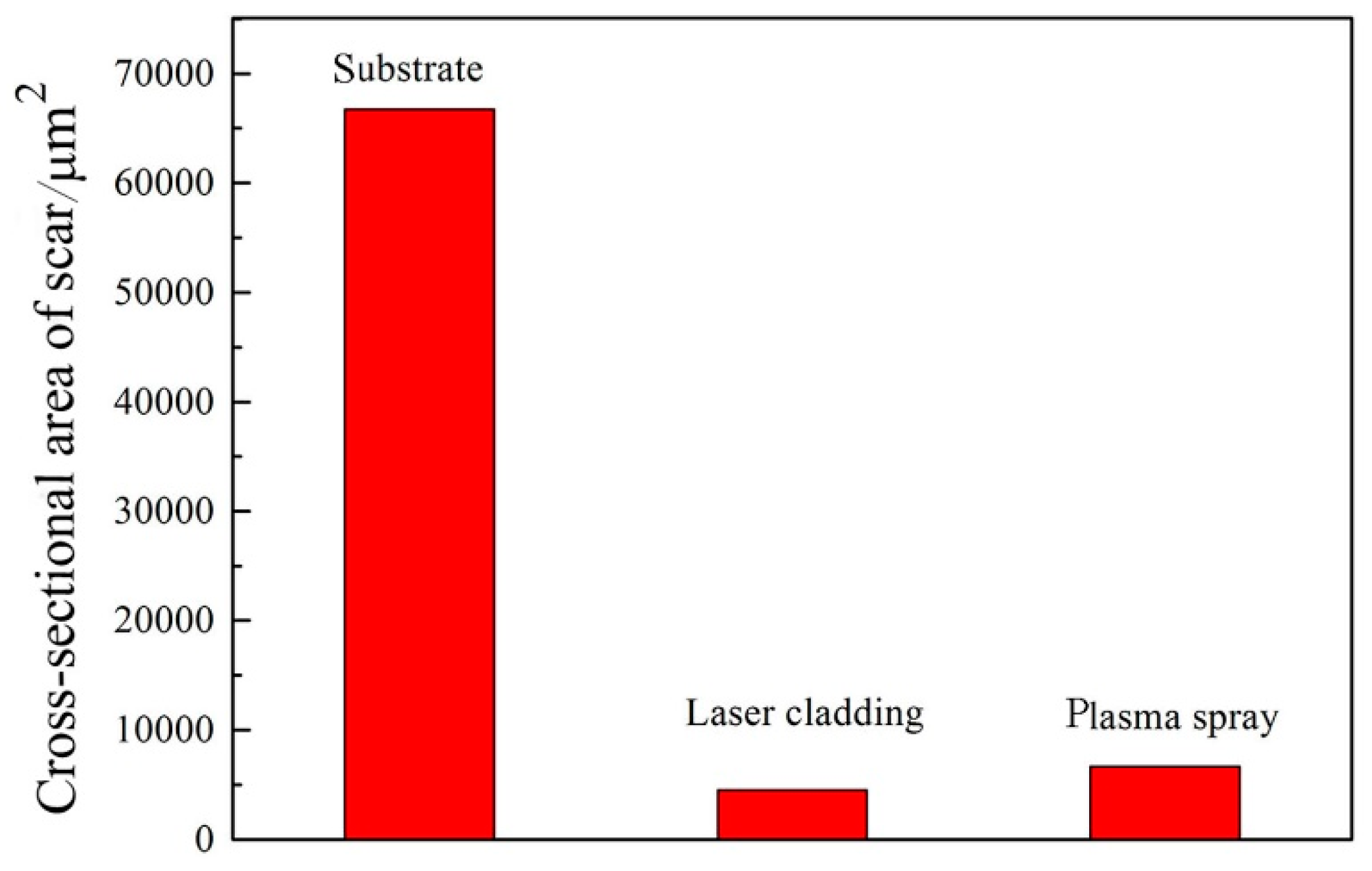
3.5. Wear Performance of the Coatings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Inoue, A. Stabilization and high strain-rate superplasticity of metallic supercooled liquid. Mater. Sci. Eng. A 1999, 267, 171–183. [Google Scholar] [CrossRef]
- Trexler, M.M.; Thadhani, N.N. Mechanical properties of bulk metallic glasses. Prog. Mater. Sci. 2010, 55, 759–839. [Google Scholar] [CrossRef]
- Gu, X.; Poon, S.J.; Shiflet, G.J. Mechanical properties of iron-based bulk metallic glasses. J. Mater. Res. 2007, 22, 344–351. [Google Scholar] [CrossRef]
- Gu, X.; Poon, S.J.; Shiflet, G.J.; Widom, M. Ductility improvement of amorphous steels: Roles of shear modulus and electronic structure. Acta Mater. 2008, 56, 88–94. [Google Scholar] [CrossRef]
- Keryvin, V.; Hoang, V.; Shen, J. Hardness, toughness, brittleness and cracking systems in an iron-based bulk metallic glass by indentation. Intermetallics 2009, 17, 211–217. [Google Scholar] [CrossRef]
- Inoue, A.; Kong, F.; Zhu, S.; Shalaan, E.-S.; Al-Marzouki, F. Production methods and properties of engineering glassy alloys and composites. Intermetallics 2015, 58, 20–30. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, C.; Wang, W.; Yasir, M.; Liu, L. Microstructure and mechanical properties of Fe-based amorphous composite coatings reinforced by stainless steel powders. J. Mater. Technol. 2015, 31, 43–47. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Z.; Li, R.; Li, M.; Daze, X.; Feng, K.; Wu, Y. Microstructure and property of Fe–Co–B–Si–C–Nb amorphous composite coating fabricated by laser cladding process. Appl. Surf. Sci. 2013, 280, 50–54. [Google Scholar] [CrossRef]
- Farmer, J.; Haslam, J.; Day, S.; Lian, T.; Saw, C.; Hailey, P.; Choi, J.-S.; Rebak, R.; Yang, N.; Payer, J.; et al. Corrosion resistance of thermally sprayed high-boron iron-based amorphous-metal coatings: Fe49.7Cr17.7Mn1.9Mo7.4W1.6B15.2C3.8Si2.4. J. Mater. Res. 2007, 22, 2297–2311. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, W.; Wang, S.; Gu, X.; Wang, J. Characterisation of three-dimensional porosity in an Fe-based amorphous coating and its correlation with corrosion behaviour. Corros. Sci. 2015, 93, 211–221. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.-W.; Chen, Q.; Liu, L. Effect of hydrostatic pressure on the corrosion behavior of HVOF-sprayed Fe-based amorphous coating. J. Alloy. Compd. 2018, 758, 108–115. [Google Scholar] [CrossRef]
- Nie, G.M.; Huang, C.; Li, B.; Zhong, J.; Wang, S. Fabrication and application status of Fe-based amorphous alloy coatings. Surf. Technol. 2017, 46, 18–26. [Google Scholar]
- Zhang, C.; Guo, R.; Yang, Y.; Wu, Y.; Liu, L. Influence of the size of spraying powders on the microstructure and corrosion resistance of Fe-based amorphous coating. Electrochim. Acta 2011, 56, 6380–6388. [Google Scholar] [CrossRef]
- Stoica, M.; Eckert, J.; Roth, S.; Zhang, Z.; Schultz, L.; Wang, W. Mechanical behavior of Fe65.5Cr4Mo4Ga4P12C5B5.5 bulk metallic glass. Intermetallics 2005, 13, 764–769. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; He, D.Y.; Wang, F.C.; Liu, Y.B. Microstructure and electrochemical behavior of Fe-based amorphous metallic coatings fabricated by atmospheric plasma spraying. J. Therm. Spray Technol. 2011, 20, 344–350. [Google Scholar] [CrossRef]
- Kobayashi, A.; Yano, S.; Kimura, H.; Inoue, A. Fe-based metallic glass coatings produced by smart plasma spraying process. Mater. Sci. Eng. B 2008, 148, 110–113. [Google Scholar] [CrossRef]
- Zhou, Y.-Y.; Ma, G.-Z.; Wang, H.-D.; Li, G.-L.; Chen, S.-Y.; Wang, H.-J.; Liu, M. Fabrication and characterization of supersonic plasma sprayed Fe-based amorphous metallic coatings. Mater. Des. 2016, 110, 332–339. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Z.; Li, R.; Li, M.; Feng, K.; Wu, Y.; Wada, T.; Kato, H. High power diode laser cladding of Fe–Co–B–Si–C–Nb amorphous coating: Layered microstructure and properties. Surf. Coat. Technol. 2013, 235, 699–705. [Google Scholar] [CrossRef]
- Katakam, S.; Kumar, V.; Santhanakrishnan, S.; Rajamure, R.; Samimi, P.; Dahotre, N.B. Laser assisted Fe-based bulk amorphous coating: Thermal effects and corrosion. J. Alloy. Compd. 2014, 604, 266–272. [Google Scholar] [CrossRef]
- Mojaver, R.; Mojtahedi, F.; Shahverdi, H.R.; Torkamany, M.J. Study on feasibility of producing an amorphous surface layer of Fe49Cr18Mo7B16C4Nb3 by pulsed Nd:YAG laser surface melting. Appl. Surf. Sci. 2013, 264, 176–183. [Google Scholar] [CrossRef]
- Wu, X.; Hong, Y. Fe-based thick amorphous-alloy coating by laser cladding. Surf. Coat. Technol. 2001, 141, 141–144. [Google Scholar] [CrossRef]
- Ye, X.; Shin, Y.C. Synthesis and characterization of Fe-based amorphous composite by laser direct deposition. Surf. Coat. Technol. 2014, 239, 34–40. [Google Scholar] [CrossRef]
- Koga, G.; Schulz, R.; Savoie, S.; Nascimento, A.; Drolet, Y.; Bolfarini, C.; Kiminami, C.; Botta, W. Microstructure and wear behavior of Fe-based amorphous HVOF coatings produced from commercial precursors. Surf. Coat. Technol. 2017, 309, 938–944. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, G.; Wang, Y.; Li, H.; Qin, Z.; Lu, X. Crack-free Fe-based amorphous coating synthesized by laser cladding. Mater. Lett. 2017, 210, 46–50. [Google Scholar] [CrossRef]
- Chew, Y.; Pang, J.H.L.; Bi, G.; Song, B. Thermo-mechanical model for simulating laser cladding induced residual stresses with single and multiple clad beads. J. Mater. Process. Technol. 2015, 224, 89–101. [Google Scholar] [CrossRef]

| Element | C | Si | Mn | P | Cu | Ni | Cr | S | Fe |
|---|---|---|---|---|---|---|---|---|---|
| wt % | 0.44 | 0.21 | 0.53 | 0.028 | 0.02 | 0.01 | 0.03 | 0.007 | Bal. |
| Element | Cr | Mo | C | B | Fe |
|---|---|---|---|---|---|
| wt % | 25 | 20 | 3 | 3 | Bal. |
| Argon Flow (L/min) | Hydrogen Flow (L/min) | Voltage (V) | Current (A) | Powder Feed (g/min) | Spray Distance (mm) | Spray Thickness (mm) |
|---|---|---|---|---|---|---|
| 160 | 20 | 140 | 370 | 30 | 120 | 0.2 |
| Laser Wavelength (nm) | Laser Power (W) | Scanning Speed (mm/s) | Spot Diameter (mm) | Argon Flow (L/min) |
|---|---|---|---|---|
| 1070 | 2500 | 20 | 3 | 25 |
| Loading Force (N) | Sliding Speed (m/s) | Sliding Frequency (Hz/s) | Sliding Total Stroke (m) | Friction Pair (mm) | Friction Time (s) |
|---|---|---|---|---|---|
| 25 | 0.1 | 20 | 250 | 5 | 2400 |
| Substrate and Coating | Ecorr/mV | Icorr/A·cm−2 |
|---|---|---|
| 45 steel | −448.5 | 1.049 × 10−4 |
| Laser cladding-produced coating | −300.8 | 3.583 × 10−5 |
| Plasma-sprayed coating | −332.8 | 5.218 × 10−5 |
| 45 Steel | Laser Cladding Coating | Plasma-Sprayed Coating | |
|---|---|---|---|
| Average Friction Coefficient | 0.2992 | 0.2915 | 0.2778 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Gan, Y.; Liu, C.; Shi, Y.; Zhao, Y.; Kou, S. Corrosion and Wear Resistance of Fe-Based Amorphous Coatings. Coatings 2020, 10, 73. https://doi.org/10.3390/coatings10010073
Li G, Gan Y, Liu C, Shi Y, Zhao Y, Kou S. Corrosion and Wear Resistance of Fe-Based Amorphous Coatings. Coatings. 2020; 10(1):73. https://doi.org/10.3390/coatings10010073
Chicago/Turabian StyleLi, Guang, Youyi Gan, Chenheng Liu, Yu Shi, Yanchun Zhao, and Shengzhong Kou. 2020. "Corrosion and Wear Resistance of Fe-Based Amorphous Coatings" Coatings 10, no. 1: 73. https://doi.org/10.3390/coatings10010073
APA StyleLi, G., Gan, Y., Liu, C., Shi, Y., Zhao, Y., & Kou, S. (2020). Corrosion and Wear Resistance of Fe-Based Amorphous Coatings. Coatings, 10(1), 73. https://doi.org/10.3390/coatings10010073





