Effect of Sodium Phosphate and Calcium Nitrate Sealing Treatment on Microstructure and Corrosion Resistance of Wire Arc Sprayed Aluminum Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Process of the Coating
2.2. Treatment of the Coatings
2.3. Corrosion Studies
2.4. Characterization of the Coatings and Corrosion Products
3. Results and Discussion
3.1. Characterization of the Coatings
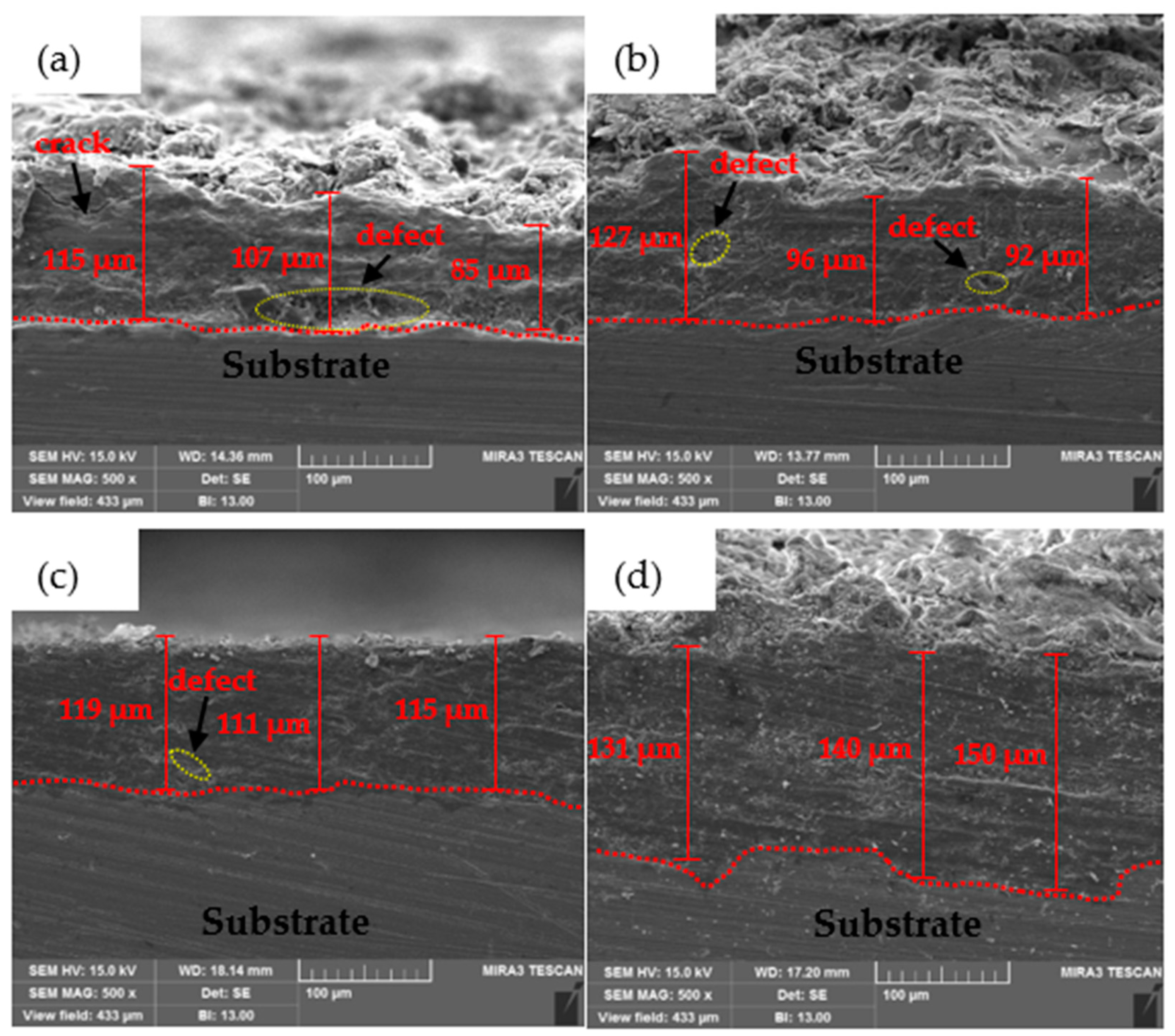
3.1.1. Coating Thickness and Bond Adhesion Analysis
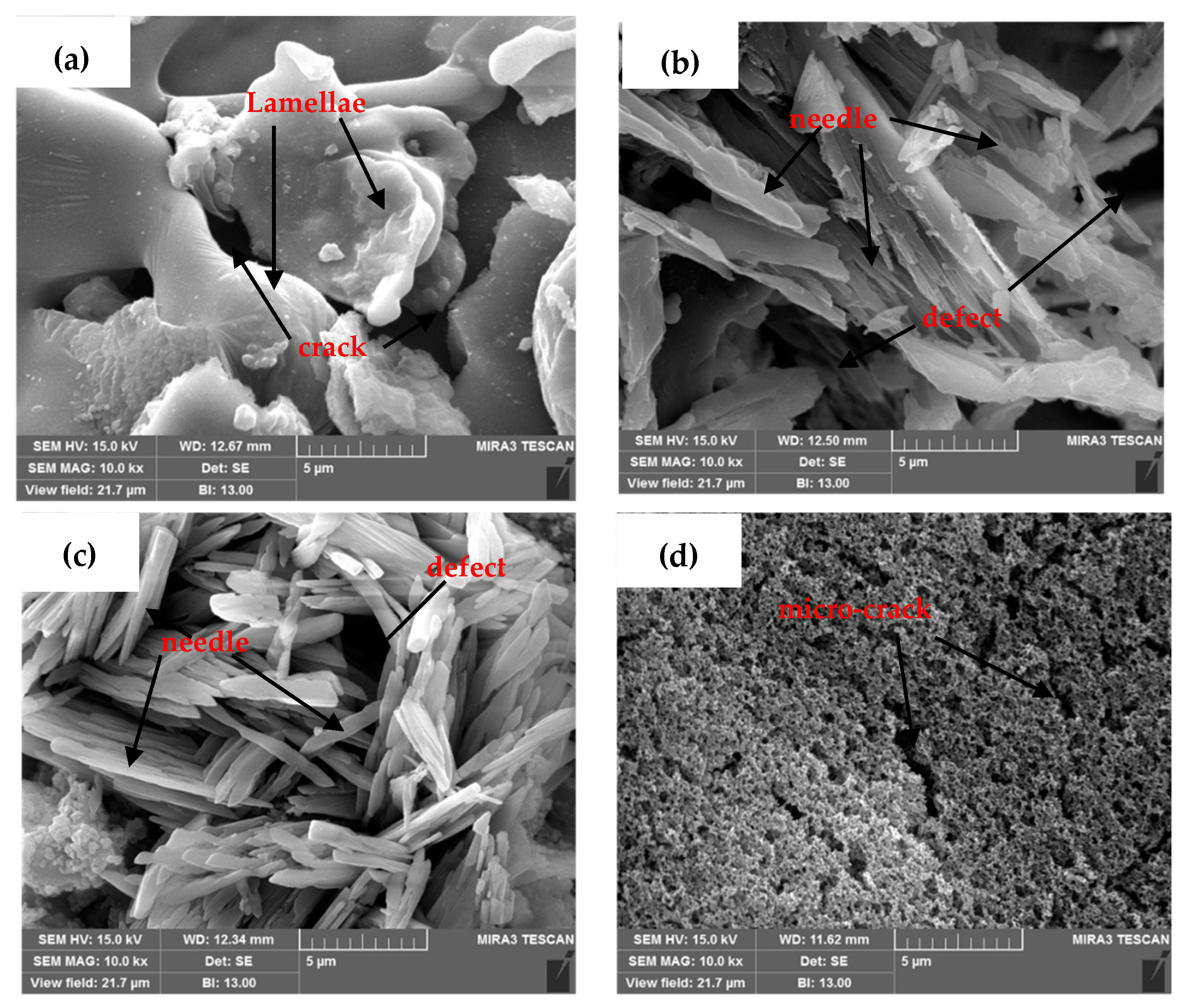
3.1.2. Scanning Electron Microscopy (SEM) of the Coatings
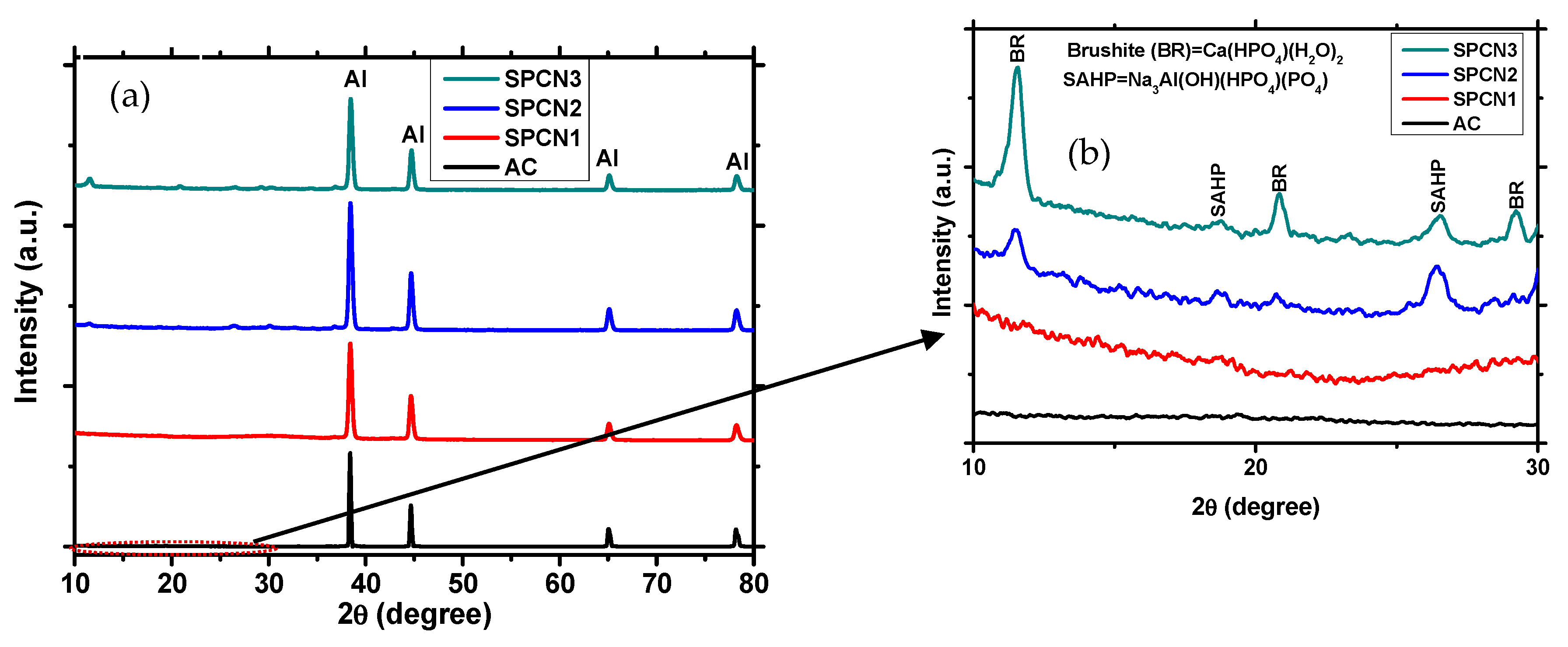
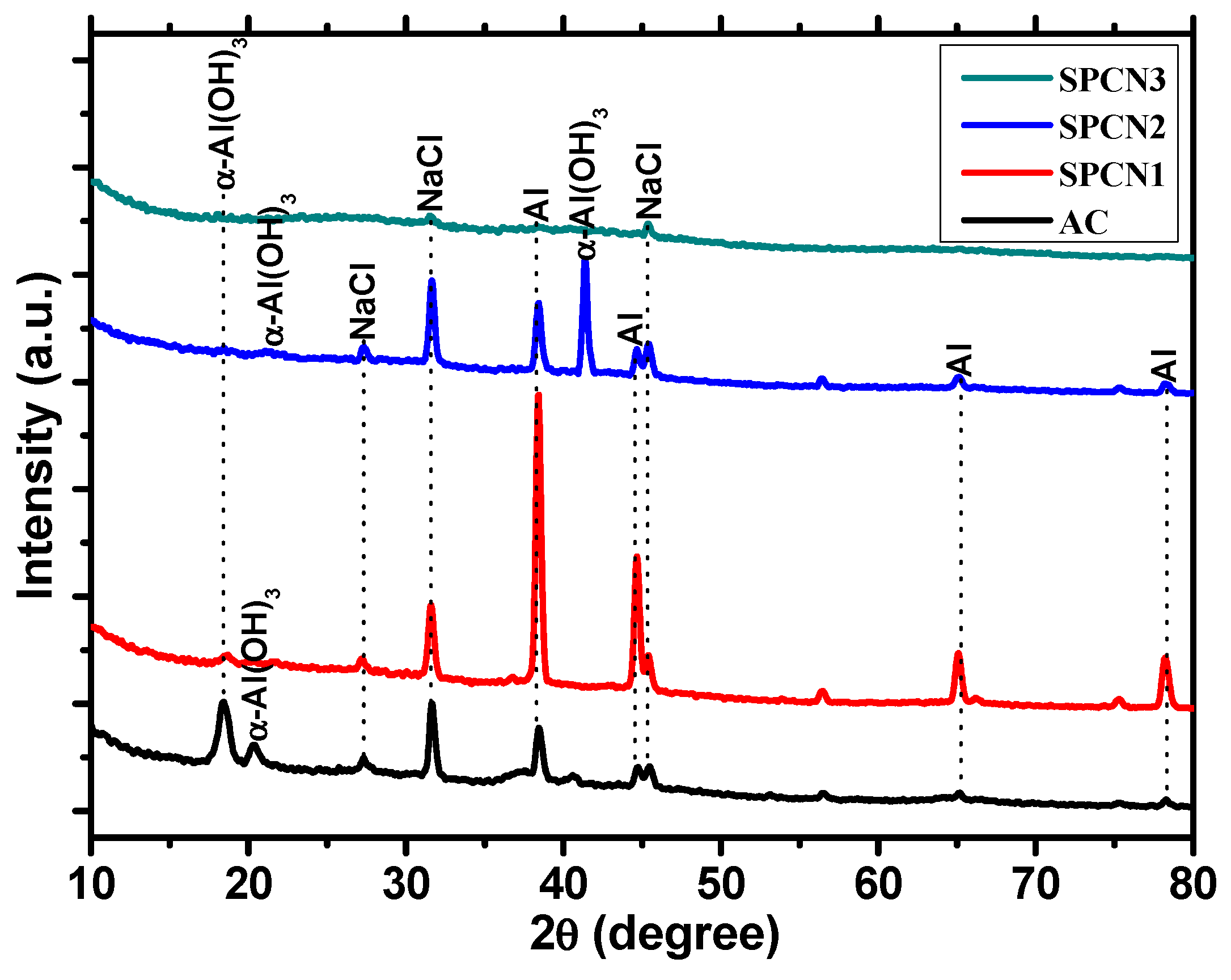
3.1.3. X-ray Diffraction (XRD) of the Coatings
3.2. Corrosion Studies
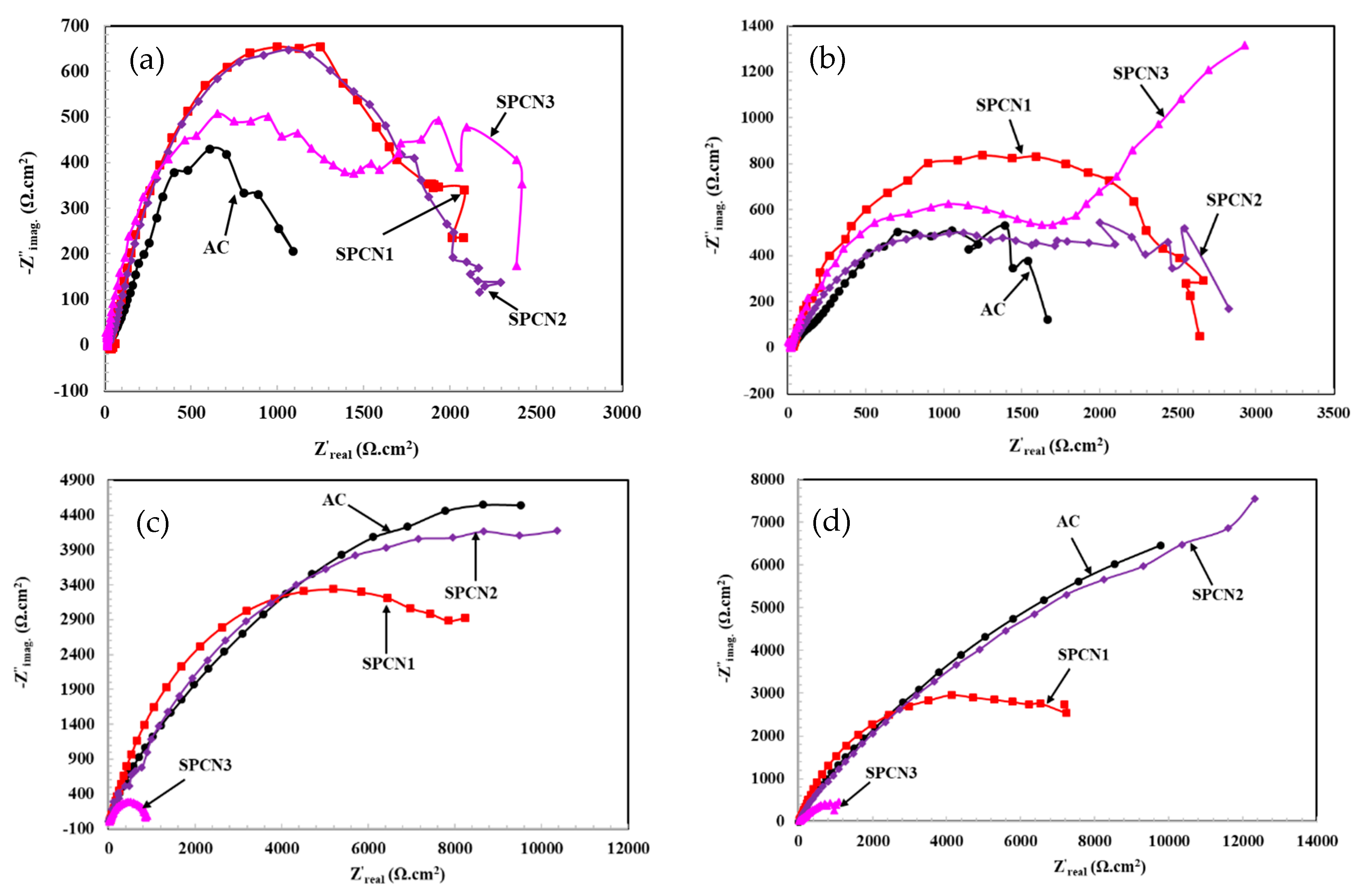
3.2.1. Electrochemical Impedance Spectroscopy (EIS)
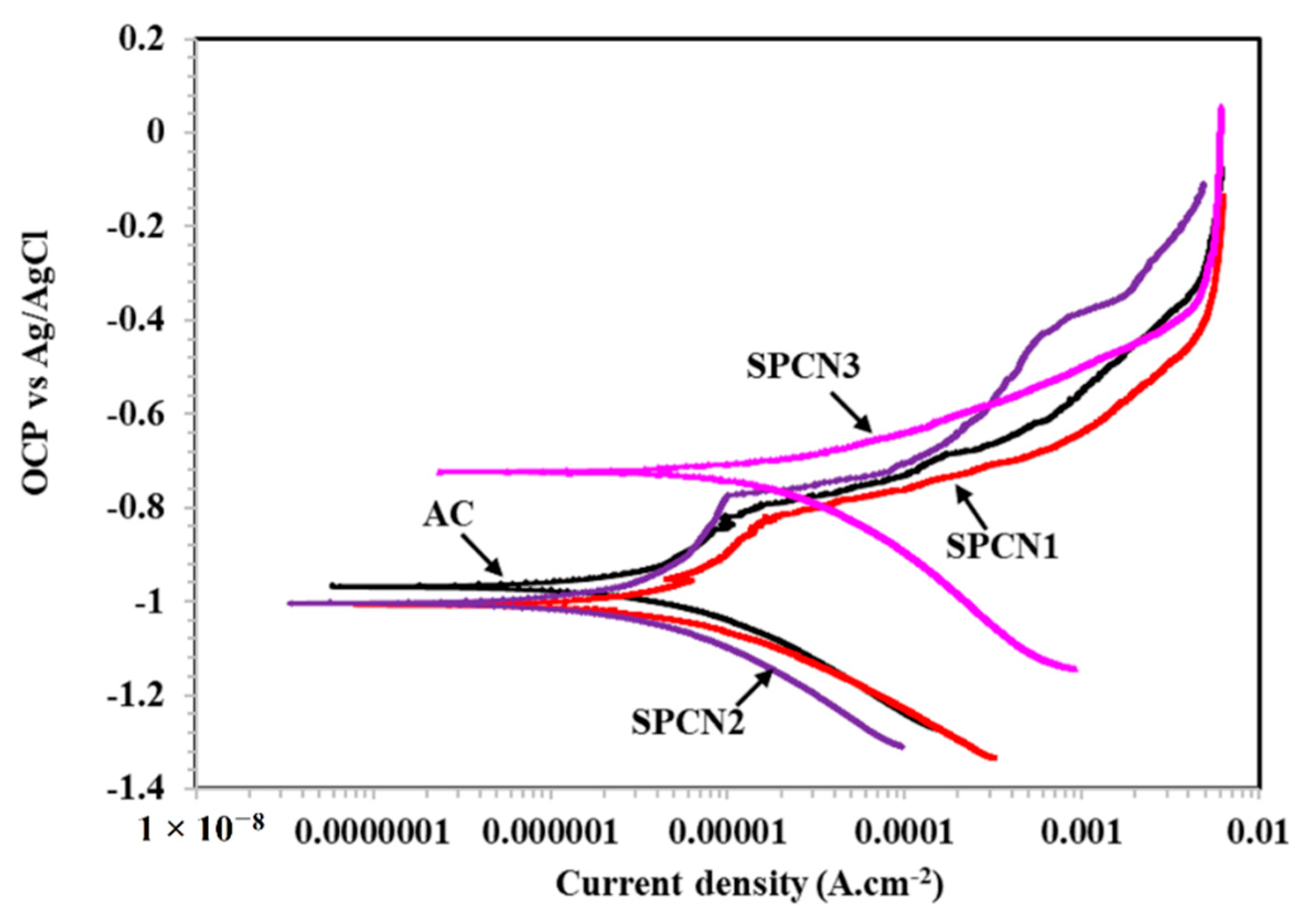
3.2.2. Potentiodynamic Polarization Results
3.3. Characterization of the Corrosion Products
3.3.1. SEM of the Corrosion Products
3.3.2. XRD of Corrosion Products
4. Conclusions
- The treatment of Al coating exhibited reduction in defects by 19%, 38%, and 71% for SPCN1, SPCN2, and SPCN3, respectively compared to AC.
- After treatment with different concentrations of NaH2PO4 and 0.1 M Ca(NO3)2, brushite and sodium Aluminum hydrogen phosphate (SAHP) composite oxide film was formed on the coating surface. Due to the formation of SAHP film on treated coating, it exhibits cracking.
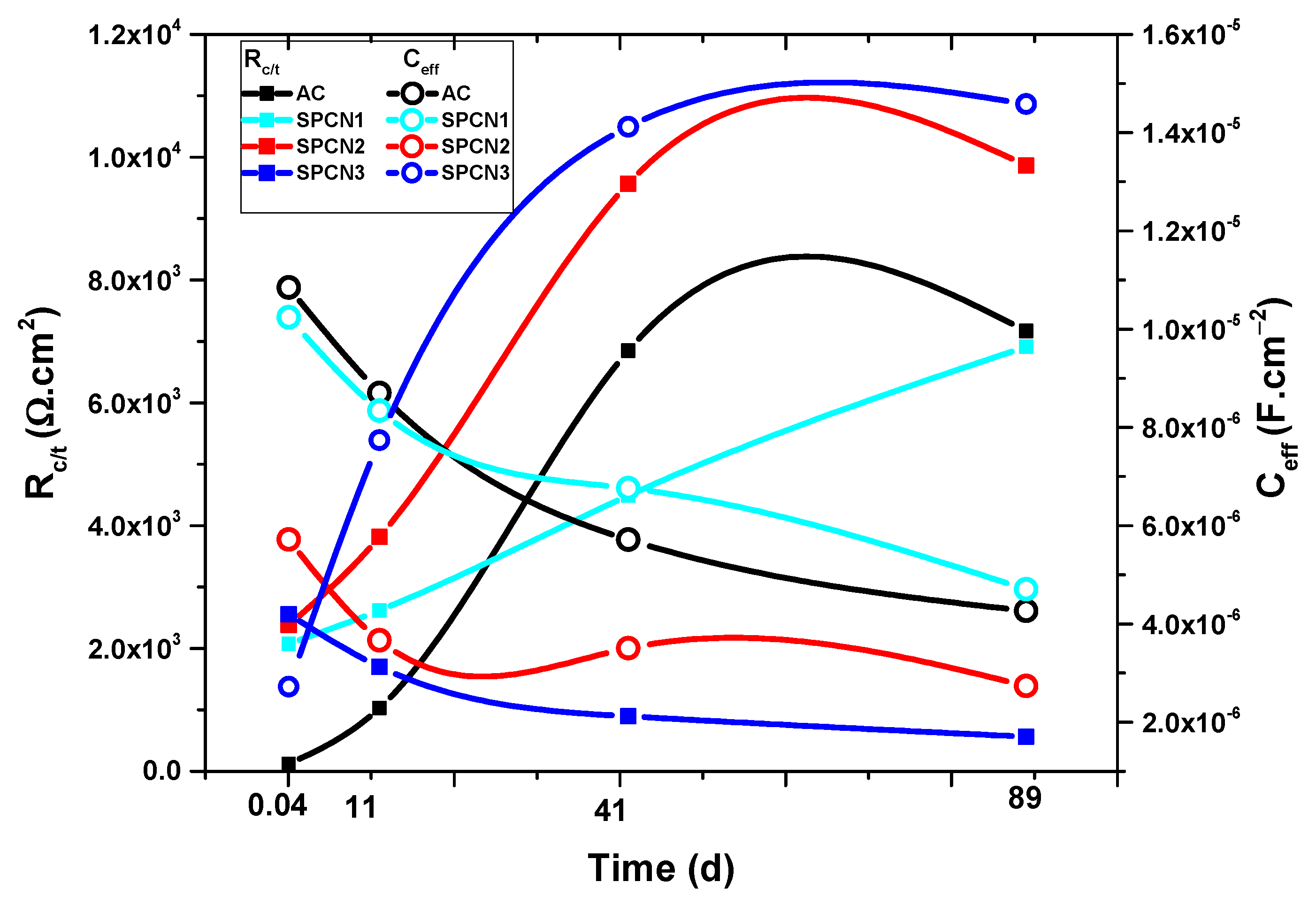
- The EIS results show that initially SPCN3 exhibited highest Rt value followed by SPCN2, SPCN1, and AC, but at longer durations of exposure it shows least in value owing to the dissolution of the treated film. High amounts of SAHP make the solution acidic which enhances the deterioration of the coating in 3.5 wt.% NaCl solution.
- After 89 days of exposure, SPCN2 shows highest Rt and Rct values whereas SPCN3 is the lowest owing to the deposition of stable and protective corrosion products.
- SPCN2 shows lowest corrosion rate whereas SPCN3 is the highest.
- Morphology of corrosion products formed on SPCN2 after 89 days of exposure in 3.5 wt.% NaCl solution show compact and less porous while SPCN3 shows heavy cracking.
- Highest amount of α-Al(OH3)-Bayerite present in corrosion products of SPCN2 after 89 days of exposure in 3.5 wt.% NaCl solution stifle the ingress of solution. Therefore, enhancement in corrosion resistance is observed.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmitt, G.; Schütze, M.; Hays, G.F.; Burn, W.; Han, E.-H.; Pourbaix, A.; Hacobson, G. Global Needs for Knowledge Dissemination, Research, and Development in Materials Deterioration and Corrosion Control; World Corrosion Organization (WCO): New York, NY, USA, 2009. [Google Scholar]
- McCafferty, E. Introduction in Corrosion Science; Springer: New York, NY, USA, 2010. [Google Scholar]
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. Npj Mater. Degrad. 2017, 1, 1–10. [Google Scholar] [CrossRef]
- Qian, Y.; Li, Y.; Jungwirth, S.; Seely, N.; Fang, Y.; Shi, X. The application of anti-corrosion coating for preserving the value of equipment asset in chloride-laden environments: A review. Int. J. Electrochem. Sci. 2015, 10, 10756–10780. [Google Scholar]
- Etzrodt, G. Pigments, inorganic, 5. Anticorrosive pigments. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Weinheim, Germany, 2012; Volume 27, pp. 343–357. [Google Scholar]
- Buxbaum, G.; Pfaff, G. (Eds.) Industrial Inorganic Pigments; Wiley: Weinheim, Germany, 2005. [Google Scholar]
- Zhang, H.B.; Zuo, Y. The improvement of corrosion resistance of Ce conversion films on aluminum alloy by phosphate post-treatment. Appl. Surf. Sci. 2008, 254, 4930–4935. [Google Scholar] [CrossRef]
- Hinton, B.R.W.; Arnott, D.R.; Ryan, N.E. The inhibition of aluminium alloy corrosion by cerous cation. Maters Forum 1984, 7, 211–217. [Google Scholar]
- Hughes, A.E.; Scholes, F.H.; Glenn, A.M.; Lau, D.; Muster, T.H. Factors influencing the deposition of Ce-based conversion coatings, part I: The role of Al3+ ions. Surf. Coat. Technol. 2009, 203, 2927–2936. [Google Scholar] [CrossRef]
- Hou, B.-R.; Zhang, J.-L.; Sun, H.-Y.; Li, Y.; Xiang, B. Corrosion of C–Mn steel in simulated tidal and immersion zones. Br. Corros. J. 2001, 36, 310–312. [Google Scholar] [CrossRef]
- Smith, M.; Bowley, C.; Williams, L. In situ protection of splash zones—30 years on. Mater. Perform. 2002, 41, 30–33. [Google Scholar]
- Wigren, J.; Pejryd, L. Tungsten carbide coatings on jet engine components: Safe aviation. Sulzer Tech. Rev. 2000, 2, 36–39. [Google Scholar]
- Dorfman, M.R.; Erning, U.; Mallon, J. Benefits of clearance control thermal spray coatings: Sealing the gap. Anti Corros. Methods Mater. 2002, 49, 137–140. [Google Scholar] [CrossRef]
- Meuter, P. Protecting pumps against abrasive wear. World Pumps 2006, 482, 18–20. [Google Scholar] [CrossRef]
- Barbezat, G.; Herber, R. Breakthrough in improving car engine performance through coatings. Sulzer Tech. Rev. 2001, 2, 8–11. [Google Scholar]
- Pecoskie, R.P.; Parker, D.W. Coatings protect severe-service ball valves. Adv. Mater. Process. 1993, 144, 22–25. [Google Scholar]
- Muhamad, H.A.M.; Hayati, S.N.; Kiyai, A.S.; Binti, M.S.N. Thermal arc spray overview. Mater. Sci. Eng. 2013, 46, 1–10. [Google Scholar]
- Li, Y.; Liu, J.; Duan, J.; Hou, B. Thermally sprayed aluminium and zinc coatings for tidal zone cathodic protection of offshore platform pile leg. Mater. Perform. 2006, 45, 16–20. [Google Scholar]
- Yung, T.Y.; Chen, T.C.; Tsai, K.C.; Lu, W.F.; Huang, J.Y.; Liu, T.Y. Thermal spray coatings of Al, ZnAl and inconel 625 alloys on SS304L for anti-saline corrosion. Coatings 2019, 9, 32. [Google Scholar] [CrossRef]
- Lee, H.-S.; Singh, J.K.; Ismail, M.A.; Bhattacharya, C. Corrosion resistance properties of aluminum coating applied by arc thermal metal spray in SAE J2334 solution with exposure periods. Metals 2016, 6, 55. [Google Scholar] [CrossRef]
- Lee, H.S.; Singh, J.K.; Park, J.H. Pore blocking characteristics of corrosion products formed on aluminum coating produced by arc thermal metal spray process in 3.5wt.% NaCl solution. Constr. Build. Mater. 2016, 113, 905–916. [Google Scholar] [CrossRef]
- Min-Su, H.A.N.; Yong-Bin, W.O.O.; Seok-Cheol, K.O.; Jeong, Y.-J.; Jang, S.-K.; Kim, S.-J. Effects of thickness of Al thermal spray coating for STS 304. Trans. Nonferrous Met. Soc. China 2009, 19, 925–929. [Google Scholar]
- Jiang, Q.; Miao, Q.; Liang, W.; Ying, F.; Tong, F.; Yi, X.; Ren, B.; Yao, Z.; Zhang, P. Corrosion behavior of arc sprayed Al–Zn–Si–RE coatings on mild steel in 3.5 wt.% NaCl solution. Electrochim. Acta 2014, 115, 644–656. [Google Scholar] [CrossRef]
- Persson, D.; Thierry, D.; LeBozec, N. Corrosion product formation on Zn55Al coated steel upon exposure in a marine atmosphere. Corros. Sci. 2011, 53, 720–726. [Google Scholar] [CrossRef]
- Kim, S.-J.; Jeong, J.-Y.; Kim, J.-I. Investigation of optimum materials selection in thermal spray coating for the corrosion protection of STS 304. J. Ceram. Process. Res. 2007, 8, 296–299. [Google Scholar]
- Lee, H.-S.; Kwon, S.-J.; Singh, J.K.; Ismail, M.A. Influence of Zn and Mg alloying on the corrosion resistance properties of Al coating applied by arc thermal spray process in simulated weather solution. Acta Metall. Sin. (Engl. Lett.) 2018, 31, 591–603. [Google Scholar] [CrossRef]
- Lee, H.-S.; Singh, J.K.; Ismail, M.A.; Bhattacharya, C.; Seikh, A.H.; Alharthi, N.; Hussain, R.R. Corrosion mechanism and kinetics of Al–Zn coating deposited by arc thermal spraying process in saline solution at prolong exposure periods. Sci. Rep. 2019, 9, 3399. [Google Scholar] [CrossRef] [PubMed]
- Tailor, S.; Modi, A.; Modi, S.C. Synthesis, microstructural, corrosion and antimicrobial properties of Zn and Zn-Al coatings. Surf. Eng. 2019, 35, 736–742. [Google Scholar] [CrossRef]
- Heidersbach, R. Metallurgy and Corrosion Control in Oil and Gas Production, 1st ed.; Willey: Hoboken, NJ, USA, 2011. [Google Scholar]
- NORSOK. M-501, Surface Preparation and Protective Coating; Standards Norway: Oslo, Norway, 2012. [Google Scholar]
- Fauchais, P.; Vardelle, A. Thermal sprayed coatings used against corrosion and corrosive wear. In Advanced Plasma Spray Applications, 1st ed.; Jazi, H.S., Ed.; Intech: Rijeka, Croatia, 2012; pp. 1–38. [Google Scholar]
- Ramezanzadeh, B.; Akbarian, M.; Ramezanzadeh, M.; Mahdavian, M.; Alibakhshi, E.; Kardarar, P. Corrosion protection of steel with zinc phosphate conversion coating and post-treatment by hybrid organic-inorganic sol-gel based silane film. J. Electrochem. Soc. 2017, 164, C224–C230. [Google Scholar] [CrossRef]
- Lee, H.S.; Singh, J.K.; Ismail, M.A. An effective and novel pore sealing agent to enhance the corrosion resistance performance of Al coating in artificial ocean water. Sci. Rep. 2017, 7, 41935. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Singh, J.K. Influence of calcium nitrate on morphology and corrosion characteristics of ammonium phosphate treated aluminum coating deposited by arc thermal spraying process. Corros. Sci. 2019, 146, 254–268. [Google Scholar] [CrossRef]
- Jeong, H.-R.; Lee, H.-S.; Jalalzai, P.; Kwon, S.-J.; Singh, J.K.; Hussain, R.R.; Alyousef, R.; Alabduljabbar, H.; Aslam, F. Sodium phosphate post-treatment on Al coating: Morphological and corrosion study. J Therm. Spray Technol. 2019, 28, 1511–1531. [Google Scholar] [CrossRef]
- Pawlowski, L. The Science and Engineering of Thermal Spray Coating; Wiley: New York, NY, USA, 1995. [Google Scholar]
- Guenbour, A.; Benbachir, A.; Kacemi, A. Evaluation of the corrosion performance of zinc-phosphate-painted carbon steel. Surf. Coat. Technol. 1999, 113, 36–43. [Google Scholar] [CrossRef]
- Cinca, N.; Lima, C.R.C.; Guilemany, J.M. An overview of intermetallics research and application: Status of thermal spray coatings. J. Mater. Res. Technol. 2013, 2, 75–86. [Google Scholar] [CrossRef]
- Bettridge, D.F.; Ubank, R.G. Quality control of high-temperature protective coatings. Mater. Sci. Technol. 1986, 2, 232–242. [Google Scholar] [CrossRef]
- Steffens, H.D.; Babiak, Z.; Wewel, M. Recent developments in arc spraying. IEEE Trans. Plasma Sci. 1990, 18, 974–975. [Google Scholar] [CrossRef]
- Muhamad, H.A.M.; Hayati, S.N.; Kiyai, A.S.; Binti, M.S.N. Critical process and performance parameter of thermal arc spray coatings. Int. J. Mater. Eng. Innov. 2014, 5, 12–27. [Google Scholar]
- Davis, J.R. Surface Engineering for Corrosion and Wear Resistance; ASM International USA, Materials Park: Cleveland, OH, USA, 2001. [Google Scholar]
- KS F4716, Cement Filling Compound for Surface Preparation; Korean Agency for Technology and Standards (KATS): Seoul, Korea, 2001.
- Natarajan, U.V.; Rajeswari, S. Influence of calcium precursors on the morphology and crystallinity of sol-gel-derived hydroxyapatite nanoparticles. J. Korean Cryst. Growth Cryst. Technol. 2008, 310, 4601–4611. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Chen, R.; Zhang, F.; Han, E.-H. A novel phosphate conversion film on Mg–8.8Li alloy. Surf. Coat. Technol. 2009, 203, 1107–1113. [Google Scholar] [CrossRef]
- Brown, P.W. Phase relationships in the ternary system CaO-P2O5-H2O at 25 °C. J. Am. Ceram. Soc. 1992, 75, 17–22. [Google Scholar] [CrossRef]
- Dale, E.A.; Butler, K.H.; Thomas, M.J.B. Process for making brushite form of calcum hydrogen phosphate. U.S. Patent 3505012, 7 April 1970. [Google Scholar]
- Lii, K.-H.; Wang, S.-L. Na3M(OH)(HPO4)(PO4), M5Al, Ga: Two phosphates with a chain structure. J. Solid State Chem. 1997, 128, 21–26. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Lagno, F.; Demopoulos, G.P. The stability of hydrated Aluminium phosphate, AlPO4·1.5H2O. Environ. Technol. 2006, 27, 1217–1224. [Google Scholar] [CrossRef]
- Menon, M.; Parulkar, B.G.; Drach, G.W. Urinary Lithiasis, Etiology, Diagnosis, and Medical Management, Campbell’s Urology, 7th ed.; W.B. Saunders Co: London, UK, 1998; pp. 2661–2733. [Google Scholar]
- Xiong, Q.Y.; Zhou, Y.; Xiong, J.P. The study of a phosphate conversion coating on magnesium alloy AZ91D: II. Effects of components and their content in phosphating bath. Int. J. Electrochem. Sci. 2015, 10, 8454–8464. [Google Scholar]
- Dong, K.; Song, Y.; Shan, D.; Han, E.-H. Corrosion behavior of a self-sealing pore micro-arc oxidation film on AM60 magnesium alloy. Corros. Sci. 2015, 100, 275–283. [Google Scholar] [CrossRef]
- Yu, X.; Cao, C. Electrochemical study of the corrosion behavior of Ce sealing of anodized 2024 aluminum alloy. Thin Solid Films 2003, 423, 252–256. [Google Scholar] [CrossRef]
- Yu, X.; Yan, C.; Cao, C. Corrosion behavior of the rare earth sealing anodized coating on aluminum alloy LY12. J. Mater. Sci. Technol. 2002, 18, 471–474. [Google Scholar]
- Tao, Y.; Xiong, T.; Sun, C.; Kong, L.; Cui, X.; Li, T.; Song, G.L. Microstructure and corrosion performance of a cold sprayed aluminium coating on AZ91D magnesium alloy. Corros. Sci. 2010, 52, 3191–3197. [Google Scholar] [CrossRef]
- Ma, Y.; Li, N.; Li, D.; Zhang, M.; Huang, X. Characteristics and corrosion studies of vanadate conversion coating formed on Mg–14 wt.% Li–1 wt.% Al–0.1 wt.% Ce alloy. Appl. Surf. Sci. 2012, 261, 59–67. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Serra, R.; Montemor, M.F.; Yasakau, K.A.; Salvado, I.M.M.; Ferreira, M.G.S. Nanostructured sol-gel coatings doped with cerium nitrate as pretreatments for AA2024-T3 Corrosion protection performance. Electrochim. Acta 2005, 51, 208–217. [Google Scholar] [CrossRef]
- Fontinha, I.R.; Salta, M.M.; Zheludkevich, M.L.; Ferreira, M.G.S. EIS study of amine cured epoxy-silica-zirconia sol-gel coatings for corrosion protection of the aluminium alloy EN AW 6063. Port. Electrochim. Acta 2013, 31, 307–319. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Shchukin, D.G.; Yasakau, K.A.; Mo¨hwald, H.; Ferreira, M.G.S. Anticorrosion coatings with self-healing effect based on nanocontainers impregnated with corrosion inhibitor. Chem. Mater. 2007, 19, 402–411. [Google Scholar] [CrossRef]
- Kumar, C.S.; Rao, V.S.; Raja, V.S.; Sharma, A.K.; Mayanna, S.M. Corrosion behavior of solar reflector coatings on AA 2024T3—An electrochemical impedance spectroscopy study. Corros. Sci. 2002, 44, 387–393. [Google Scholar] [CrossRef]
- Hitzig, J.; Juttner, K.; Lorenz, W.J.; Paatsch, W. AC-impedance measurements on porous aluminium oxide films. Corros. Sci. 1984, 24, 945–952. [Google Scholar] [CrossRef]
- Mansfeld, F.; Kendig, M.W. Evaluation of anodized aluminum surfaces with electrochemical impedance spectroscopy. J. Electrochem. Soc. 1988, 135, 828–833. [Google Scholar] [CrossRef]
- Orazem, M.E.; Pébère, N.; Tribollet, B. Enhanced graphical representation of electrochemical impedance data. J. Electrochem. Soc. 2006, 153, B129–B136. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Huang, V.M.-W.; Vivier, V.; Orazem, M.E.; Pébère, N.; Tribollet, B. The apparent constant-phase-element behavior of a disk electrode with faradaic reactions: A global and local impedance analysis. J. Electrochem. Soc. 2007, 154, C99–C107. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Park, I.C.; Kim, S.J. Electrochemical characteristics in seawater for cold thermal spray-coated Al–Mg alloy layer. Acta Metall. Sin. (Engl. Lett.) 2016, 29, 727–734. [Google Scholar] [CrossRef]
- Dean, S.W. Electrochemical methods of corrosion testing. In Electrochemical Techniques for Corrosion; Baboian, R., Ed.; NACE: Houston, TX, USA, 1977. [Google Scholar]
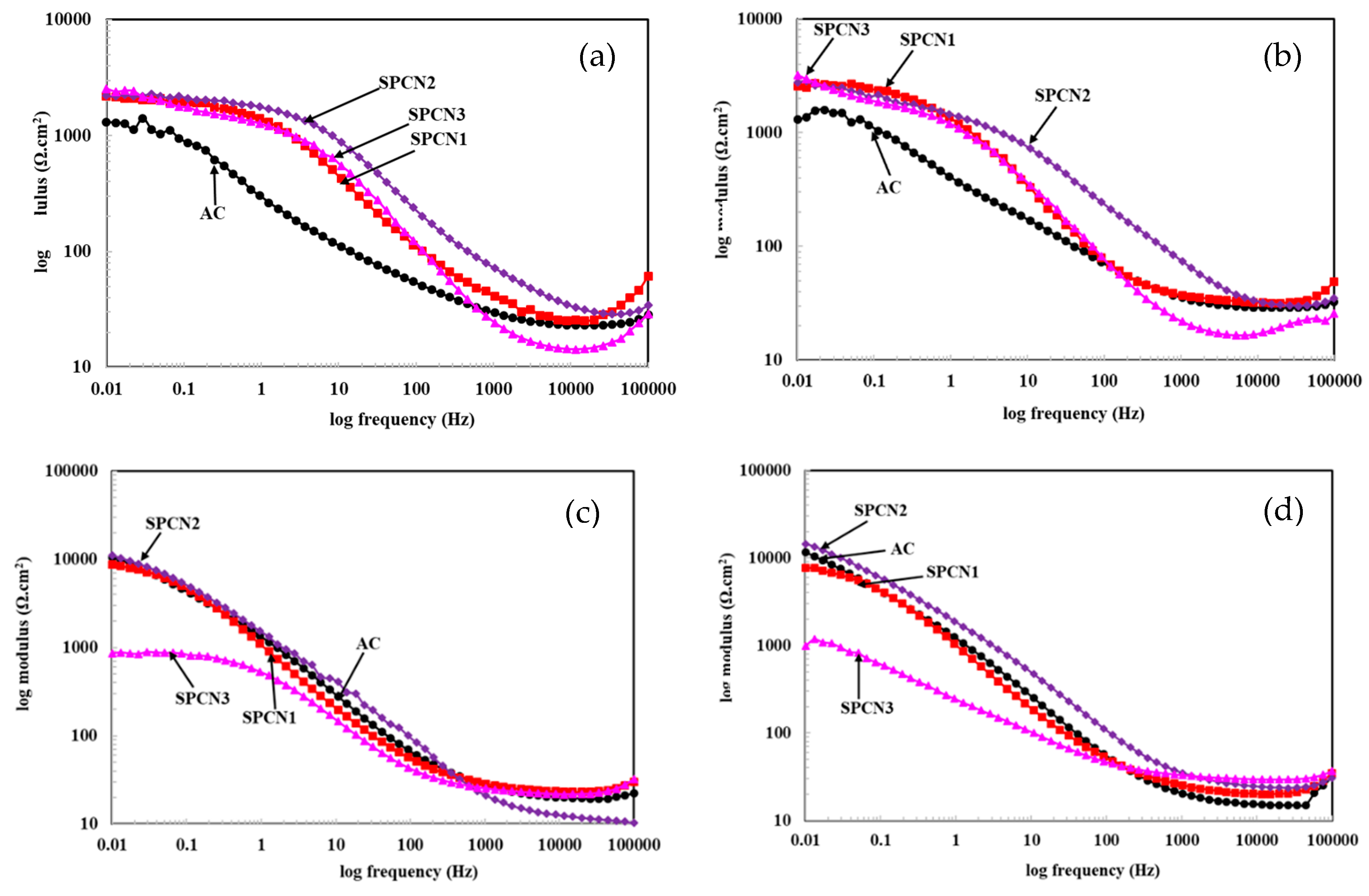
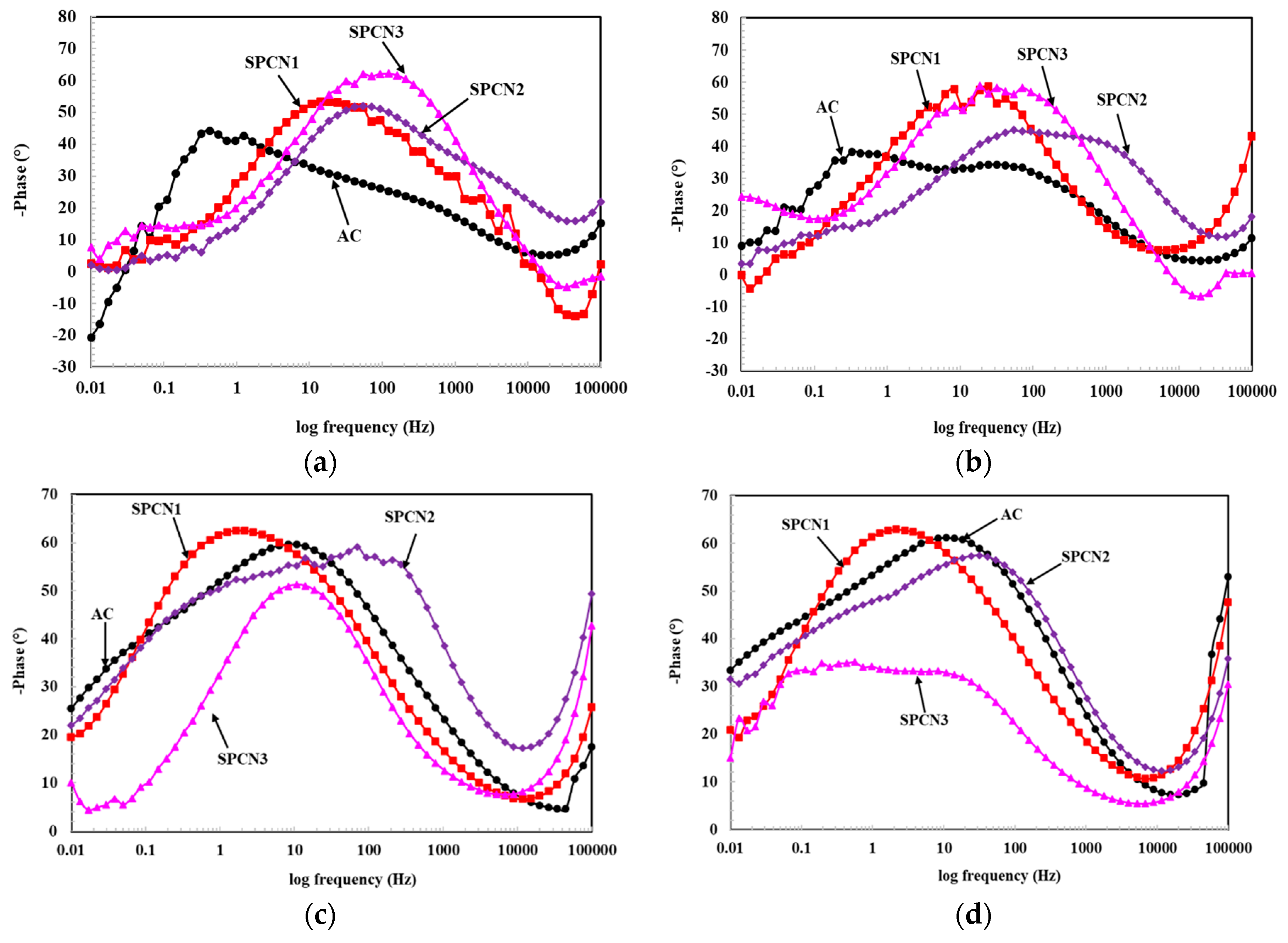
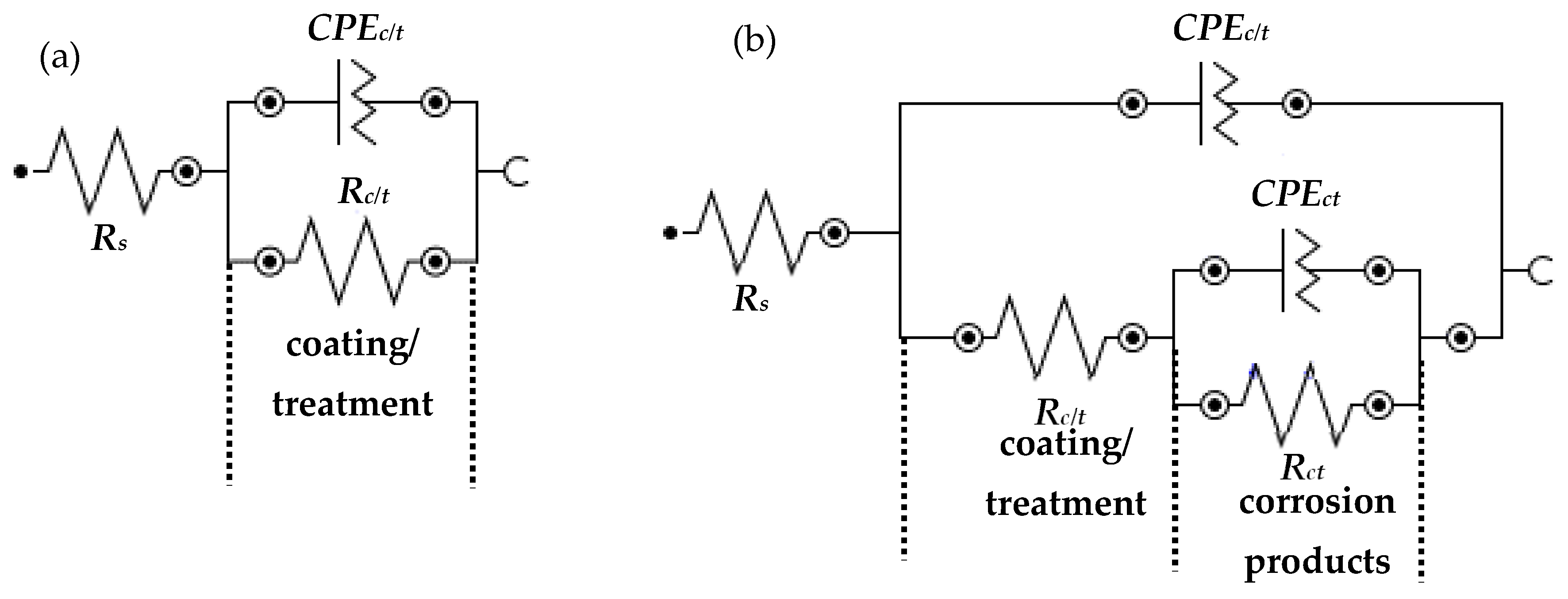

| Sample ID | Elements (wt.%) | |||||
|---|---|---|---|---|---|---|
| N | O | Na | P | Ca | Al | |
| AC | – | 2.20 | – | – | – | Rest |
| SPCN1 | 1.35 | 14.38 | 0.99 | 8.84 | 7.71 | Rest |
| SPCN2 | 1.44 | 20.77 | 1.51 | 13.16 | 7.89 | Rest |
| SPCN3 | 1.37 | 36.89 | 7.26 | 25.80 | 7.95 | Rest |
| Sample ID | Volume Fraction (%) | ||
|---|---|---|---|
| Al | BR | SAHP | |
| AC | 100 | 0 | 0 |
| SPCN1 | 89.26 | 3.68 | 7.06 |
| SPCN2 | 80.41 | 11.14 | 8.45 |
| SPCN3 | 71.95 | 19.18 | 8.87 |
| Sample ID | Time | Electrochemical Parameters | |||||
|---|---|---|---|---|---|---|---|
| Rs (Ω·cm2) | CPEc/t | Rct (kΩ·cm2) | CPEct | ||||
| Qc/t (1 × 10−5) (Ω−1·cm−2·s−n) | nc/t | Qct (1 × 10−5) (Ω−1·cm−2·s−n) | nct | ||||
| AC | 1 h | 23.15 | 27.5 | 0.61 | 0.49 | 51.9 | 0.65 |
| SPCN1 | 29.00 | 12.7 | 0.69 | – | – | – | |
| SPCN2 | 27.24 | 7.27 | 0.71 | – | – | – | |
| SPCN3 | 23.79 | 4.05 | 0.72 | 0.53 | 39.8 | 0.67 | |
| AC | 11 days | 27.79 | 12.5 | 0.68 | 0.51 | 44.6 | 0.67 |
| SPCN1 | 31.62 | 9.1 | 0.71 | – | – | – | |
| SPCN2 | 25.29 | 4.1 | 0.74 | 0.11 | 54.7 | 0.65 | |
| SPCN3 | 16.02 | 11.5 | 0.7 | 1.48 | 37.6 | 0.68 | |
| AC | 41 days | 19.41 | 6.1 | 0.74 | 3.68 | 21.1 | 0.77 |
| SPCN1 | 24.43 | 7.1 | 0.73 | 4.25 | 16.8 | 0.77 | |
| SPCN2 | 9.01 | 3.8 | 0.77 | 1.50 | 23.6 | 0.69 | |
| SPCN3 | 21.98 | 20.3 | 0.67 | 0.25 | 39.0 | 0.60 | |
| AC | 89 days | 18.82 | 4.1 | 0.76 | 4.54 | 15.5 | 0.78 |
| SPCN1 | 20.65 | 5.2 | 0.74 | 0.75 | 29.1 | 0.67 | |
| SPCN2 | 22.4 | 2.1 | 0.79 | 4.57 | 16.5 | 0.79 | |
| SPCN3 | 29.8 | 21.9 | 0.65 | 0.29 | 38.8 | 0.60 | |
| Sample ID | Electrochemical Parameters | ||
|---|---|---|---|
| Ecorr (V) vs. Ag/AgCl | Icorr (µA·cm−2) | CR (µm·year−1) | |
| AC | −0.957 | 3.99 | 43.49 |
| SPCN1 | −1.003 | 4.85 | 52.87 |
| SPCN2 | −1.005 | 2.79 | 30.41 |
| SPCN3 | −0.722 | 19.13 | 208.52 |
| Sample ID | Elements (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| N | O | Na | P | Ca | Cl | Al | |
| AC | – | 48.85 | 1.50 | – | – | 7.95 | Rest |
| SPCN1 | 1.29 | 46.89 | 1.93 | 3.25 | 0.13 | 11.56 | Rest |
| SPCN2 | 1.31 | 42.69 | 2.73 | 7.61 | 1.80 | 9.76 | Rest |
| SPCN3 | 1.24 | 47.14 | 1.40 | 0.95 | 0.24 | 13.65 | Rest |
| Sample ID | Volume Fraction (%) | ||
|---|---|---|---|
| Al | NaCl | α-Al(OH)3 | |
| AC | 28.06 | 32.28 | 39.66 |
| SPCN1 | 48.12 | 23.48 | 28.40 |
| SPCN2 | 30.25 | 25.80 | 43.95 |
| SPCN3 | 35.29 | 37.03 | 27.68 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-S.; Kumar, A.; Mandal, S.; Singh, J.K.; ASLAM, F.; Alyousef, R.; Albduljabbar, H. Effect of Sodium Phosphate and Calcium Nitrate Sealing Treatment on Microstructure and Corrosion Resistance of Wire Arc Sprayed Aluminum Coatings. Coatings 2020, 10, 33. https://doi.org/10.3390/coatings10010033
Lee H-S, Kumar A, Mandal S, Singh JK, ASLAM F, Alyousef R, Albduljabbar H. Effect of Sodium Phosphate and Calcium Nitrate Sealing Treatment on Microstructure and Corrosion Resistance of Wire Arc Sprayed Aluminum Coatings. Coatings. 2020; 10(1):33. https://doi.org/10.3390/coatings10010033
Chicago/Turabian StyleLee, Han-Seung, Ashutosh Kumar, Soumen Mandal, Jitendra Kumar Singh, Fahid ASLAM, Rayed Alyousef, and Hisham Albduljabbar. 2020. "Effect of Sodium Phosphate and Calcium Nitrate Sealing Treatment on Microstructure and Corrosion Resistance of Wire Arc Sprayed Aluminum Coatings" Coatings 10, no. 1: 33. https://doi.org/10.3390/coatings10010033
APA StyleLee, H.-S., Kumar, A., Mandal, S., Singh, J. K., ASLAM, F., Alyousef, R., & Albduljabbar, H. (2020). Effect of Sodium Phosphate and Calcium Nitrate Sealing Treatment on Microstructure and Corrosion Resistance of Wire Arc Sprayed Aluminum Coatings. Coatings, 10(1), 33. https://doi.org/10.3390/coatings10010033








