Preparation and Photocatalytic Property of Ag Modified Titanium Dioxide Exposed High Energy Crystal Plane (001)
Abstract
1. Introduction
2. Experimental
2.1. Preparation of High Energy Crystal Surface TiO2
2.2. Preparation of Ag-TiO2
2.3. Evaluation of Photocatalytic Performance
2.4. Characterization
3. Results and Discussion
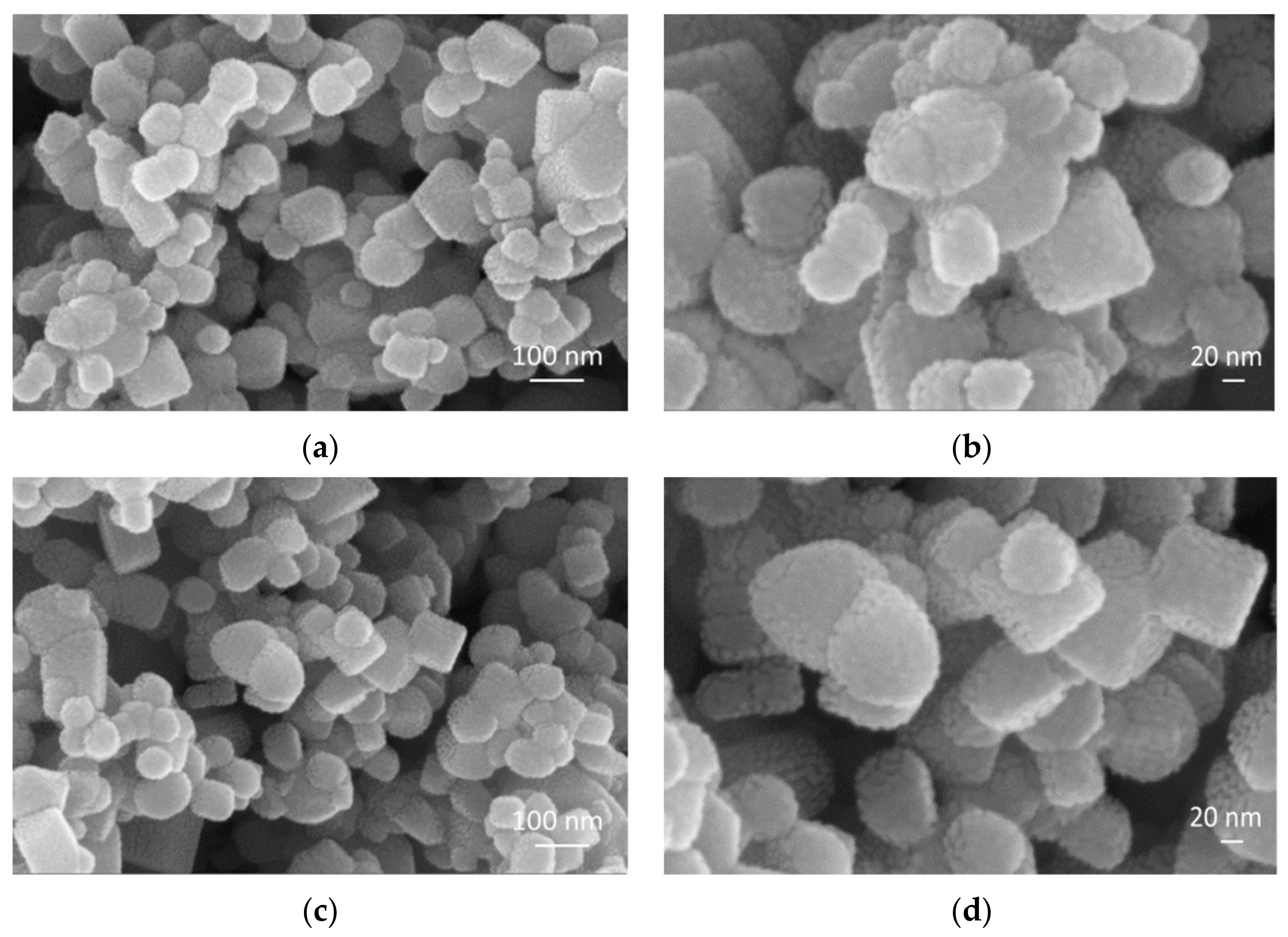
3.1. FESEM Analysis
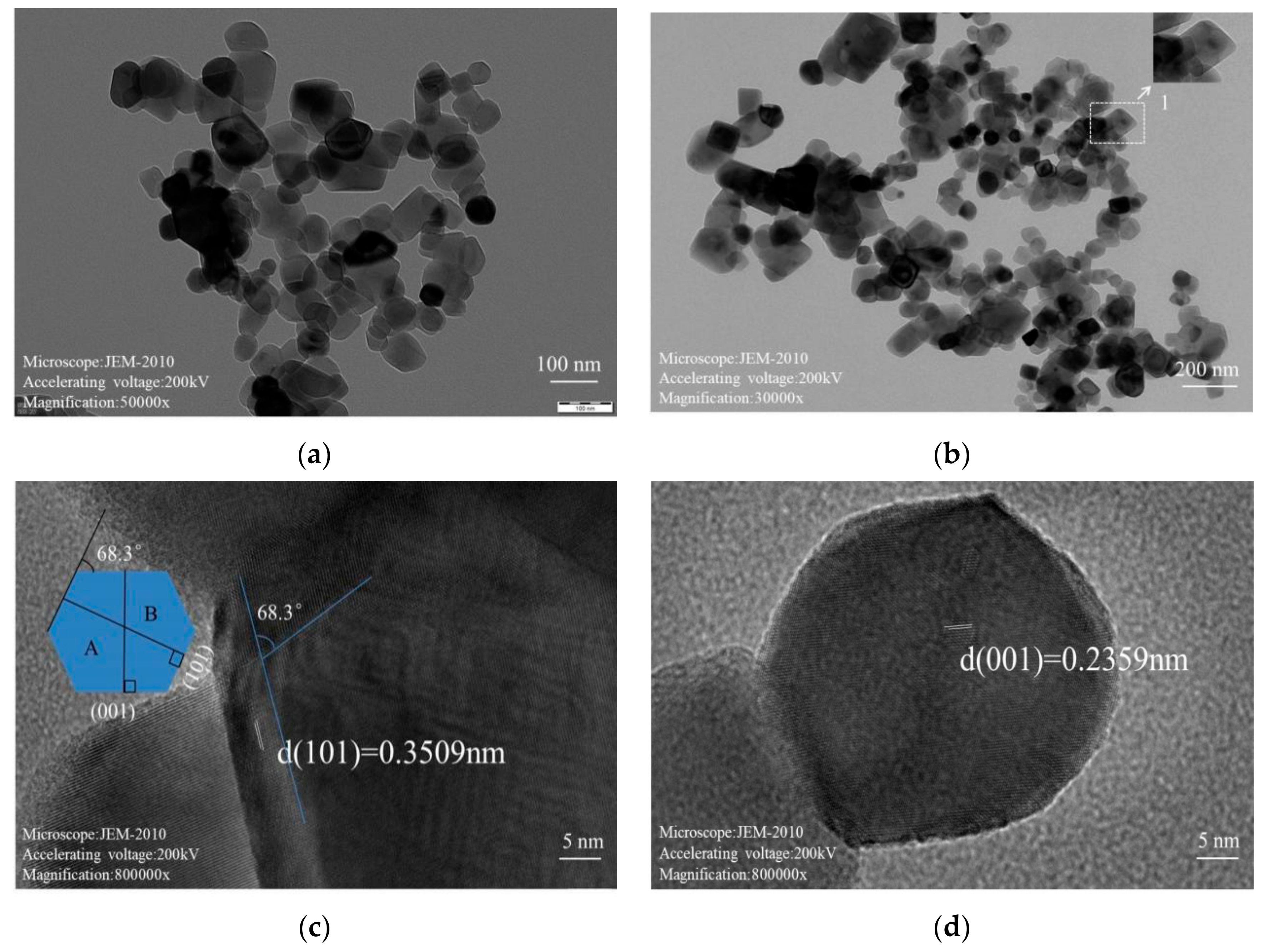
3.2. TEM Analysis
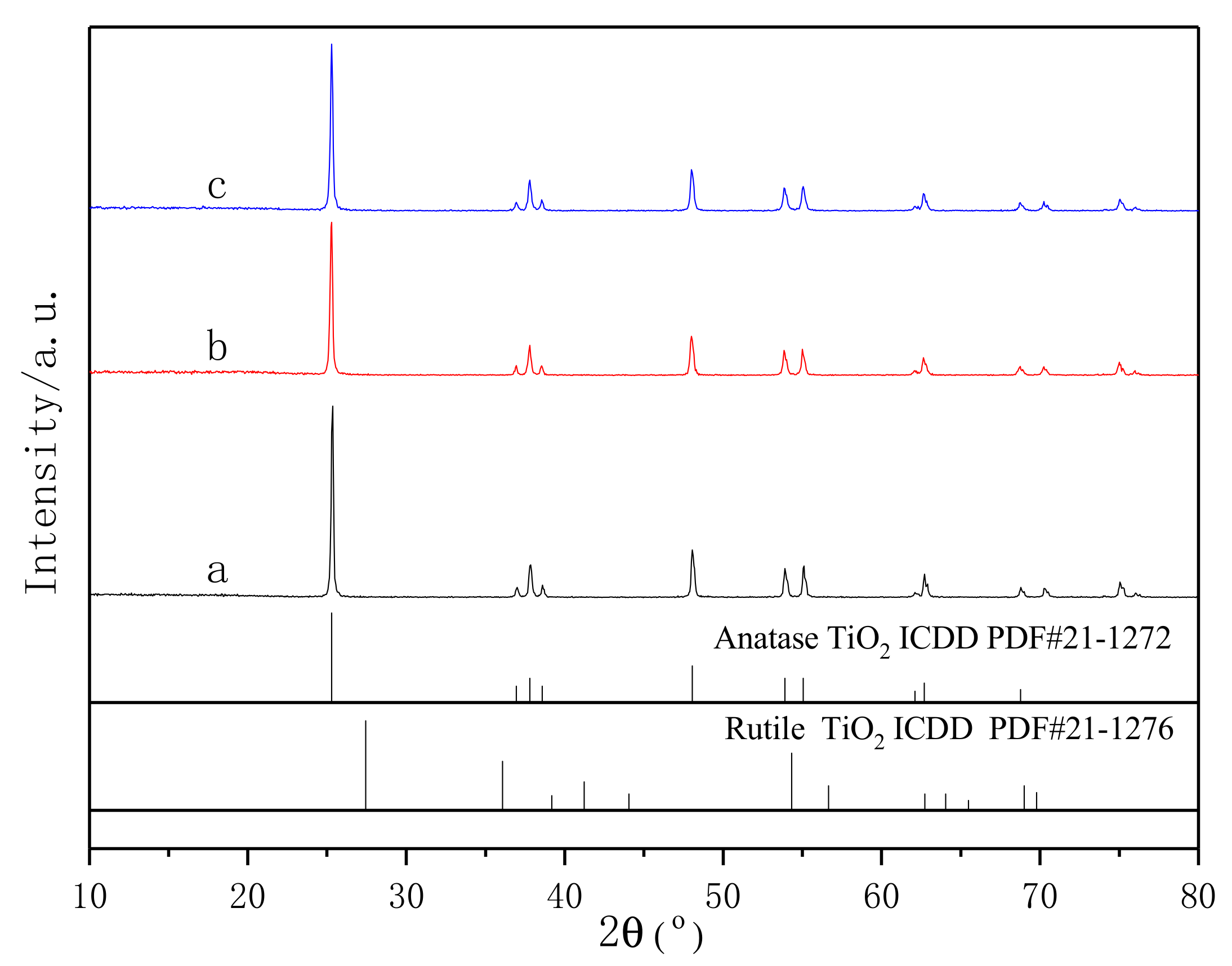
3.3. XRD Analysis
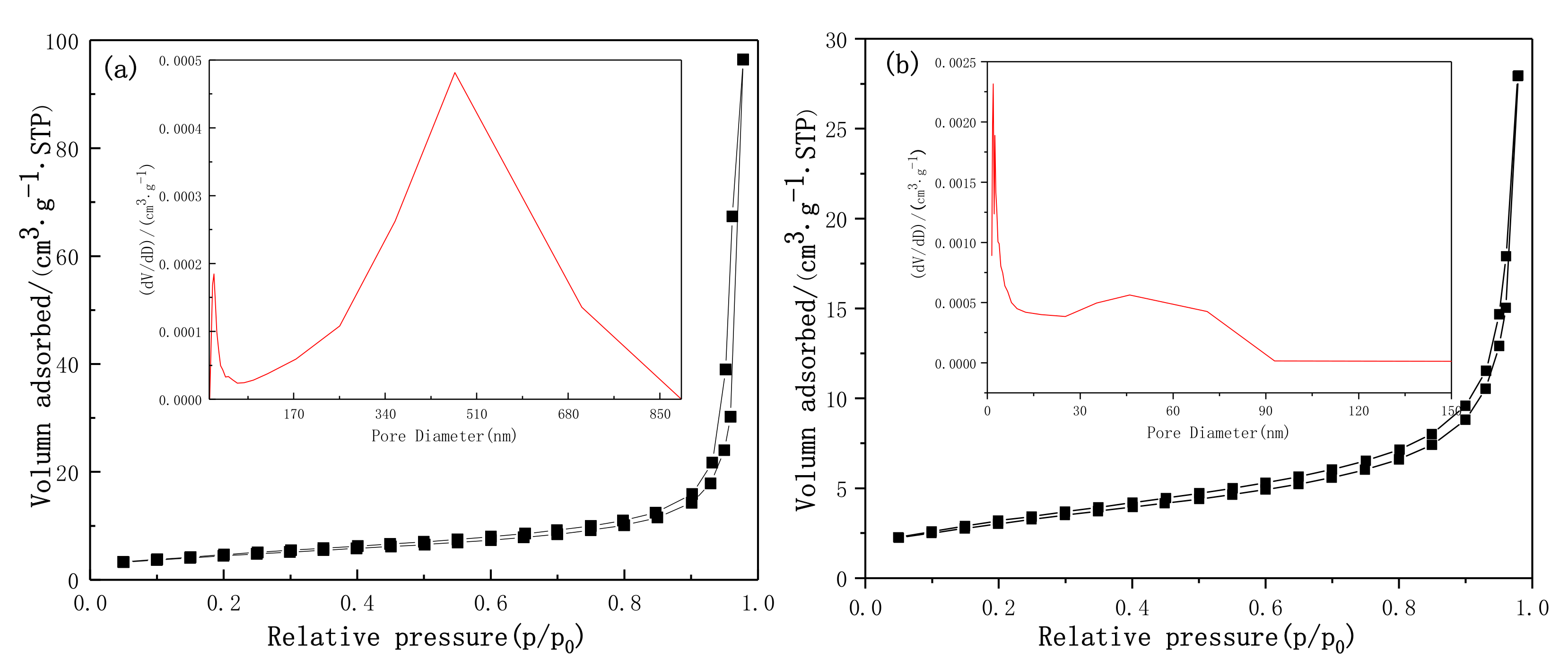
3.4. BET Analysis
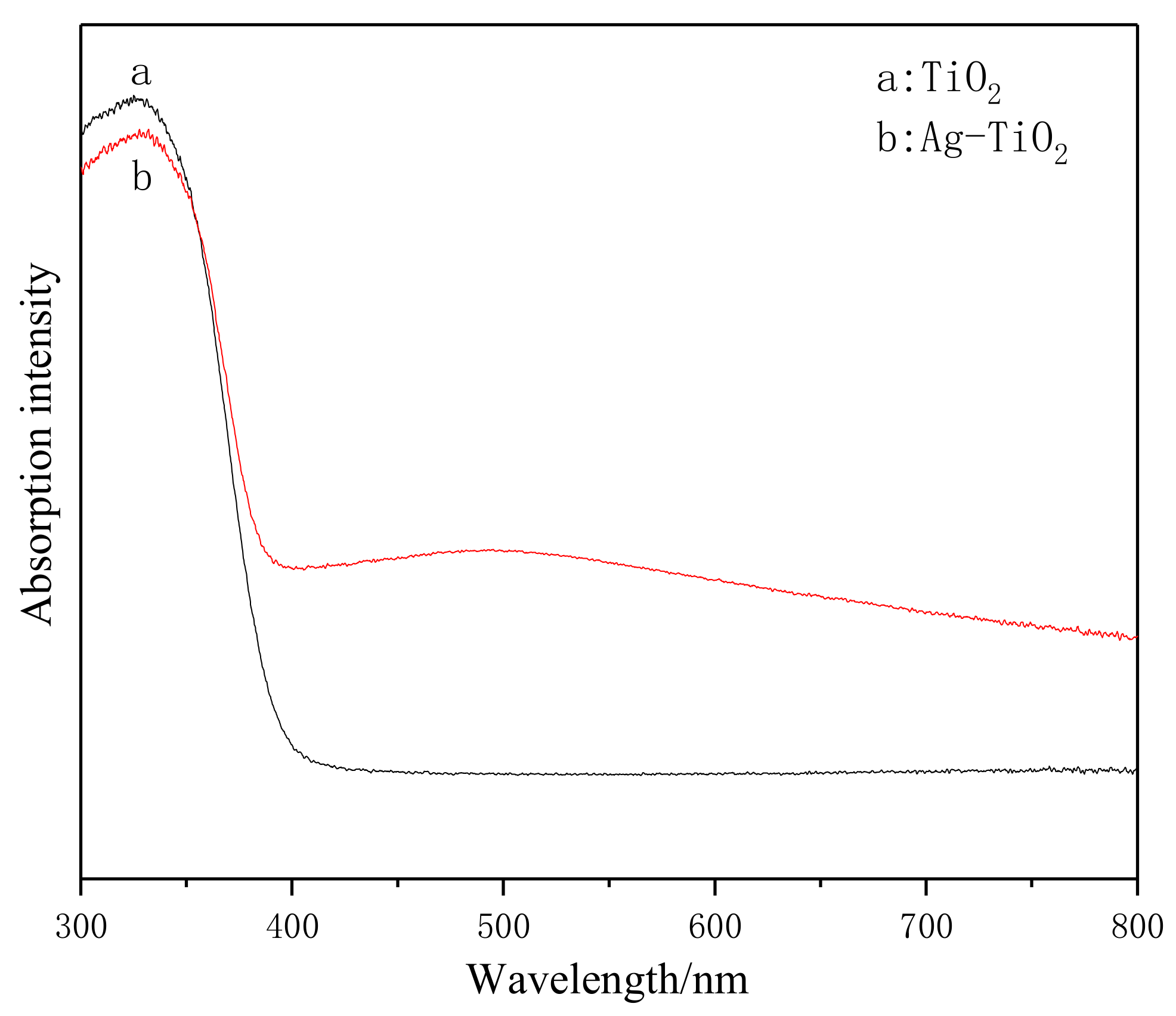
3.5. UV-Vis-Abs Analysis
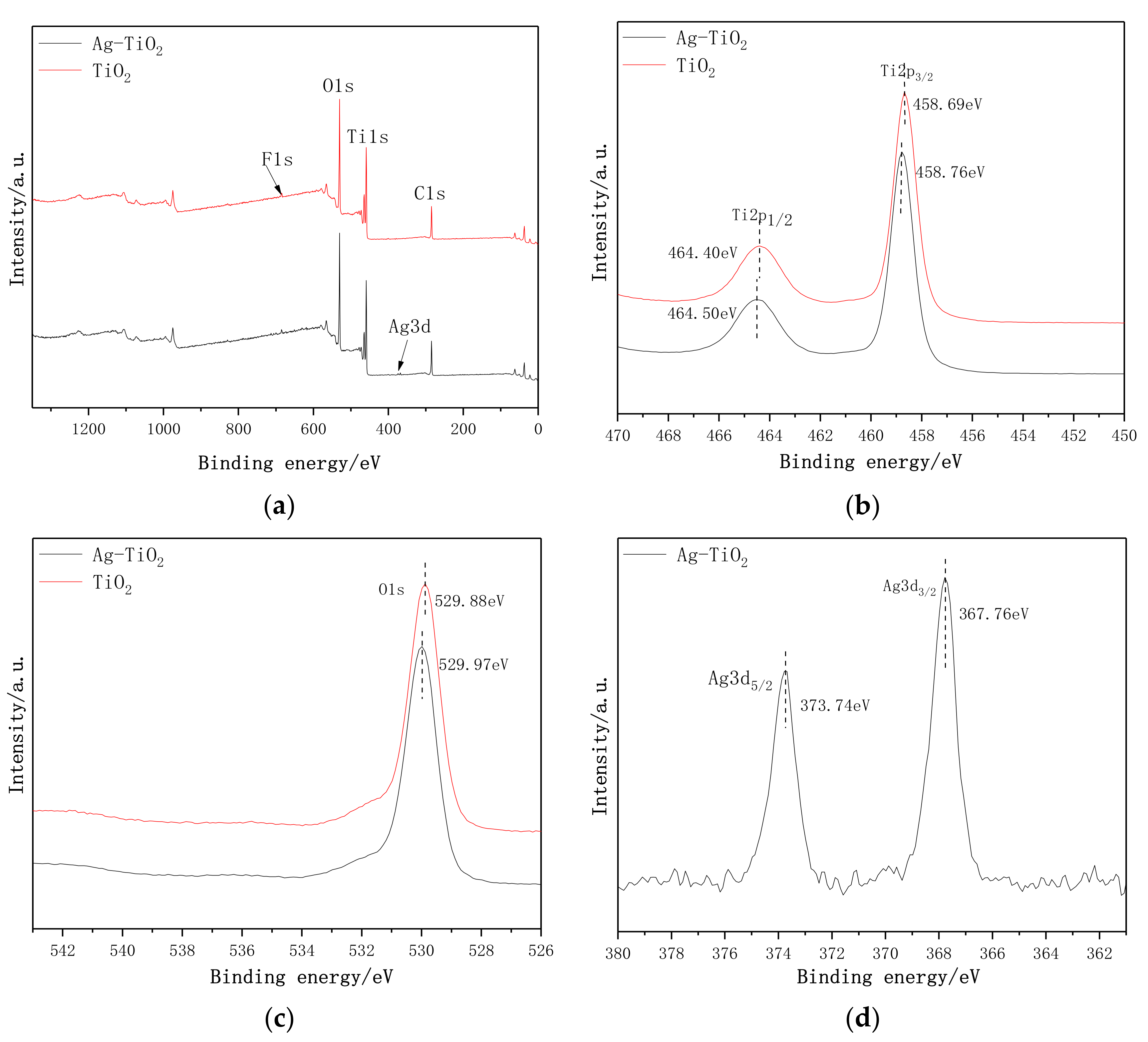
3.6. XPS Analysis
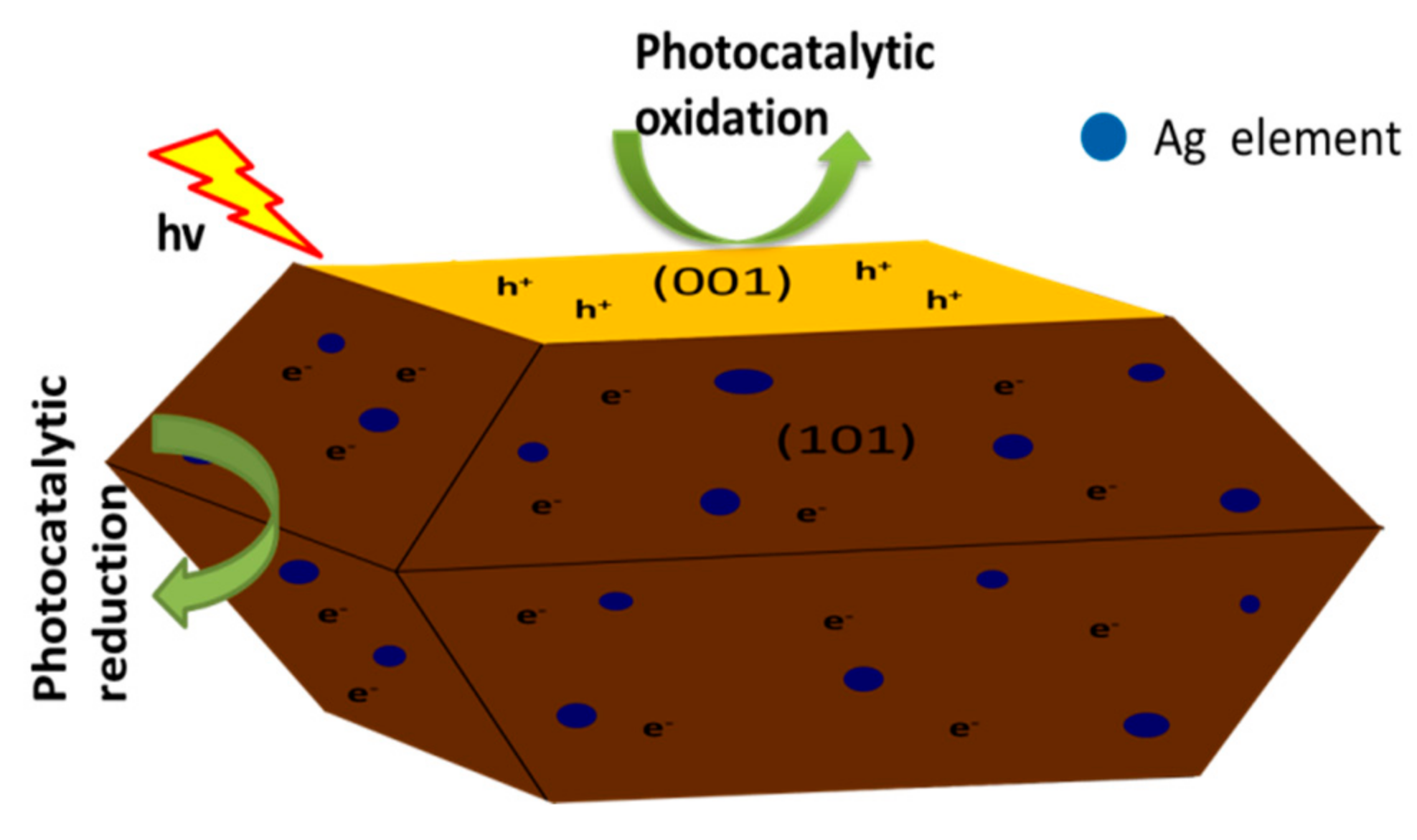
3.7. Photocatalytic Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yan, Q.; Huang, G.F.; Li, D.F.; Zhang, M.; Pari, A.L.; Huang, W.Q. Facile synthesis and superior photocatalytic and electrocatalytic performances of porous B-doped g-C3N4 nanosheets. J. Mater. Sci. Technol. 2018, 34, 2515–2520. [Google Scholar] [CrossRef]
- Aris, M.; Agus, P. Removal efficiency of nitrite and sulfide pollutants by electrochemical process by using Ti/RuIrO2 anode. Indones. J. Chem. 2018, 18, 286–293. [Google Scholar]
- Trellu, C.; Oturan, N.; Pechaud, Y.; Hullebush, E.D.V.; Esposito, G.; Outran, M.A. Anodic oxidation of surfactants and organic compounds entrapped in micelles-selective degradation mechanisms and soil washing solution reuse. Water Res. 2017, 118, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Boltersdorf, J.; Sullivan, I.; Shelton, T.L.; Wu, Z.K.; Gray, M.; Zoellner, B.; Osterloh, F.E.; Maggard, P.A. Flux synthesis optical and photocatalytic properties of n-type Sn2TiO4: Hydrogen and oxygen evolution under visible light. Chem. Mater. 2016, 28, 8876–8889. [Google Scholar] [CrossRef]
- Hernandez-Alonso, M.D.; Fresno, F.; Suarez, S.; Coronado, M. Development of alternative photocatalysts to TiO2: Challenges and opportunities. Energy Environ. Sci. 2009, 2, 1231–1257. [Google Scholar] [CrossRef]
- Wang, T.; Costan, J.; Centeno, A.; Pang, J.S.; Darvill, D.; Ryan, M.P.; Xie, F. Broad and enhanced fluorescence using zinc-oxide nanoflower arrays. J. Mater. Chem. 2015, 3, 2656–2663. [Google Scholar]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.L. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef]
- Hosseinpour, Z.; Alemi, A.; Khandar, A.A. A controlled solvothermal synthesis of cus hierarchical structures and their natural-light-induced photocatalytic properties. New J. Chem. 2015, 39, 5470–5476. [Google Scholar] [CrossRef]
- Qi, K.Z.; Liu, S.Y.; Qiu, M. Photocatalytic performance of TiO2 nanocrystal with/without oxygen defects. Chin. J. Catal. 2018, 39, 867–875. [Google Scholar] [CrossRef]
- Low, J.X.; Chen, B.; Yu, J.G. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Wei, S.H.; Ni, S.; Xu, X.X. A new approach to inducing Ti3+ in anatase TiO2 for efficient photocatalytic hydrogen production. Chin. J. Catal. 2018, 39, 510–516. [Google Scholar] [CrossRef]
- Liu, F.; Feng, N.D.; Wang, Q. Transfer channel of photoinduced hole on TiO2 surface as revealed by solid-state nmr and esr spectroscopy. J. Am. Chem. Soc. 2017, 139, 10020–10028. [Google Scholar] [CrossRef]
- Moreau, M.M.L.; Granja, L.P.; Fuertes, M.C.; Martinez, E.D.; Ferrari, V.; Levy, P.E.; Soler-lllia, G.J.A.A. Three-dimensional electrochemical lithography in mesoporous TiO2 thin films. J. Phys. Chem. 2015, 119, 28954–28960. [Google Scholar] [CrossRef]
- Hoffman, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Li, F.L.; Cai, H.Q.; Chen, L.S. The study of antioxidation and reducing ability of polysaccharides from pogonatherum crinitum. J. Org. Chem. Res. 2015, 3, 16–24. [Google Scholar] [CrossRef]
- Saharudin, K.A.; Sreekantan, S.; Basiron, N.; Khor, Y.L.; Harun, N.H.; Mydin, R.B.S.M.N.; Adki, H.M.; Seeni, A.; Vignesh, K. Bacteriostatic activity of lldpe nanocomposite embedded with sol-gel synthesized TiO2/ZnO coupled oxides at various ratios. Polymers 2018, 10, 878. [Google Scholar] [CrossRef]
- Shen, W.; Nitta, A.; Chen, Z.; Eda, T.; Yoshida, A.; Nation, S.C. NOx storage and reduction over potassium titanate nanobelt-based catalyst with high storage capacity. J. Catal. 2011, 280, 161–167. [Google Scholar] [CrossRef]
- Buchalska, M.; Kobielusz, M.; Matuszek, A.; Pacia, M.; Wojtyla, S.; Macyk, W. On oxygen activation at rutile- and anatase-TiO2. ACS Catal. 2015, 5, 7424–7431. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, Y.P.; Zhang, J.; Pan, C.X. Raman spectroscopy: A new approach to measure the percentage of anatase TiO2 exposed (001) facets. J. Phys. Chem. 2012, 116, 7515–7519. [Google Scholar] [CrossRef]
- Kumari, R.; Majumdar, J.D. Heat-treated TiO2 plasma spray deposition for bioactivity improvement in ti-6Al-4V Alloy. J. Mater. Eng. Perform. 2017, 26, 6207–6218. [Google Scholar] [CrossRef]
- Moitzheim, S.; Balder, J.E.; Poodt, P.; Unnikrishnan, S.; Gendt, S.D.; Vereecken, P.M. Chlorine doping of amorphous TiO2 for increased capacity and faster Li+-Ion storage. Chem. Mater. 2017, 29, 10007–10018. [Google Scholar] [CrossRef]
- Du, J.M.; Zhang, J.L.; Liu, Z.M.; Han, B.X.; Jiang, T.; Huang, Y. Controlled synthesis of Ag/TiO2 core−shell nanowires with smooth and bristled surfaces via a one-step solution route. Langmuir 2006, 22, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, R.G.; Xu, Q.; Han, H.X.; Li, C. Roles of (001) and (101) facets of anatase TiO2 in photocatalytic reactions. Acta Phys. Chim. Sin. 2013, 29, 1566–1571. [Google Scholar]
- Luo, T.; Wan, X.J.; Jiang, S.X.; Zhang, L.Y.; Hong, Z.Q.; Liu, J. Preparation and photocatalytic performance of fibrous Tb3+-doped TiO2 using collagen fiber as template. Appl. Phys. Mater. Sci. Process. 2018, 124, 304. [Google Scholar] [CrossRef]
- Shi, Y.L.; Sun, H.J.; Nguyen, M.C.; Wang, A.Z.; Ho, K.M.; Saidi, W.A.; Zhao, J. Structures of defects on anatase TiO2 (001) surfaces. Nanoscale 2017, 9, 11553–11565. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Xu, J.Y.; Shen, H.; Lu, B.L. Etching behavior on (001) face of relaxor ferroelectric crystal pznt. J. Synth. Cryst. 2011, 40, 334–337. [Google Scholar]
- Hong, X.X.; Kang, Y.Y.; Zhen, C.; Kang, X.D.; Yin, L.C.; Wang, L.Z.; Irvine, J.T.S.; Liu, G.; Cheng, H.M. Maximizing the visible light photo electron chemical activity of B/N-doped anatase TiO2 microspheres with exposed dominant (001) facets. Sci. Chin. Mater. 2018, 61, 831–838. [Google Scholar] [CrossRef]
- Jia, X.W.; Wang, M. Surface photocatalytic research of Fe-doped TiO2 (001) based on the first-principles. IOP Conf. Ser. Mater. Sci. Eng. 2018, 392, 32–40. [Google Scholar] [CrossRef]
- Liu, S.W.; Yu, J.G.; Jaroniec, M. Anatase TiO2 with dominant high-energy {001} facets: Synthesis, properties, and applications. Chem. Mater. 2011, 23, 4085–4093. [Google Scholar] [CrossRef]
- Yu, J.G.; Qi, L.F.; Jaroniec, M. Hydrogen production by photocatalytic water splitting over Pt/TiO2 nanosheets with exposed (001) facets. J. Phys. Chem. 2010, 114, 13118–13125. [Google Scholar]
- Liu, S.W.; Yu, J.G.; Jaroniec, M. Tunable photocatalytic selectivity of hollow TiO2 microspheres composed of anatase polyhedra with exposed {001} facets. J. Am. Chem. Soc. 2010, 132, 11914–11916. [Google Scholar] [CrossRef]
- Liu, M.; Li, H.M.; Zeng, Y.G.; Huang, T.C. Anatase TiO2 single crystals with dominant (001) facets: Facile fabrication from Ti powders and enhanced photocatalytical activity. Appl. Sur. Sci. 2013, 274, 117–123. [Google Scholar] [CrossRef]
- Yu, L.Q.; Dong, K.T.; Yang, C.; Wang, Q.Q.; Hou, Y.L. Facile synthesis and dehydrogenation properties of Fe3B nanoalloys. Mater. Lett. 2014, 132, 4–7. [Google Scholar] [CrossRef]
- Tan, L.L.; Ong, W.J.; Chai, S.P.; Mohamed, A.R. Noble metal modified reduced graphene oxide/TiO2 ternary nanostructures for efficient visible-light-driven photoreduction of carbon dioxide into methane. Appl. Catal. Environ. 2015, 166, 251–259. [Google Scholar] [CrossRef]
- Hotsenpiller, P.A.M.; Bolt, J.D.; Farneth, W.E.; Lowekamp, J.B.; Rohrer, G.S. Orientation dependence of photochemical reactions on TiO2 thin film surfaces. Abstr. Pap. Am. Chem. Soc. 1998, 216, 747. [Google Scholar]
- Yang, X.L.; Wang, Y.; Xu, X.; Ding, X.; Chen, H. Surface plasmon resonance-induced visible-light photocatalytic performance of silver/silver molybdate composites. Chin. J. Catal. 2017, 38, 260–269. [Google Scholar] [CrossRef]
- Xin, B.F.; Jing, L.Q.; Ren, Z.Y.; Wang, B.Q.; Fu, H.G. Effects of simultaneously doped and deposited Ag on the photocatalytic activity and surface states of TiO2. J. Phys. Chem. 2005, 109, 2805–2809. [Google Scholar] [CrossRef]
- Liu, H.; Liu, T.T.; Dong, X.N. Preparation and photocatalytic activity of noble metal ag selectively loading on high energy facet of TiO2 single crystal. J. Shanxi Univ. Sci. Technol. 2016, 34, 40–45. [Google Scholar]
- Zhang, Y.; Li, L.D.; Liu, H.Y. Photocatalytic reduction activity of {001} TiO2 codoped with F and Fe under visible light for bromate removal. J. Nanomater. 2016, 2016, 7. [Google Scholar] [CrossRef]
- Wang, J.Y.; Liu, B.S.; Nakata, K. Effects of crystallinity, {001}/{101} ratio, and Au decoration on the photocatalytic activity of anatase TiO2 crystals. Chin. J. Catal. 2019, 40, 403–412. [Google Scholar] [CrossRef]
- Howard, C.J.; Sabine, T.M.; Dickson, F. Structural and thermal parameters for rutile and anatase. Acta Crystal. 1991, 47, 462–468. [Google Scholar] [CrossRef]
- Bae, Y.S.; Yazaydın, A.O.; Snurr, R.Q. Evaluation of the BET method for determining surface areas of MOFs and zeolites that contain ultra-micropores. Langmuir 2010, 26, 5475–5483. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.C.; Ji, Z.Y.; Zou, W.X.; Gu, X.R.; Deng, Y.; Gao, F.; Tang, C.J.; Dong, L. In site loading transition metal oxide clusters on TiO2 nanosheets as Co-catalysts for exceptional high photoactivity. ACS Catal. 2013, 3, 2052–2061. [Google Scholar] [CrossRef]
- Zhong, M.; Wei, Z.H.; Si, P.Z.; Li, H.; Wei, G.Y.; Han, G.R.; Ge, H.L. Preparation of iron-doped titania nanocrystalline grains and its photocatalytic property. J. Chin. Ceram. Soc. 2010, 38, 68–73. [Google Scholar]
- Zhang, Y.Y.; Gu, D.; Zhu, L.Y. Highly ordered Fe3+/TiO2 nanotube arrays for efficient photocatalytic degradation of nitrobenzene. Appl. Surf. Sci. 2017, 420, 896–904. [Google Scholar] [CrossRef]
- Yu, J.G.; Ma, T.T.; Liu, S.W. Enhanced photocatalytic activity of mesoporous TiO2 aggregates by embedding carbon nanotbes as electron-transfer channel. Phys. Chem. Chem. Phys. 2011, 13, 3491–3501. [Google Scholar] [CrossRef]


| Samples | Crystallite Size D(101)/nm | TEM Particle Size/nm |
|---|---|---|
| Ag-TiO2 | 38.97 | – |
| TiO2 | 41.44 | 80 |
| Nano-TiO2 | 45.64 | – |
| Samples | Surface Area (m2/g) | Pore Volume (cc/g) | Pore Diameter Dv(d)(nm) |
|---|---|---|---|
| TiO2 | 18.590 | 0.150 | 469.386 |
| Ag-TiO2 | 12.570 | 0.044 | 1.936 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.-Y.; You, J.; Li, Q.-W.; Dong, Z.-H.; Zhong, Y.-J.; Han, Y.-L.; You, Y.-H. Preparation and Photocatalytic Property of Ag Modified Titanium Dioxide Exposed High Energy Crystal Plane (001). Coatings 2020, 10, 27. https://doi.org/10.3390/coatings10010027
Zhang L-Y, You J, Li Q-W, Dong Z-H, Zhong Y-J, Han Y-L, You Y-H. Preparation and Photocatalytic Property of Ag Modified Titanium Dioxide Exposed High Energy Crystal Plane (001). Coatings. 2020; 10(1):27. https://doi.org/10.3390/coatings10010027
Chicago/Turabian StyleZhang, Li-Yuan, Jia You, Qian-Wen Li, Zhi-Hong Dong, Ya-Jie Zhong, Yan-Lin Han, and Yao-Hui You. 2020. "Preparation and Photocatalytic Property of Ag Modified Titanium Dioxide Exposed High Energy Crystal Plane (001)" Coatings 10, no. 1: 27. https://doi.org/10.3390/coatings10010027
APA StyleZhang, L.-Y., You, J., Li, Q.-W., Dong, Z.-H., Zhong, Y.-J., Han, Y.-L., & You, Y.-H. (2020). Preparation and Photocatalytic Property of Ag Modified Titanium Dioxide Exposed High Energy Crystal Plane (001). Coatings, 10(1), 27. https://doi.org/10.3390/coatings10010027





