Correlation between Defect Density and Corrosion Parameter of Electrochemically Oxidized Aluminum
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
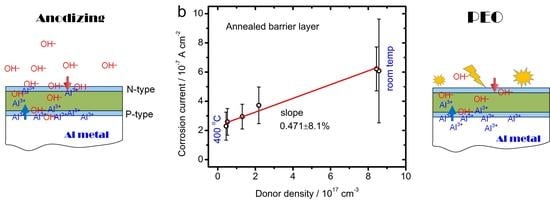
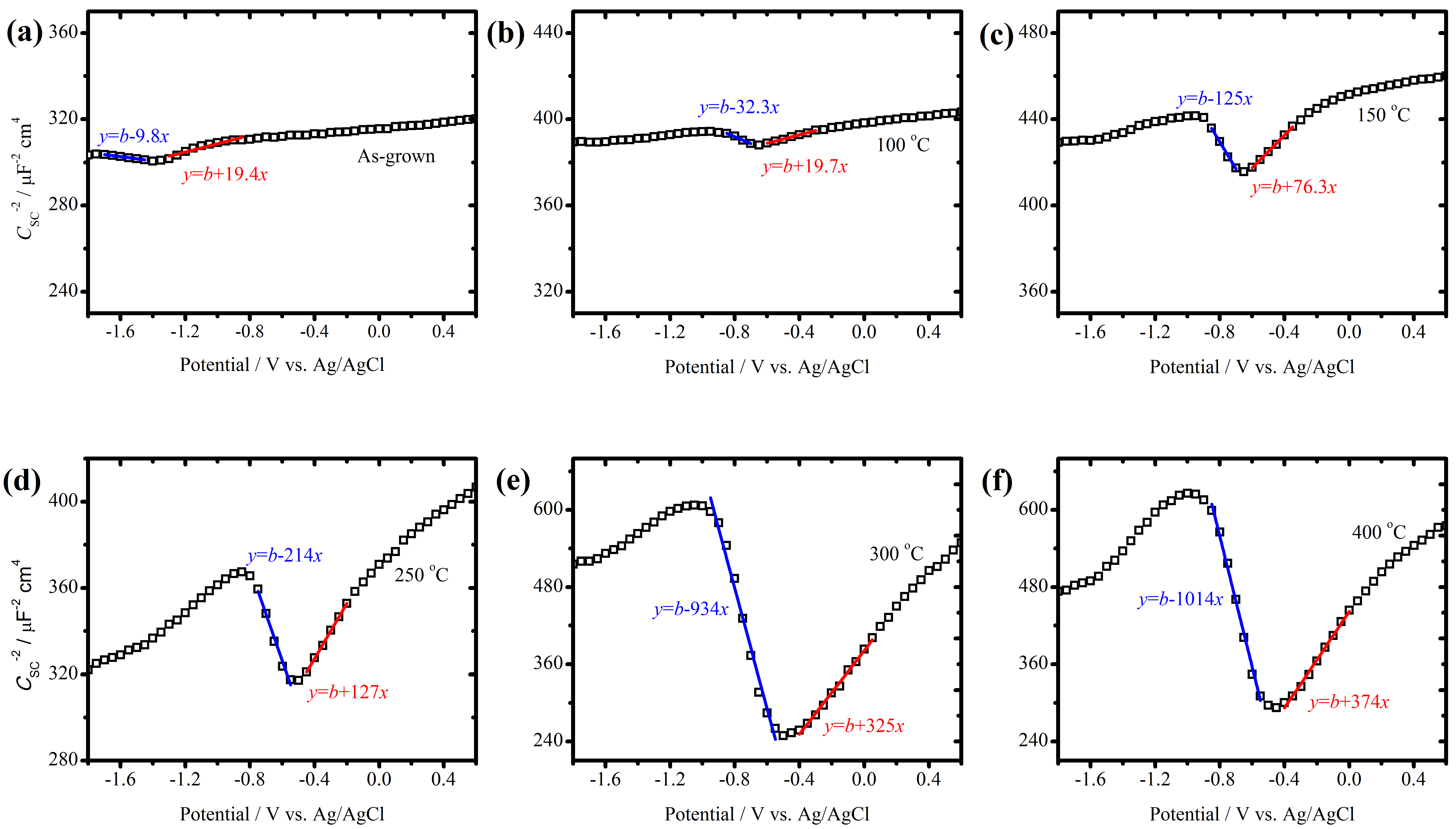
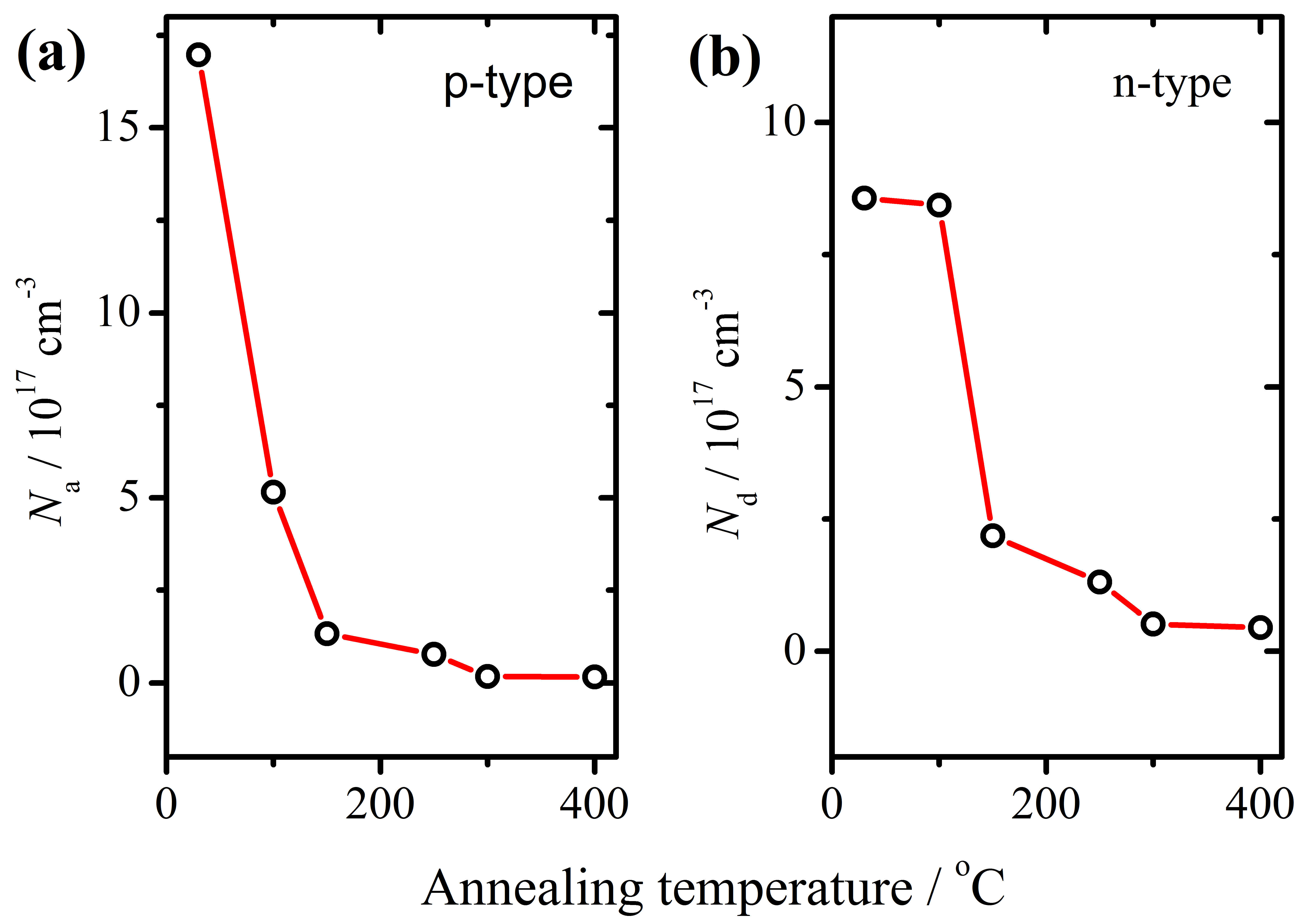
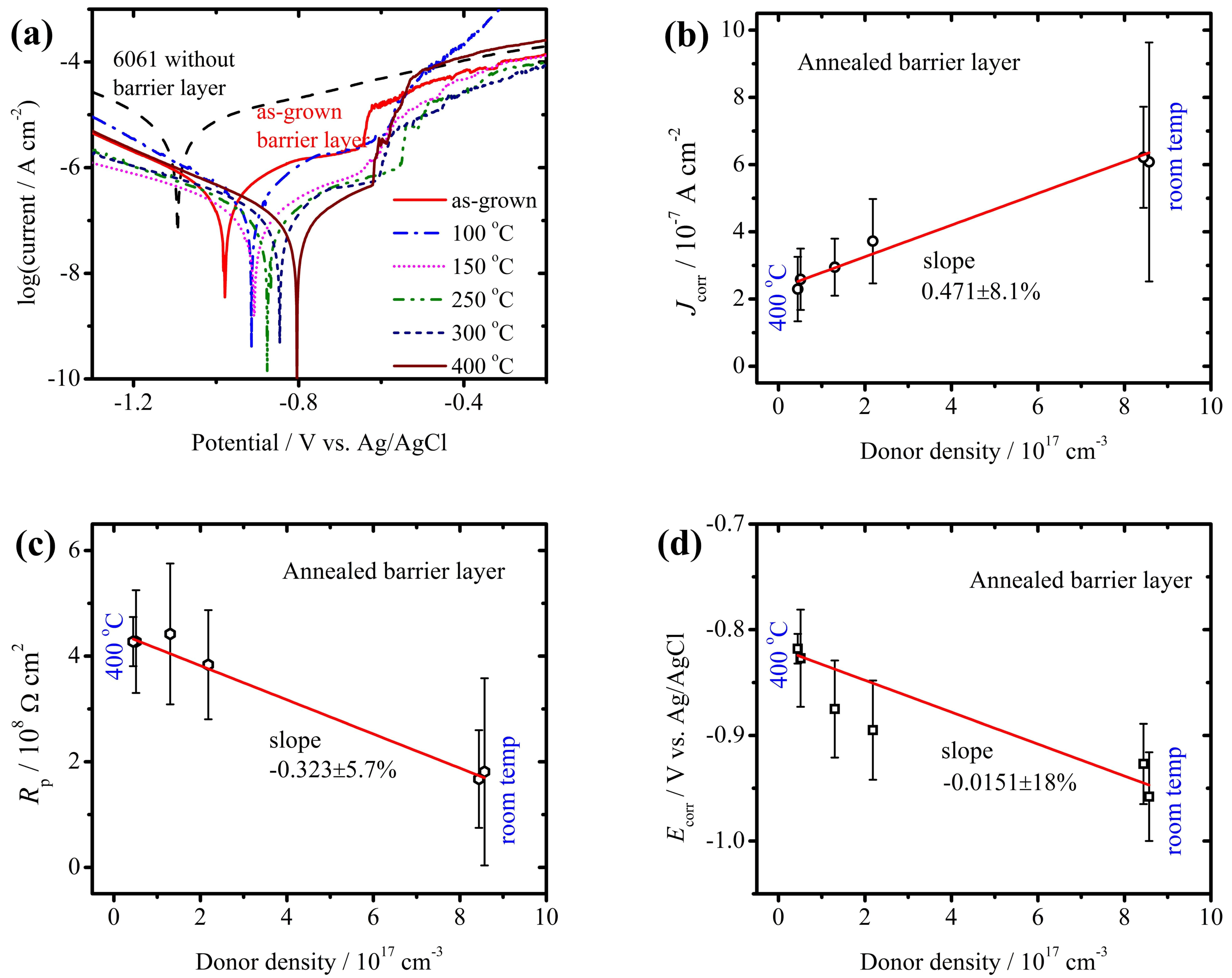
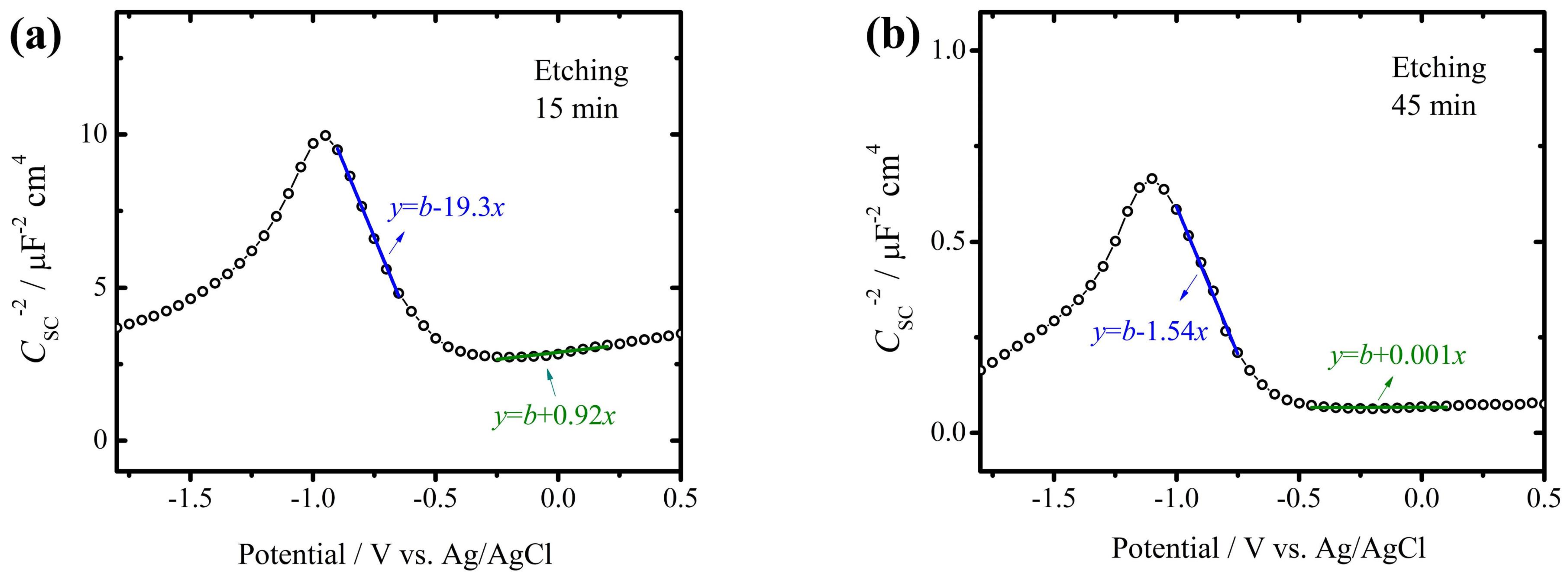
3.1. Defect Density and Corrosion Protection of AAO
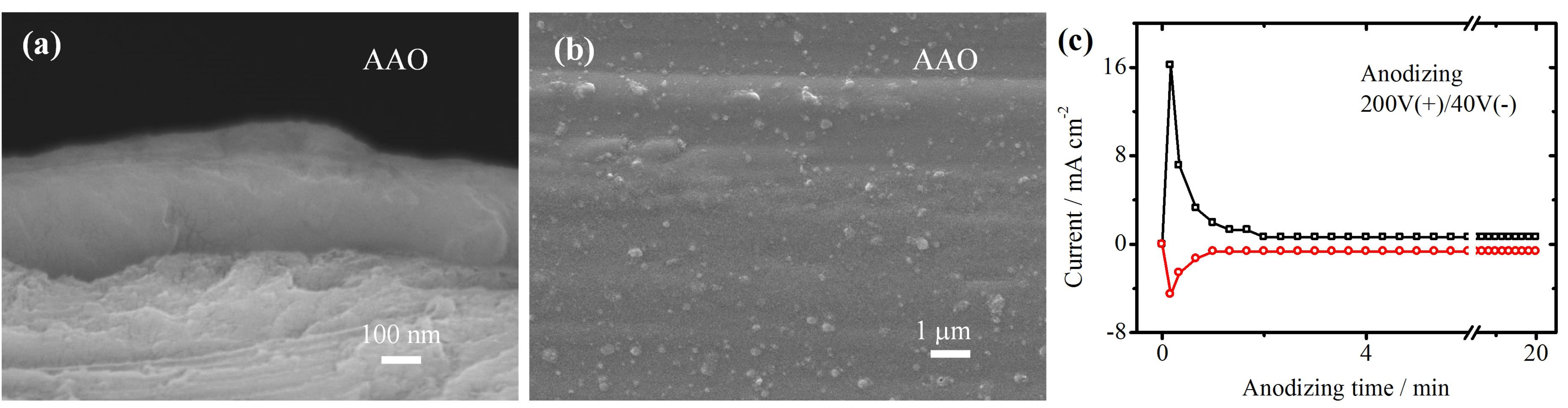
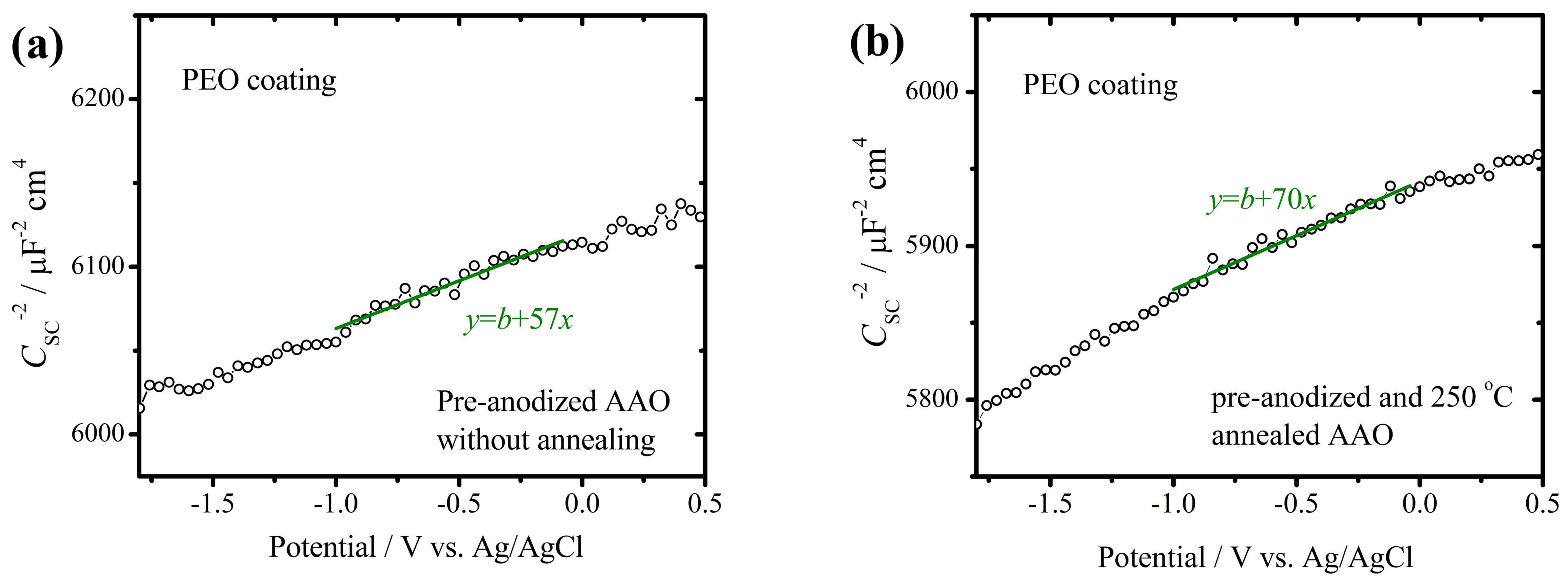
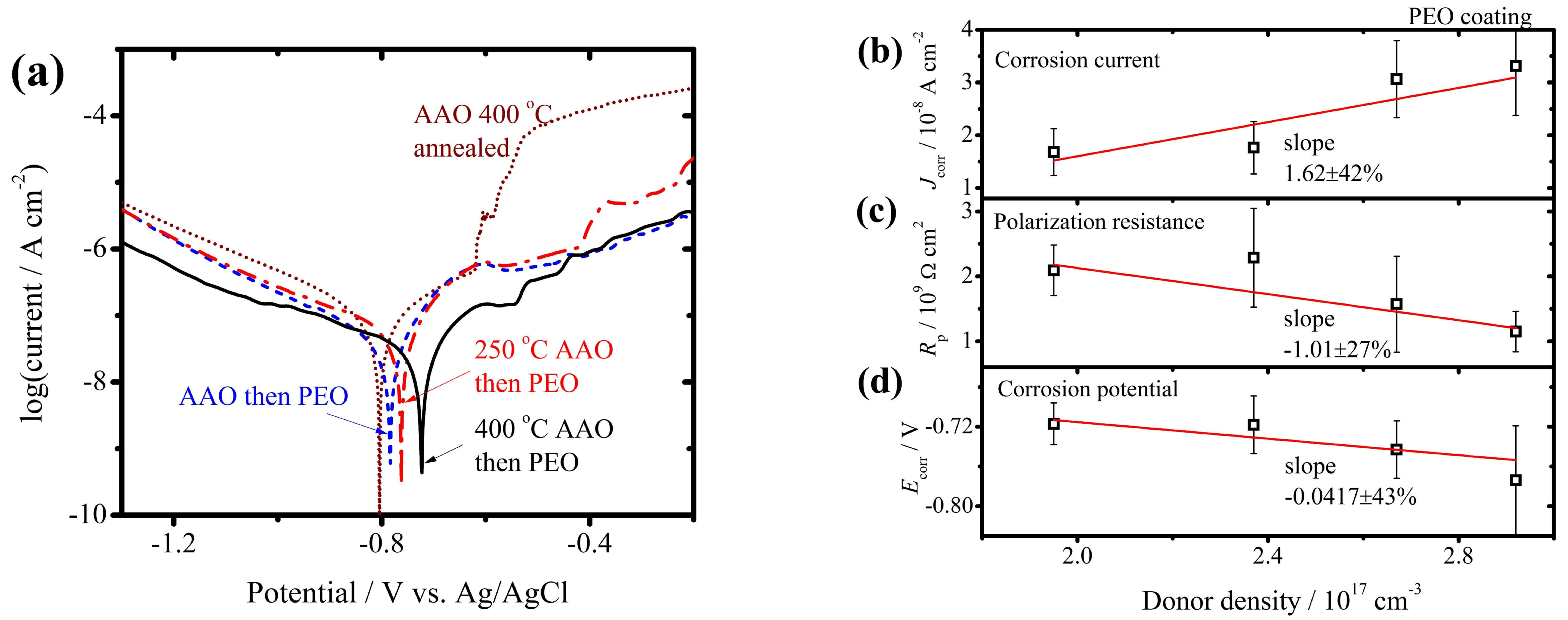
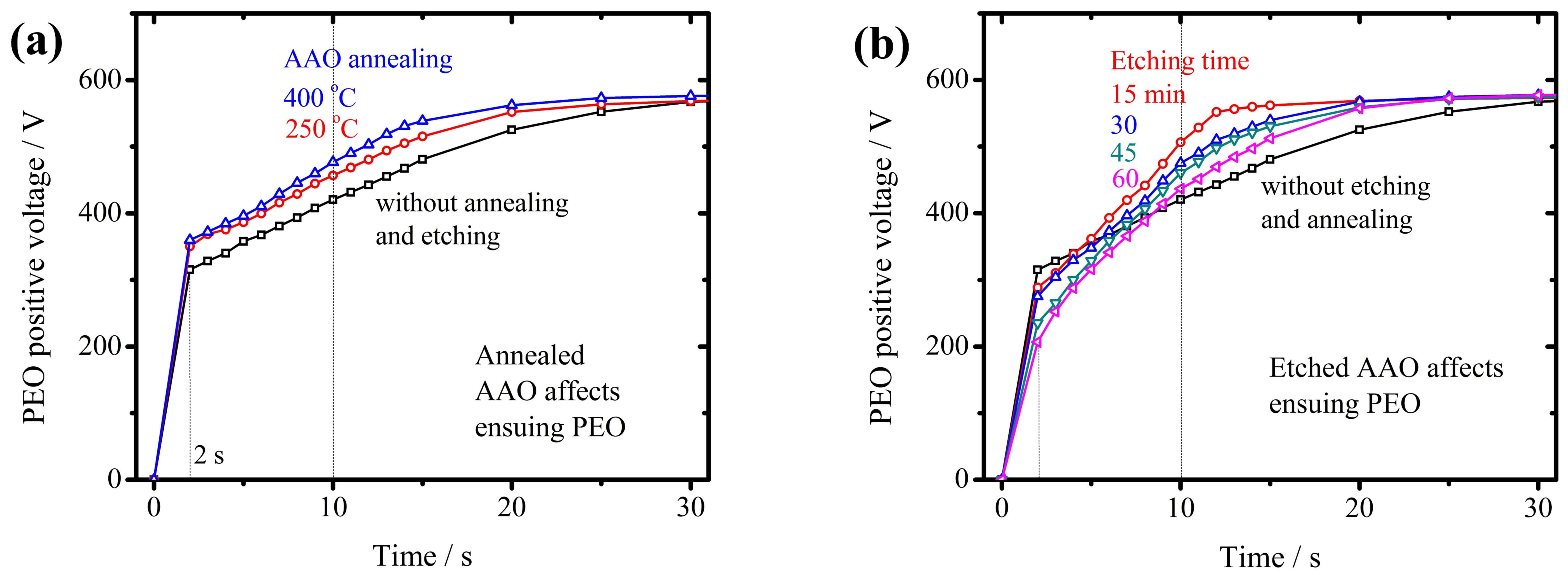
3.2. The PEO Coating with Pre-Grown AAO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2018, 63, 127–164. [Google Scholar] [CrossRef]
- Liu, C.Y.; Tsai, D.S.; Wang, J.M.; Tsai, J.T.J.; Chou, C.C. Particle size influences on the coating microstructure through green chromia inclusion in plasma electrolytic oxidation. ACS Appl. Mater. Interfaces 2017, 9, 21864–21871. [Google Scholar] [CrossRef] [PubMed]
- Tsai, D.S.; Chen, G.W.; Chou, C.C. Probe the micro arc softening phenomenon with pulse transient analysis in plasma electrolytic oxidation. Surf. Coat. Technol. 2019, 357, 235–243. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G.E.; Belenguer, P. AC PEO of aluminum with porous alumina precursor films. Surf. Coat. Technol. 2010, 205, 1668–1678. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Mohamedm, A.; Skeldon, P.; Thompson, G.E. Plasma electrolytic oxidation of pre-anodized aluminum. Corros. Sci. 2009, 51, 2897–2905. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.J. Porous anodic aluminum oxide: Anodization and templated synthesis of functional nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef]
- Thompson, G.E. Porous anodic alumina: Fabrication, characterization and applications. Thin Solid Films 1997, 297, 192–201. [Google Scholar] [CrossRef]
- Hourdakis, E.; Koutsoureli, M.; Papaioannou, G.; Nassiopoulou, A.G. Leakage current and charging discharging processes in barrier-type anodic alumina thin films for use in metal-insulator-metal capacitors. J. Appl. Phys. 2018, 123, 215301. [Google Scholar] [CrossRef]
- Mibus, M.; Jensen, C.; Hu, X.; Knospe, C.; Reed, M.L.; Zangari, G. Dielectric breakdown and failure of anodic aluminum oxide films for electrowetting systems. J. Appl. Phys. 2013, 114, 014901. [Google Scholar] [CrossRef]
- Capraz, O.O.; Overmeere, Q.V.; Shrotriya, P.; Herbert, K.R. Stress induced by electrolyte anion incorporation in porous anodic aluminum oxide. Electrochim. Acta 2017, 238, 368–374. [Google Scholar] [CrossRef]
- Capraz, O.O.; Shrotriya, P.; Skelton, P.; Thompson, G.E.; Herbert, K.R. Factors controlling stress generation during the initial growth of porous anodic aluminum oxide. Electrochim. Acta 2015, 159, 16–22. [Google Scholar] [CrossRef]
- Dou, Q.; Overmeere, Q.V.; Shrotriya, P.; Li, W.; Herbert, K.R. Stress induced by incorporation of sulfate ions into aluminum oxide films. Electrochem. Commun. 2018, 88, 39–42. [Google Scholar] [CrossRef]
- Stojadinovic, S.; Vasilic, R.; Nedic, Z.; Kasalica, B.; Belca, I.; Zekovic, L. Photoluminescent properties of barrier aluminum oxide films on aluminum. Thin Solid Films 2011, 519, 3516–3521. [Google Scholar] [CrossRef]
- Nigo, S.; Kubota, M.; Harada, Y.; Hirayama, T.; Kato, S.; Kitazawa, H.; Kido, G. Conduction band caused by oxygen vacancies in aluminum oxide for resistance random access memory. J. Appl. Phys. 2012, 112, 033711. [Google Scholar] [CrossRef]
- Chang, J.K.; Liao, C.M.; Chen, C.H.; Tsai, W.T. Microstructure and electrochemical characteristics of aluminum anodized film formed in ammonium adipate solution. J. Electrochem. Soc. 2003, 150, B266–B273. [Google Scholar] [CrossRef][Green Version]
- Sousa, C.T.; Leitao, D.C.; Proenca, M.P.; Apolinario, A.; Correia, J.G.; Ventura, J.; Araujo, J.P. Tunning pore filling of anodic alumina templates by accurate control of the bottom barrier layer thickness. Nanotechnology 2011, 22, 315602. [Google Scholar] [CrossRef]
- Diggle, J.W.; Downie, T.C.; Goulding, C.W. Anodic oxide films on aluminum. Chem. Rev. 1969, 69, 365–405. [Google Scholar] [CrossRef]
- Takahashi, H.; Kasahara, K.; Fujiwara, K.; Seo, M. The cathodic polarization of aluminum covered with anodic oxide films in a neutral borate solution. Corros. Sci. 1994, 36, 677–688. [Google Scholar] [CrossRef]
- Mibus, M.; Jensen, C.; Hu, X.; Knospe, C.; Reed, M.L.; Zangari, G. Improving dielectric performance in anodic aluminum oxide via detection and passivation of defect states. Appl. Phys. Lett. 2014, 104, 244103. [Google Scholar] [CrossRef]
- Vrublevsky, I.; Jagminas, A.; Schreckenbach, J.; Goedel, W.A. Electronic properties of electrolyte/anodic alumina junction during porous anodizing. Appl. Surf. Sci. 2007, 253, 4680–4687. [Google Scholar] [CrossRef]
- Vrublevsky, I.; Jagminas, A.; Schreckenbach, J.; Goedel, W.A. Embedded space charge in porous alumina films formed in phosphoric acid. Electrochim. Acta 2007, 53, 300–304. [Google Scholar] [CrossRef]
- Vrublevsky, I.; Parkoun, V.; Schreckenbach, J.; Goedel, W.A. Dissolution behavior of the barrier layer of porous oxide films on aluminum formed in phosphoric acid studied by a re-anodizing technique. Appl. Surf. Sci. 2006, 252, 5100–5108. [Google Scholar] [CrossRef]
- Vrublevsky, I.; Parkoun, V.; Sokol, V.; Schreckenbach, J. Study of chemical dissolution of the barrier oxide layer of porous alumina films formed in oxalic acid using a re-anodizing technique. Appl. Surf. Sci. 2004, 236, 270–277. [Google Scholar] [CrossRef]
- Benfedda, B.; Hamadou, L.; Benbrahim, N.; Kadri, A.; Chainet, E.; Charlot, F. Electrochemical impedance investigation of anodic alumina barrier layer. J. Electrochem. Soc. 2012, 159, C372–C381. [Google Scholar] [CrossRef]
- Hassel, A.W.; Lohrengel, M.M. Initial stages of cathodic breakdown of thin anodic aluminum oxide films. Electrochim. Acta 1995, 40, 433–437. [Google Scholar] [CrossRef]
- Hassel, A.W.; Diesing, D. Modification of trap distributions in anodic aluminum tunnel barriers. J. Electrochem. Soc. 2007, 154, C558–C561. [Google Scholar] [CrossRef]
- Schultze, J.W.; Lohrengel, M.M. Stability, reactivity and breakdown of passive films. Electrochim. Acta 2000, 45, 2499–2513. [Google Scholar] [CrossRef]
- Yeh, S.C.; Tsai, D.S.; Wang, J.M.; Chou, C.C. Coloration of aluminum alloy surface with dye emulsions while growing a plasma electrolytic oxide layer. Surf. Coat. Technol. 2016, 287, 61–66. [Google Scholar] [CrossRef]
- Hunter, M.S.; Fowle, J. Determination of barrier layer thickness of anodic oxide coatings. J. Electrochem. Soc. 1954, 101, 481–485. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, H.-R.; Tsai, D.-S.; Chou, C.-C. Correlation between Defect Density and Corrosion Parameter of Electrochemically Oxidized Aluminum. Coatings 2020, 10, 20. https://doi.org/10.3390/coatings10010020
Lou H-R, Tsai D-S, Chou C-C. Correlation between Defect Density and Corrosion Parameter of Electrochemically Oxidized Aluminum. Coatings. 2020; 10(1):20. https://doi.org/10.3390/coatings10010020
Chicago/Turabian StyleLou, Hao-Ren, Dah-Shyang Tsai, and Chen-Chia Chou. 2020. "Correlation between Defect Density and Corrosion Parameter of Electrochemically Oxidized Aluminum" Coatings 10, no. 1: 20. https://doi.org/10.3390/coatings10010020
APA StyleLou, H.-R., Tsai, D.-S., & Chou, C.-C. (2020). Correlation between Defect Density and Corrosion Parameter of Electrochemically Oxidized Aluminum. Coatings, 10(1), 20. https://doi.org/10.3390/coatings10010020