Accelerated Hermeticity Testing of Biocompatible Moisture Barriers Used for the Encapsulation of Implantable Medical Devices
Abstract
1. Introduction
2. Materials and Methods
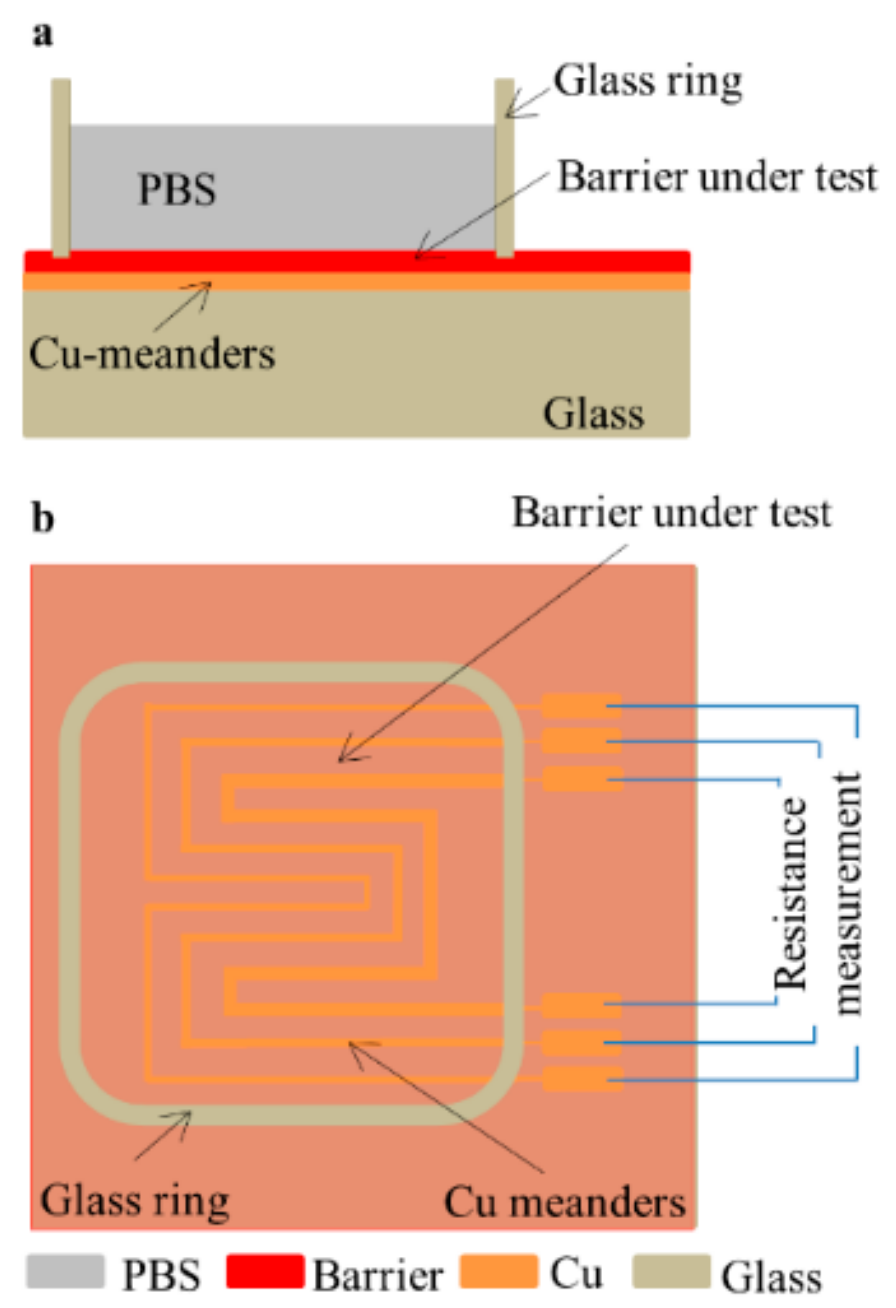
2.1. Fabrication of Cu Meander Patterns
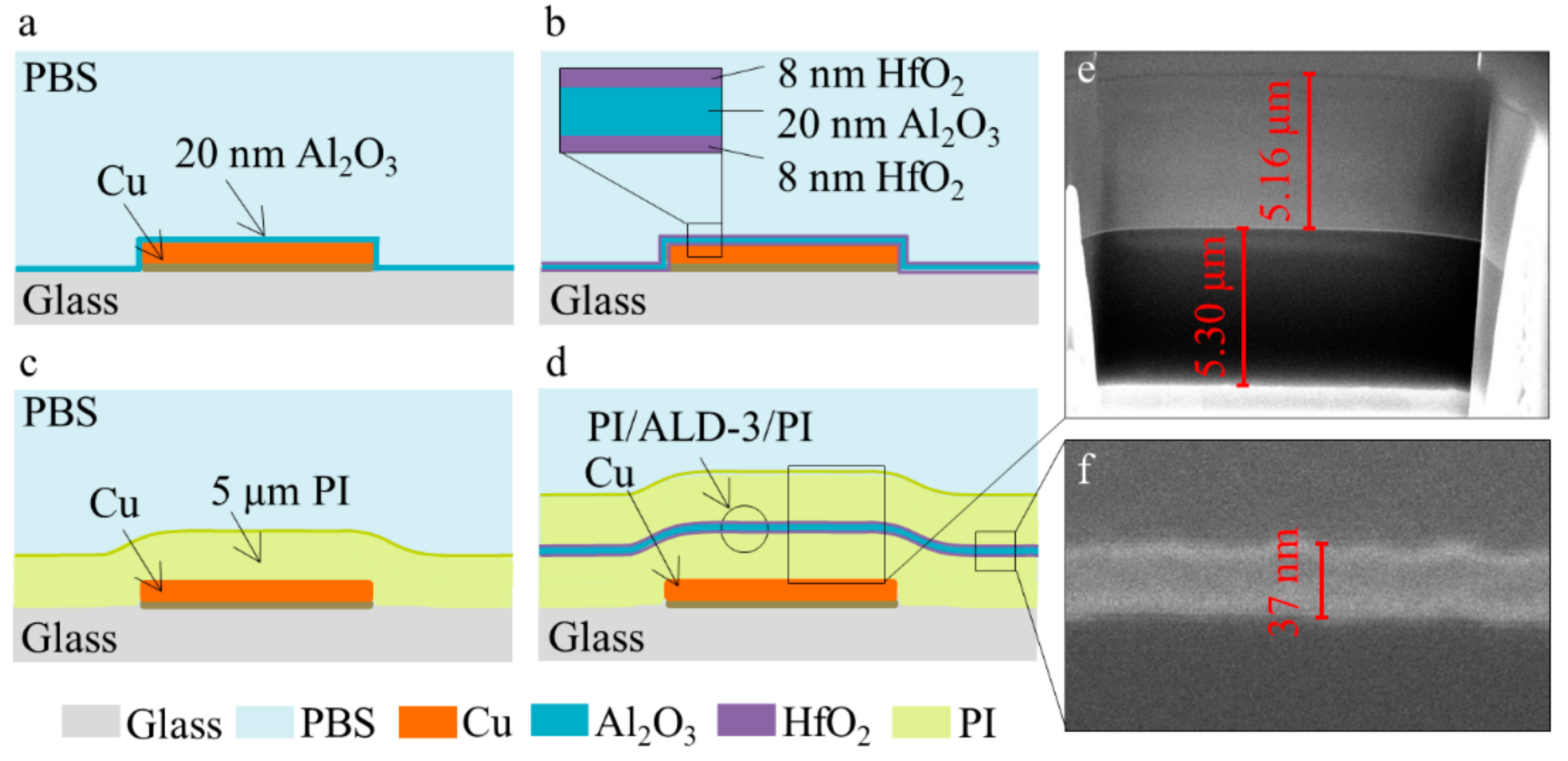
2.2. Fabrication of Barrier Layers: ALD Al2O3 and HfO2, PI
2.3. Experimental Setup
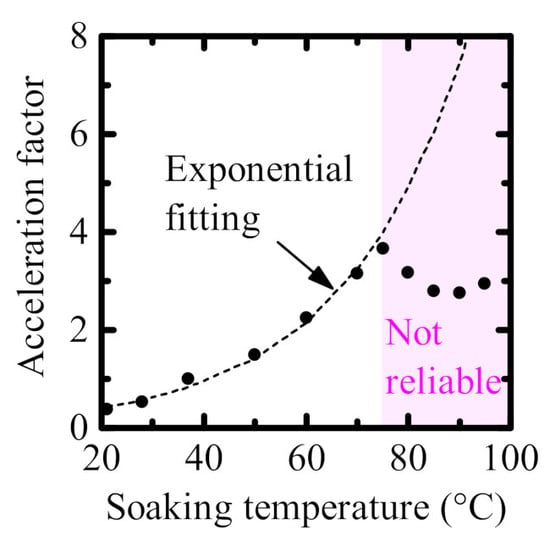
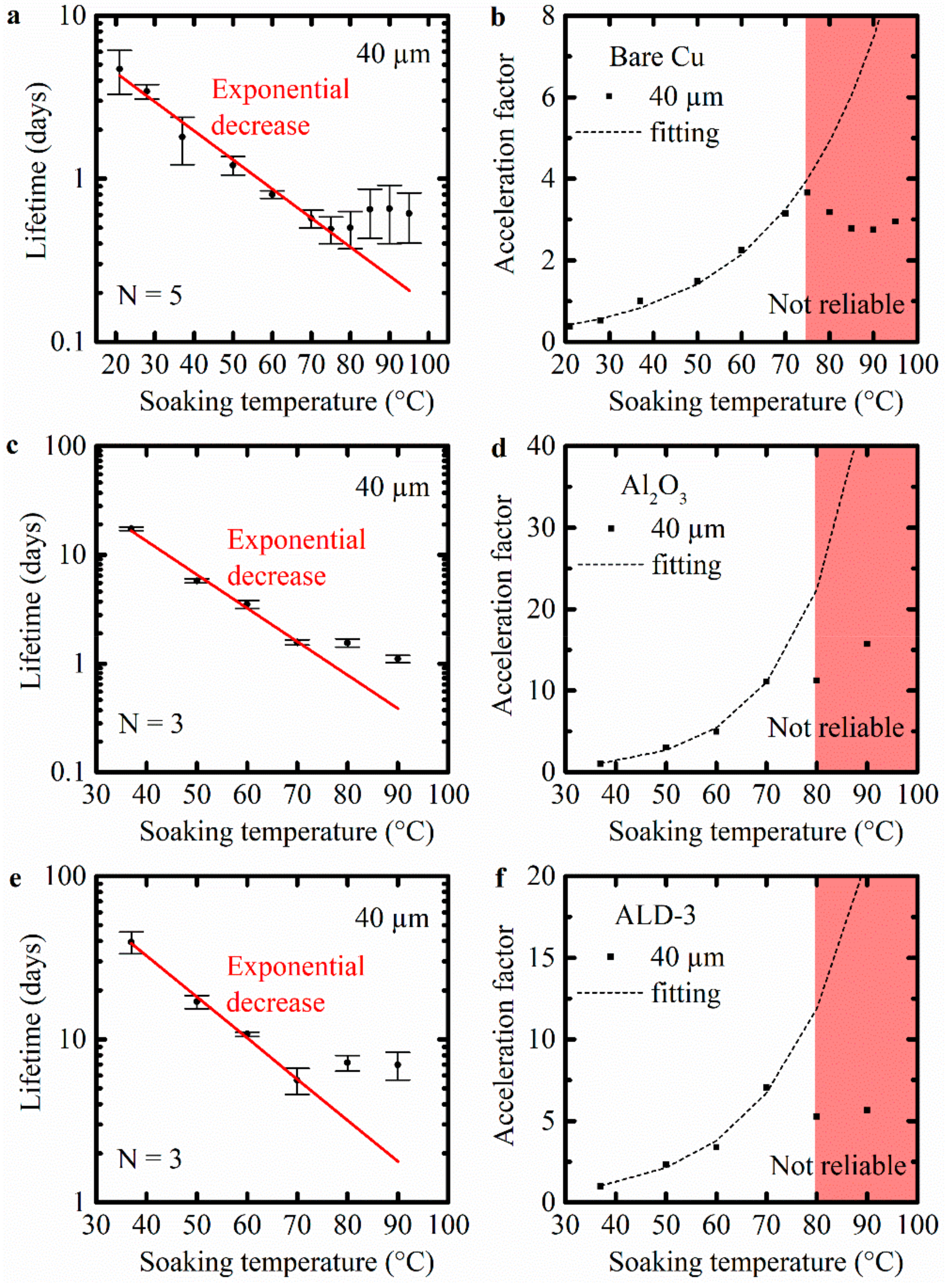
2.4. Acceleration Factor
3. Results and Discussion
- Uncoated bare Cu meanders as reference;
- ALD Al2O3 (20 nm);
- ALD-3 (8 nm HfO2/20 nm Al2O3/8 nm HfO2);
- PI;
- PI + ALD-3 + PI.
3.1. Evaluation of the Corrosion of Bare Cu Meanders
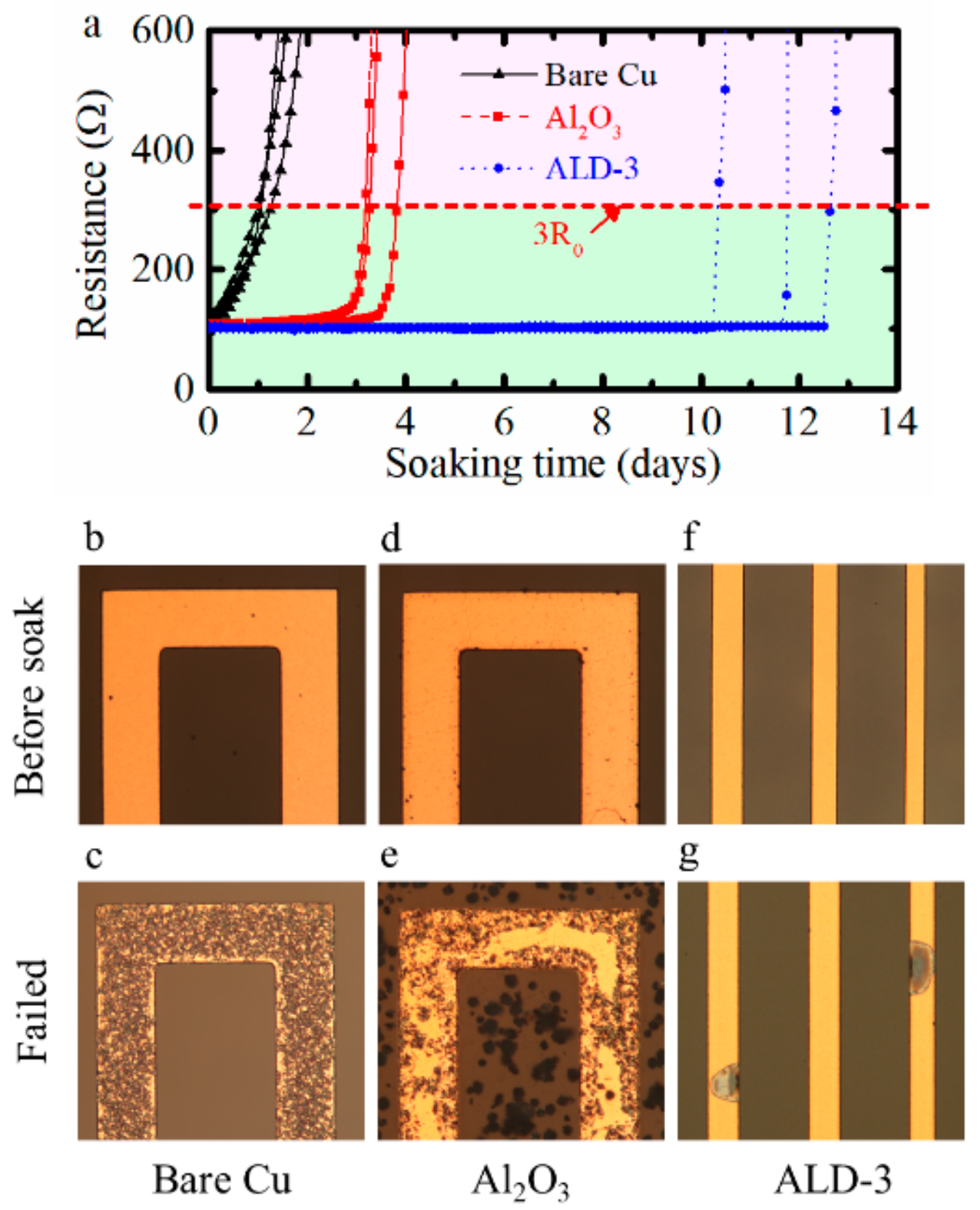
3.2. Evaluation of the Performance of Protected Cu Meanders: ALD Al2O3 and ALD-3 as Barrier Layers
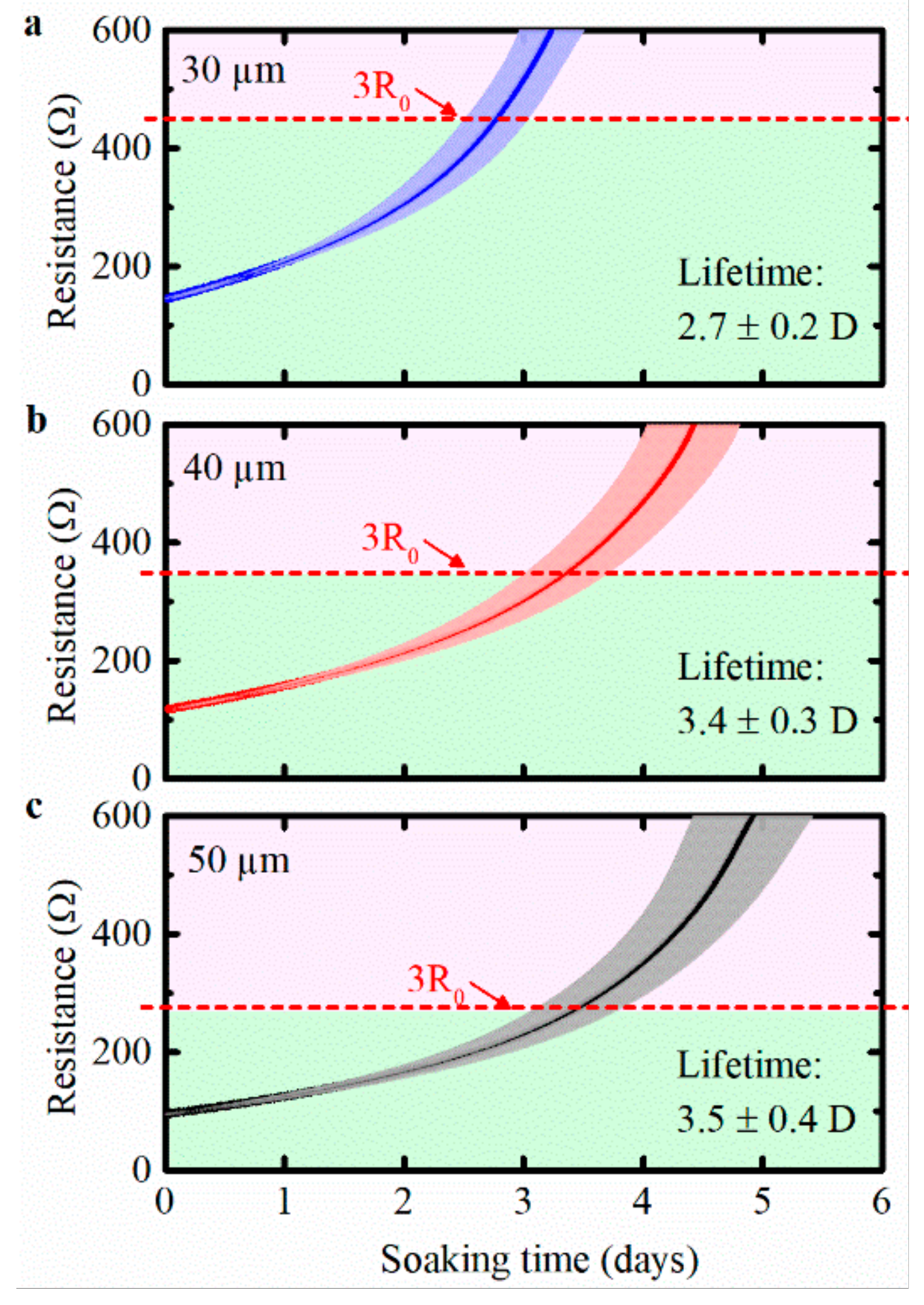
3.3. Corrosion Failure of Meanders for Three Test Cases: Uncoated Cu Meanders, ALD Al2O3, and ALD-3
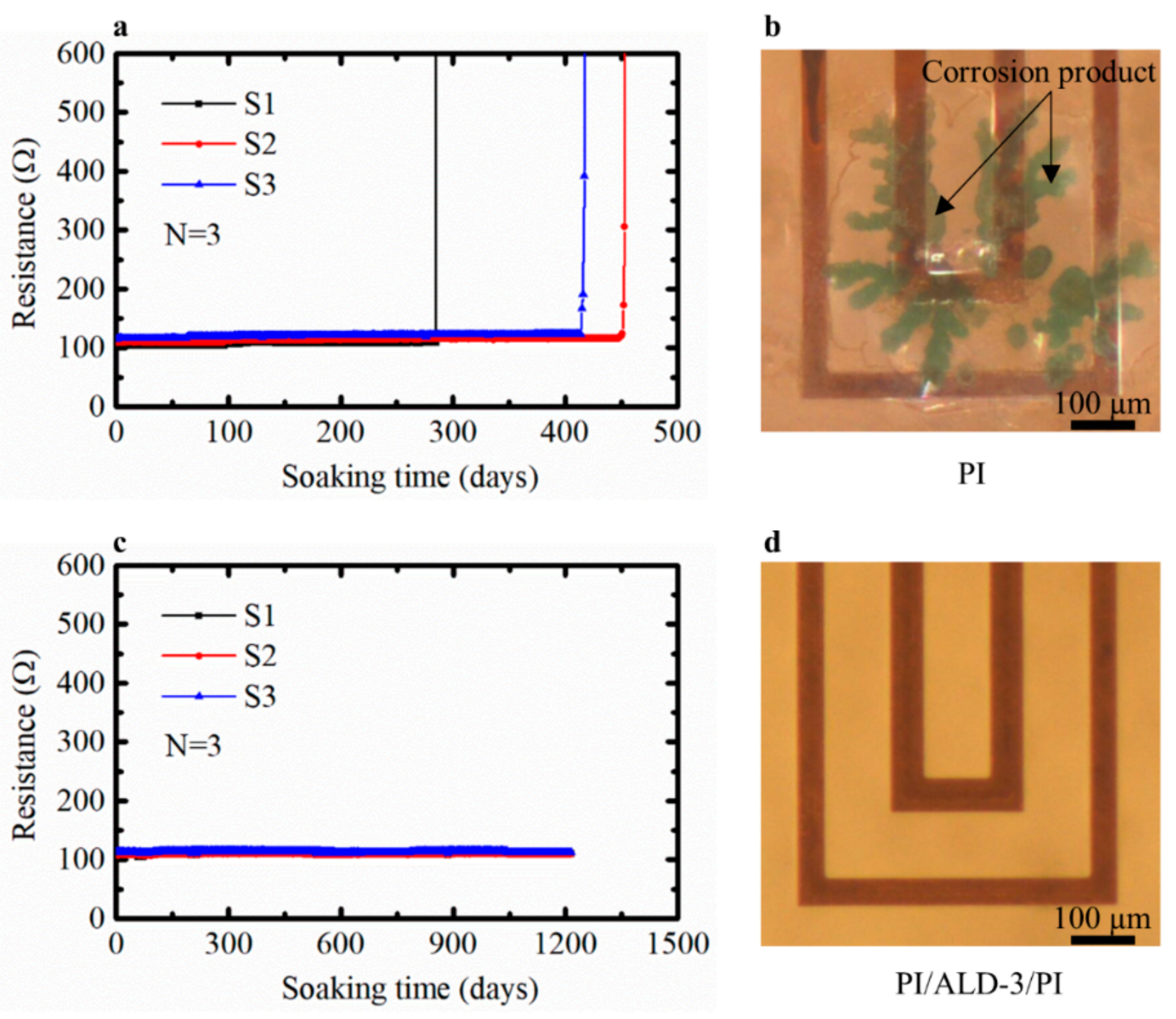
3.4. PI Combined with ALD-3 to Protect Cu Meanders
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| ALD | atomic layer deposition |
| ALD-3 | HfO2/Al2O3/HfO2 |
| FBR | foreign body reaction |
| PDMS | polydimethylsiloxane |
| WVTR | water vapor transmission rate |
| OLED | organic light-emitting diode |
| PI | Polyimide |
| BPDA-PPD | biphenyltetracarboxylic dianhydride and paraphenylenediamine |
| RH | relative humidity |
| EIS | electrochemical impedance spectroscopy |
| FIB-SEM | focused ion beam scanning electron microscopes |
| PBS | phosphate buffered saline |
| CTE | coefficient of thermal expansion. |
References
- Lacour, S.P.; Courtine, G.; Guck, J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 2016, 1, 1–14. [Google Scholar] [CrossRef]
- Xue, Y.; Shiuan, Y.; McIlvried, L.A.; Copits, B.A.; Noh, K.N.; Zhang, J.; Samineni, V.K.; Payne, M.A.; Won, S.M.; Kim, B.H.; et al. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature 2018, 565, 361–365. [Google Scholar]
- Jiang, G.; Zhou, D.D. Implantable Neural Prostheses; Springer: New York, NY, USA, 2010; ISBN 978-0-387-98119-2. [Google Scholar]
- De Op Beeck, M.; Verplancke, R.; Schaubroeck, D.; Cuypers, D.; Cauwe, M. Ultra-thin biocompatible implantable chip for bidirectional communication with peripheral nerves. In Proceedings of the 2017 IEEE Biomedical Circuits and Systems Conference, Turin, Italy, 19–21 October 2017; pp. 1–4. [Google Scholar]
- Liu, Y.; Liu, J.; Chen, S.; Lei, T.; Kim, Y.; Niu, S.; Wang, H.; Wang, X.; Foudeh, A.M.; Tok, J.B.H.; et al. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng. 2019, 3, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Scholten, K.; Meng, E. Materials for microfabricated implantable devices: A review. Lab Chip 2015, 15, 4256–4272. [Google Scholar] [CrossRef]
- Schaubroeck, D.; Verplancke, R.; Cauwe, M.; Cuypers, D.; Baumans, K.; Op De Beeck, M. Polyimide-ALD-polyimide layers as hermetic encapsulant for implants. In Proceedings of the XXXI International Conference on Surface Modification Technologies (SMT31), Mons, Belgium, 5–7 July 2017; pp. 1–6. [Google Scholar]
- Zhang, B.; Li, W.; Yang, Y.; Chen, C.; Li, C.-F.; Suganuma, K. Fully embedded CuNWs/PDMS conductor with high oxidation resistance and high conductivity for stretchable electronics. J. Mater. Sci. 2019, 54, 6381–6392. [Google Scholar] [CrossRef]
- Xie, X.; Rieth, L.; Caldwell, R.; Diwekar, M.; Tathireddy, P.; Sharma, R.; Solzbacher, F. Long-term bilayer encapsulation performance of atomic layer deposited Al2O3 and parylene C for biomedical implantable devices. IEEE Trans. Biomed. Eng. 2013, 60, 2943–2951. [Google Scholar]
- Yang, Y.; Martens, T.; Vandecasteele, B.; Degrendele, L.; Mader, L.; De Vriese, L.; Sekitani, T.; Kaufmann, M.; Van Put, S.; Vervust, T.; et al. 3D Multifunctional Composites Based on Large-Area Stretchable Circuit with Thermoforming Technology. Adv. Electron. Mater. 2018, 4, 1800071. [Google Scholar] [CrossRef]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.J.; Park, W.T.; Yoon, Y.J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Kim, S.H.; Moon, J.H.; Kim, J.H.; Jeong, S.M.; Lee, S.H. Flexible, stretchable and implantable PDMS encapsulated cable for implantable medical device. Biomed. Eng. Lett. 2011, 1, 199–203. [Google Scholar] [CrossRef]
- Lecomte, A.; Degache, A.; Descamps, E.; Dahan, L.; Bergaud, C. In vitro and in vivo biostability assessment of chronically-implanted Parylene C neural sensors. Sens. Actuators B Chem. 2017, 251, 1001–1008. [Google Scholar] [CrossRef]
- Ahn, S.-H.; Jeong, J.; Kim, S.J. Emerging Encapsulation Technologies for Long-Term Reliability of Microfabricated Implantable Devices. Micromachines 2019, 10, 508. [Google Scholar] [CrossRef] [PubMed]
- George, S.M. George Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Liu, H.; Chen, Z.; Xiong, P.; Xu, X.; Chen, F.; Li, K.; Duan, Y. Effect of Various Oxidants on Reaction Mechanisms, Self-Limiting Natures and Structural Characteristics of Al2O3 Films Grown by Atomic Layer Deposition. Adv. Mater. Interfaces 2018, 5, 1–7. [Google Scholar] [CrossRef]
- Noked, M.; Rubloff, G.W.; Lin, C.-F.; Kozen, A.C.; Liu, C. ALD Protection of Li-Metal Anode Surfaces—Quantifying and Preventing Chemical and Electrochemical Corrosion in Organic Solvent. Adv. Mater. Interfaces 2016, 3, 1600426. [Google Scholar]
- Vanhaverbeke, C.; Cauwe, M.; Stockman, A.; Op de Beeck, M.; De Smet, H. Comparison of copper electroplating, copper wet etching and linear sweep voltammetry as techniques to investigate the porosity of atomic layer deposited Al2O3. Thin Solid Film. 2019, 686, 137424. [Google Scholar] [CrossRef]
- Iacob, N.; Chindriş, I. Low-temperature atomic layer deposition of Al2O3 thin coatings for corrosion protection of steel: Surface and electrochemical analysis. Corros. Sci. 2012, 53, 2168–2175. [Google Scholar]
- Yoon, K.H.; Kim, H.S.; Han, K.S.; Kim, S.H.; Lee, Y.E.K.; Shrestha, N.K.; Song, S.Y.; Sung, M.M. Extremely High Barrier Performance of Organic-Inorganic Nanolaminated Thin Films for Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2017, 9, 5399–5408. [Google Scholar] [CrossRef]
- Klumbies, H.; Schmidt, P.; Hähnel, M.; Singh, A.; Schroeder, U.; Richter, C.; Mikolajick, T.; Hoßbach, C.; Albert, M.; Bartha, J.W.; et al. Thickness dependent barrier performance of permeation barriers made from atomic layer deposited alumina for organic devices. Org. Electron. 2015, 17, 138–143. [Google Scholar] [CrossRef]
- Graff, G.L.; Williford, R.E.; Burrows, P.E. Mechanisms of vapor permeation through multilayer barrier films: Lag time versus equilibrium permeation. J. Appl. Phys. 2004, 96, 1840–1849. [Google Scholar] [CrossRef]
- Abdulagatov, A.I.; Yan, Y.; Cooper, J.R.; Zhang, Y.; Gibbs, Z.M.; Cavanagh, A.S.; Yang, R.G.; Lee, Y.C.; George, S.M. Al2O3 and TiO2 atomic layer deposition on copper for water corrosion resistance. ACS Appl. Mater. Interfaces 2011, 3, 4593–4601. [Google Scholar] [CrossRef]
- Kim, L.H.; Jang, J.H.; Jeong, Y.J.; Kim, K.; Baek, Y.; Kwon, H.-j.; An, T.K.; Nam, S.; Kim, S.H.; Jang, J.; et al. Highly-impermeable Al2O3/HfO2 moisture barrier films grown by low-temperature plasma-enhanced atomic layer deposition. Org. Electron. 2017, 50, 296–303. [Google Scholar] [CrossRef]
- Daubert, J.S.; Hill, G.T.; Gotsch, H.N.; Gremaud, A.P.; Ovental, J.S.; Williams, P.S.; Oldham, C.J.; Parsons, G.N. Corrosion protection of copper using Al2O3, TiO2, ZnO, HfO2, and ZrO2 Atomic layer deposition. ACS Appl. Mater. Interfaces 2017, 9, 4192–4201. [Google Scholar] [CrossRef] [PubMed]
- Dameron, A.A.; Davidson, S.D.; Burton, B.B.; Carcia, P.F.; Scott McLean, R.; George, S.M. Gas diffusion barriers on polymers using multilayers fabricated by Al2O3 and rapid SiO2 atomic layer deposition. J. Phys. Chem. C 2008, 112, 4573–4580. [Google Scholar] [CrossRef]
- Zhang, Y.; Bertrand, J.A.; Yang, R.; George, S.M.; Lee, Y.C. Electroplating to visualize defects in Al2O3 thin films grown using atomic layer deposition. Thin Solid Films 2009, 517, 3269–3272. [Google Scholar] [CrossRef]
- Loveday, D.; Peterson, P.; Rodgers, B. Evaluation of Organic Coatings with Electrochemical Impedance Spectroscopy Part 2: Application of EIS to Coatings. JCT Coat. Technol. 2004, 10, 88–93. [Google Scholar]
- Li, C.; Cauwe, M.; Yang, Y.; Schaubroeck, D.; Mader, L. Ultra-Long-Term Reliable Encapsulation Using an Atomic Layer Deposited HfO2/Al2O3/HfO2 Triple-Interlayer for Biomedical Implants. Coatings 2019, 9, 579. [Google Scholar] [CrossRef]
- Nelson, W.B. Accelerated Testing: Statistical Models, Test Plans, and Data Analysis; Wiley: Hoboken, NJ, USA, 2009; ISBN 0471697362. [Google Scholar]
- Hukins, D.W.L.; Mahomed, A.; Kukureka, S.N. Accelerated aging for testing polymeric biomaterials and medical devices. Med. Eng. Phys. 2008, 30, 1270–1274. [Google Scholar] [CrossRef]
- Hemmerich, K.J. Hemmerich General aging theory and simplified protocol for accelerated aging of medical devices. Med. Plast. Biomater. 1998, 5, 16–23. [Google Scholar]
- Minnikanti, S.; Diao, G.; Pancrazio, J.J.; Xie, X.; Rieth, L.; Solzbacher, F.; Peixoto, N. Lifetime assessment of atomic-layer-deposited Al2O3-Parylene C bilayer coating for neural interfaces using accelerated age testing and electrochemical characterization. Acta Biomater. 2014, 10, 960–967. [Google Scholar] [CrossRef]
- Meyer, J.-U.; Stieglitz, T.; Scholz, O.; Haberer, W.; Beutel, H. High Density Interconnects and Flexible Hybrid Assemblies for Active Biomedical Implants. IEEE Trans. Adv. Packag. 2001, 24, 366–374. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, K.; Vervust, T.; Vanfleteren, J. Multifunctional and miniaturized flexible sensor patch: Design and application for in situ monitoring of epoxy polymerization. Sens. Actuators B Chem. 2018, 261, 144–152. [Google Scholar] [CrossRef]
- Yang, Y.; Chiesura, G.; Plovie, B.; Vervust, T.; Luyckx, G.; Degrieck, J.; Sekitani, T.; Vanfleteren, J. Design and Integration of Flexible Sensor Matrix for in Situ Monitoring of Polymer Composites. ACS Sens. 2018, 3, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lacour, S.P.; Brooks, R.A.; Rushton, N.; Fawcett, J.; Cameron, R.E. Assessment of the biocompatibility of photosensitive polyimide for implantable medical device use. J. Biomed. Mater. Res. Part A 2009, 90, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Hassler, C.; Boretius, T.; Stieglitz, T. Polymers for neural implants. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 18–33. [Google Scholar] [CrossRef]
- Herth, E.; Guerchouche, K.; Rousseau, L.; Calvet, L.E.; Loyez, C. A biocompatible and flexible polyimide for wireless sensors. Microsyst. Technol. 2017, 23, 5921–5929. [Google Scholar] [CrossRef]
- Verplancke, R.; Cauwe, M.; Schaubroeck, D.; Cuypers, D.; Ballini, M.; Callaghan, J.O.; Goikoetxea, E.; Braeken, D.; Bashirullah, R.; Op de Beeck, M. Development of an active high-density transverse intrafascicular micro-electrode probe. J. Micromech. Microeng. 2020, 30, 015010. [Google Scholar] [CrossRef]
- Xie, X.; Rieth, L.; Williams, L.; Negi, S.; Bhandari, R.; Caldwell, R.; Sharma, R.; Tathireddy, P.; Solzbacher, F. Long-term reliability of Al2O3 and Parylene C bilayer encapsulated Utah electrode array based neural interfaces for chronic implantation. J. Neural Eng. 2014, 11, 026016. [Google Scholar] [CrossRef]
- Field, J.A.; Luna-Velasco, A.; Boitano, S.A.; Shadman, F.; Ratner, B.D.; Barnes, C.; Sierra-Alvarez, R. Cytotoxicity and physicochemical properties of hafnium oxide nanoparticles. Chemosphere 2011, 84, 1401–1407. [Google Scholar] [CrossRef]
- Finch, D.S.; Oreskovic, T.; Ramadurai, K.; Herrmann, C.F.; George, S.M.; Mahajan, R.L. Biocompatibility of atomic layer-deposited alumina thin films. J. Biomed. Mater. Res. Part A 2008, 87, 100–106. [Google Scholar] [CrossRef]
- Jeong, J.; Laiwalla, F.; Lee, J.; Ritasalo, R.; Pudas, M.; Larson, L.; Leung, V.; Nurmikko, A. Conformal Hermetic Sealing of Wireless Microelectronic Implantable Chiplets by Multilayered Atomic Layer Deposition (ALD). Adv. Funct. Mater. 2019, 29, 1–10. [Google Scholar] [CrossRef]
- Benson, B.B.; Krause, D.; Peterson, M.A. The solubility and isotopic fractionation of gases in dilute aqueous solution. I. Oxygen. J. Solut. Chem. 1979, 8, 665–690. [Google Scholar] [CrossRef]
- Tromans, D. Modeling Oxygen Solubility in Water and Electrolyte Solutions. Ind. Eng. Chem. Res. 2000, 39, 805–812. [Google Scholar] [CrossRef]
- Schott BOROFLOAT® 33—Thermal Properties. Available online: https://www.schott.com/d/borofloat/b89578c1-3509-40a2-aacf-ca3c2371ef7b/1.2/borofloat33_therm_eng_web.pdf (accessed on 20 December 2019).
- Bouras, N.; Madjoubi, M.A.; Kolli, M.; Benterki, S.; Hamidouche, M. Thermal and mechanical characterization of borosilicate glass. Phys. Procedia 2009, 2, 1135–1140. [Google Scholar] [CrossRef]
- Suhonen, T.; Varis, T.; Dosta, S.; Torrell, M.; Guilemany, J.M. Residual stress development in cold sprayed Al, Cu and Ti coatings. Acta Mater. 2013, 61, 6329–6337. [Google Scholar] [CrossRef]
- Miller, D.C.; Foster, R.R.; Jen, S.H.; Bertrand, J.A.; Cunningham, S.J.; Morris, A.S.; Lee, Y.C.; George, S.M.; Dunn, M.L. Thermo-mechanical properties of alumina films created using the atomic layer deposition technique. Sens. Actuators A Phys. 2010, 164, 58–67. [Google Scholar] [CrossRef]

| Barrier Layer on Cu Meanders | Soaking Time at 70 °C (Day) | Equivalent Soaking Time at 37 °C (Day) | Acceleration Factor |
|---|---|---|---|
| None | 0.57 | 2.17 | 3.8 |
| Al2O3 | 1.57 | 15.46 | 9.8 |
| ALD-3 | 5.61 | 39.02 | 7.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Cauwe, M.; Mader, L.; Schaubroeck, D.; Op de Beeck, M. Accelerated Hermeticity Testing of Biocompatible Moisture Barriers Used for the Encapsulation of Implantable Medical Devices. Coatings 2020, 10, 19. https://doi.org/10.3390/coatings10010019
Li C, Cauwe M, Mader L, Schaubroeck D, Op de Beeck M. Accelerated Hermeticity Testing of Biocompatible Moisture Barriers Used for the Encapsulation of Implantable Medical Devices. Coatings. 2020; 10(1):19. https://doi.org/10.3390/coatings10010019
Chicago/Turabian StyleLi, Changzheng, Maarten Cauwe, Lothar Mader, David Schaubroeck, and Maaike Op de Beeck. 2020. "Accelerated Hermeticity Testing of Biocompatible Moisture Barriers Used for the Encapsulation of Implantable Medical Devices" Coatings 10, no. 1: 19. https://doi.org/10.3390/coatings10010019
APA StyleLi, C., Cauwe, M., Mader, L., Schaubroeck, D., & Op de Beeck, M. (2020). Accelerated Hermeticity Testing of Biocompatible Moisture Barriers Used for the Encapsulation of Implantable Medical Devices. Coatings, 10(1), 19. https://doi.org/10.3390/coatings10010019