Antibacterial Activity of Essential Oils and Trametes versicolor Extract against Clavibacter michiganensis subsp. michiganensis and Ralstonia solanacearum for Seed Treatment and Development of a Rapid In Vivo Assay
Abstract
1. Introduction
2. Results
2.1. Antibacterial In Vitro Activity
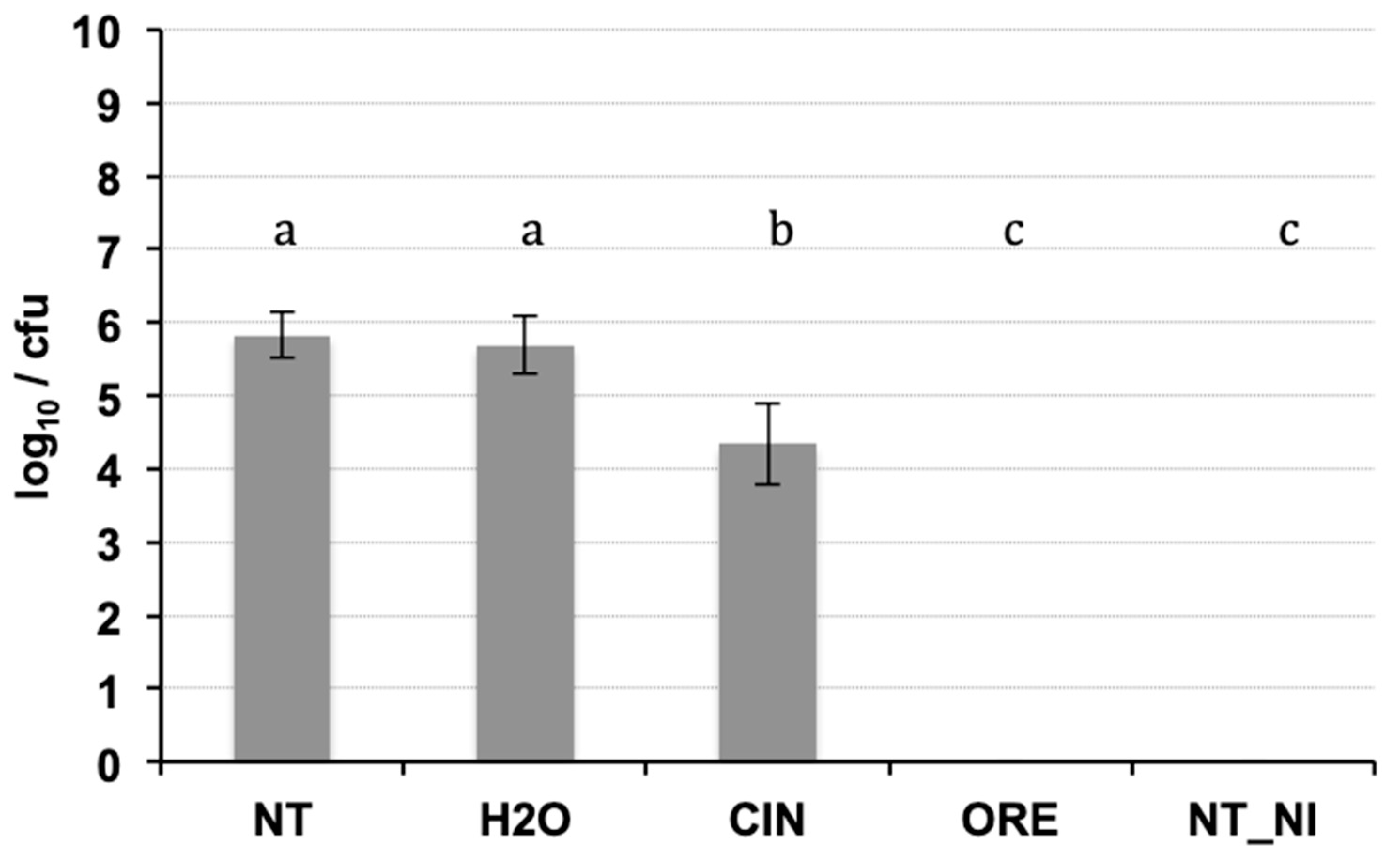
2.2. Antibacterial In Vivo Activity
2.2.1. Phytotoxicity
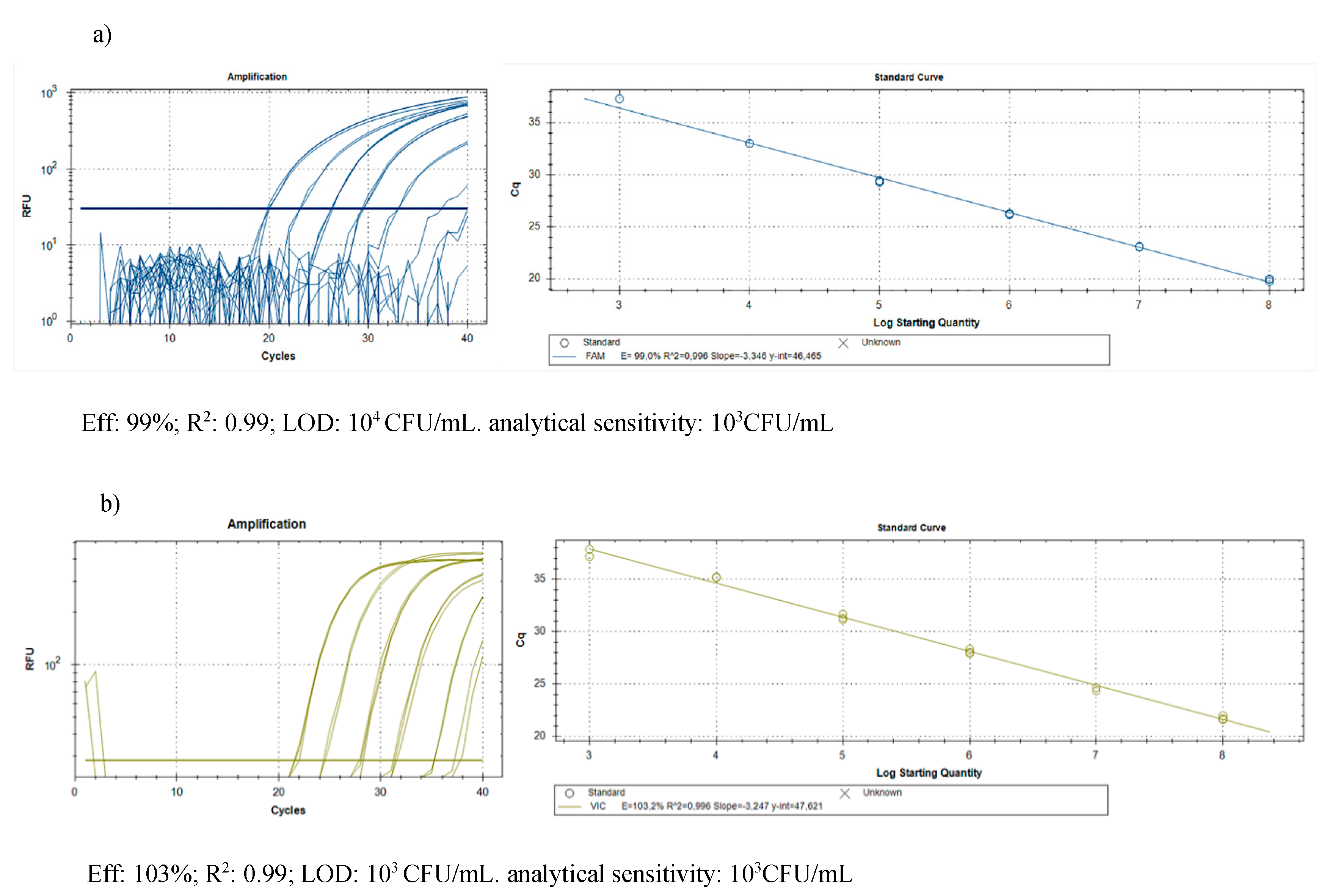
2.2.2. Molecular Assay
3. Discussion
4. Materials and Methods
4.1. Natural Compounds
4.2. Bacterial Strains
4.3. Antibacterial In Vitro Activity
4.4. Antibacterial In Vivo Activity
4.4.1. Phytotoxicity
4.4.2. Seed Inoculation and In Vivo Treatments with EOs
4.4.3. Molecular Assay
4.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Nandi, M.; Macdonald, J.; Liu, P.; Weselowski, B.; Yuan, Z.C. Clavibacter michiganensis ssp. michiganensis: bacterial canker of tomato, molecular interactions and disease management. Mol. Plant Pathol. 2018, 19, 2036–2050. [Google Scholar] [CrossRef]
- De Leòn, L.; Siverio, F.; Lopez, M.M.; Rodrıguez, A. Clavibacter michiganensis subsp. michiganensis, a seed borne tomato pathogen: healthy seeds are still the goal. Plant. Dis. 2011, 95, 1339. [Google Scholar]
- Tancos, M.A.; Chalupowicz, L.; Barash, I.; Manulis-Sasson, S.; Smart, C.D. Tomato fruit and seed colonization by Clavibacter michiganensis subsp. michiganensis through external and internal routes. Appl. Environ. Microbiol. 2013, 79, 6948–6957. [Google Scholar] [CrossRef]
- EPPO, European and Mediterranean Plant Protection Organization. Clavibacter michiganensis subsp. michiganensis. EPPO Bull. 2016, 46, 202–225. [Google Scholar] [CrossRef]
- Chang, R.; Ries, S.; Pataky, J. Dissemination of Clavibacter michiganensis subsp. michiganensis by practices used to produce tomato transplants. Phytopathology 1991, 81, 1276–1281. [Google Scholar] [CrossRef]
- Kaneshiro, W.S.; Ingram, D.M.; Alvarez, A.M. Clavibacter michiganensis subsp. michiganensis threshold levels required for transmission by naturally-infested tomato seed. Phytopathology 2008, 98, S78. [Google Scholar]
- Xu, X.; Miller, S.A.; Baysal-Gurel, F.; Gartemann, K.H.; Eichenlaub, R.; Rajashekara, G. Bioluminescence imaging of Clavibacter michiganensis subsp. michiganensis infection of tomato seeds and plants. Appl. Environ. Microbiol. 2010, 76, 3978–3988. [Google Scholar] [CrossRef] [PubMed]
- Fatmi, M.; Schaad, N.W.; Bolkan, H.A. Seed treatments for eradicating Clavibacter michiganensis subsp. michiganensis from naturally infected tomato seeds. Plant Dis. 1991, 75, 383–385. [Google Scholar] [CrossRef]
- Ricker, M.D.; Riedel, R.M. Effect of secondary spread of Clavibacter michiganensis subsp. michiganensis on yield of northern processing tomatoes. Plant Dis. 1993, 77, 364–366. [Google Scholar] [CrossRef]
- EFSA PLH Panel (EFSA Panel on Plant Health). Scientific Opinion on the pest categorisation of Clavibacter michiganensis subsp. michiganensis (Smith) Davis et al. EFSA J. 2014, 12, 3721. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- USDA—United States Department of Agriculture. Agricultural Bioterrorism Protection Act of 2002: Biennial Review and Republication of the Selected Agent and Toxin List: Amendments to the Select Agent and Toxin Regulations: Final Rule (7 CFR Part 331/9 CFR Part 121). Fed. Regist. 2012, 77, 1–27. [Google Scholar]
- Kelman, A.; Hartman, G.L.; Hayward, A.C. Bacterial Wilt: The Disease and Its Causative Agent, Pseudomonas Solanacearum; Hayward, A.C., Hartman, G.I., Eds.; CAB International: Wallingford, UK, 1994. [Google Scholar]
- Martins, O.M.; Nabizadeh-Ardeakani, F.; Rudolph, K. Seeds from infected tomato plants appear to be free from contamination by Ralstonia solanacearum, when tested by PCR or microbiological assays. In Bacterial Wilt Disease and the Ralstonia solanacearum Species Complex; Allen, C., Prior, P., Hayward, A.C., Eds.; APS Press: St. Paul, MN, USA, 2005; pp. 95–101. [Google Scholar]
- Dey, P.; Hossain, I.; Hossain, M.D. Isolation and identification of seed borne Ralstonia solanacearum from tomato and brinjal in Bangladesh. J Agric. Vet. Sci. 2017, 10, 32–39. [Google Scholar]
- Shakya, D.D. Occurrence of Pseudomonas solanacearum in tomato seeds imported into Nepal, Bacterial wilt. In Aciar Proceedings; Australian Centre for International Agricultural Research: Canberra, Australia, 1993. [Google Scholar]
- Roopali, S. Seed transmission of Pseudomonas solanacearum in tomato and eggplant. Bact. Wilt Newsl. 1994, 11, 12–13. [Google Scholar]
- Kasselaki, A.M.; Goumas, D.; Tamm, L.; Fuchs, J.; Cooper, J.; Leifert, C. Effect of alternative strategies for the disinfection of tomato seed infected with bacterial canker (Clavibacter michiganensis subsp. michiganensis). NJAS Wagen. J. Life Sci. 2011, 58, 145–147. [Google Scholar] [CrossRef]
- Basim, H.; Yegen, O.; Zeller, W. Antibacterial effect of essential oil of Thymbra spicata L. var. spicata on some plant pathogenic bacteria. J. Plant Dis. Prot. 2000, 107, 279–284. [Google Scholar]
- Orzali, L.; Corsi, B.; Forni, C.; Riccioni, L. Chitosan in Agriculture: A New Challenge for Managing Plant Disease. In Biological Activities and Application of Marine Polysaccharides; Emad, S., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Sturchio, E.; Donnarumma, L.; Annesi, T.; Milano, F.; Casorri, L.; Masciarelli, E.; Zanellato, M.; Meconi, C.; Boccia, P. Essential oils: An alternative approach to management of powdery mildew diseases. Phytopathol. Mediterr. 2014, 53, 385–395. [Google Scholar]
- Sarwar, M. Biopesticides: an effective and environmental friendly insect-pests inhibitor line of action. Int. J. Adv. Res. Technol. 2015, 1, 10–15. [Google Scholar]
- Simonetti, G.; Pucci, N.; Brasili, E.; Valletta, A.; Sammarco, I.; Carnevale, E.; Loreti, S. In vitro antimicrobial activity of plant extracts against Pseudomonas syringae pv. actinidiae causal agent of bacterial canker in kiwifruit. Plant Biosyst. Inter. J. 2019, 154, 100–106. [Google Scholar] [CrossRef]
- Lo Cantore, P.; Shanmugaiah, V.; Iacobellis, N.S. Antibacterial activity of essential oil components and their potential use in seed disinfection. J. Agric. Food Chem. 2009, 57, 9454–9461. [Google Scholar] [CrossRef]
- Riccioni, L.; Orzali, L. Activity of tea tree (Melaleuca alternifolia, Cheel) and thyme (Thymus vulgaris, Linnaeus.) essential oils against some pathogenic seed borne fungi. J. Essent. Oil Res. 2011, 23, 43–47. [Google Scholar] [CrossRef]
- Arshad, Z.; Hanif, M.A.; Qadri, R.W.K.; Khan, M. Role of essential oils in plant diseases protection: A review. Int. J. Chem. Biol. Sci. 2014, 6, 11–17. [Google Scholar]
- Scarpari, M.; Reverberi, M.; Parroni, A.; Scala, V.; Fanelli, C.; Pietricola, C.; Zjalic, S.; Maresca, V.; Tafuri, A.; Ricciardi, M.R.; et al. Tramesan, a novel polysaccharide from Trametes versicolor. Structural characterization and biological effects. PLoS ONE 2017, 12, e0171412. [Google Scholar] [CrossRef]
- Marinelli, E.; Orzali, L.; Lotti, E.; Riccioni, L. Activity of Some Essential Oils against Pathogenic Seed Borne Fungi on Legumes. Asian J. Plant Pathol. 2012, 6, 66–74. [Google Scholar] [CrossRef][Green Version]
- Nguefack, J.; Leth, V.; Dongmo, J.B.L.; Torp, J.; Zollo, P.H.A.; Nyasse, S. Use of three essential oils as seed treatments against seed-borne fungi of rice (Oryza sativa L.). Am. Eurasian J. Agric. Environ. Sci. 2008, 4, 554–560. [Google Scholar]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Maruzzella, J.C.; Reine, S.; Solat, H.; Zeitlin, H. The action of essential oils on phytopathogenic bacteria. Plant Dis. Rep. 1963, 47, 23–26. [Google Scholar]
- Maiti, D.; Kole, R.C.; Sen, C. Antimicrobial efficacy of some essential oils. J. Plant Dis. Prot. 1985, 92, 64–68. [Google Scholar]
- Scortichini, M.; Rossi, M.P. In Vitro susceptibility of Erwinia amylovora (Burrill) to geraniol and citronellol. J. Appl. Bacteriol. 1991, 71, 113–118. [Google Scholar] [CrossRef]
- Satish, S.; Raveesha, K.A.; Janardhana, G.R. Antibacterial activity of plant extracts on phytopathogenic Xanthomonas campestris pathovars. Lett. Appl. Microbiol. 1999, 28, 145–147. [Google Scholar] [CrossRef]
- Daferera, D.J.; Ziogas, B.N.; Polissiou, M.G. The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp michiganensis. Crop Prot. 2003, 22, 39–44. [Google Scholar] [CrossRef]
- Iacobellis, N.S.; Lo Cantore, P.; Capasso, F.; Senatore, F. Antibacterial activity of Cuminum cyminum L. and Carum carvi L. essential oils. J. Agr. Food Chem. 2005, 53, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Pucci, N.; Orzali, L.; Modesti, V.; Lumia, V.; Brunetti, A.; Pilotti, M.; Loreti, S. Essential oils with inhibitory capacities on Pseudomonas syringae pv. actinidiae, the causal agent of kiwifruit bacterial canker. Asian J. Plant Pathol. 2018, 12, 16–26. [Google Scholar]
- Scarpari, M.; Bello, C.; Pietricola, C.; Zaccaria, M.; Bertocchi, L.; Angelucci, A.; Ricciardi, M.R.; Scala, V.; Parroni, A.; Fabbri, A.; et al. Aflatoxin control in maize by Trametes versicolor. Toxins 2014, 6, 3426–3437. [Google Scholar] [CrossRef]
- Scarpari, M.; Parroni, A.; Zaccaria, M.; Fattorini, L.; Bello, C.; Fabbri, A.A.; Bianchi, G.; Scala, V.; Zjalic, S.; Fanelli, C. Trametes versicolor bioactive compounds stimulate Aspergillus flavus antioxidant system and inhibit aflatoxin synthesis. Plant Biosyst. 2016, 150, 653–659. [Google Scholar] [CrossRef]
- Guggenheim, A.G.; Wright, K.M.; Zwickey, H.L. Immune modulation from five major mushrooms: application to integrative oncology. Integr. Med. Clin. J. 2014, 13, 32–44. [Google Scholar]
- Ricciardi, M.R.; Licchetta, R.; Mirabilii, S.; Scarpari, M.; Parroni, A.; Fabbri, A.A.; Cescutti, P.; Reverberi, M.; Fanelli, C.; Tafuri, A. Preclinical antileukemia activity of tramesan: a newly identified bioactive fungal metabolite. Oxid. Med. Cell. Longev. 2017. [Google Scholar] [CrossRef]
- Reverberi, M.; Fabbri, A.A.; Zjalic, S.; Ricelli, A.; Punelli, F.; Fanelli, C. Antioxidant enzymes stimulation in Aspergillus parasiticus by Lentinula edodes inhibits aflatoxin production. Appl. Microbiol. Biotechnol. 2005, 69, 207–215. [Google Scholar] [CrossRef]
- Zjalic, S.; Reverberi, M.; Ricelli, A.; Granito, V.M.; Fanelli, C.; Fabbri, A.A. Trametes versicolor: A possible tool for aflatoxin control. Int. J. Food Microbiol. 2006, 107, 243–249. [Google Scholar] [CrossRef]
- Radaelli, M.; Silva, B.P.D.; Weidlich, L.; Hoehne, L.; Flach, A.; Costa, L.A.M.A.D.; Ethur, E.M. Antimicrobial activities of six essential oils commonly used as condiments in Brazil against Clostridium perfringens. Braz. J. Microbiol. 2016, 47, 424–430. [Google Scholar] [CrossRef]
- Valdés, R.A.; Castillo, F.D.H.; Cabello, J.C.A.; Fuentes, Y.M.O.; Morales, G.G.; Cantú, D.J.; Aguilar, C.N. Review of antibacterial activity of plant extracts and growth-promoting microorganism (GPM) against phytopathogenic bacterial tomato crop. Eur. J. Biotechnol. Genet. Eng. 2012, 4, 11–36. [Google Scholar]
- Mbega, E.R.; Mortensen, C.N.; Mabagala, R.B.; Wulff, E.G. The effect of plant extracts as seed treatments to control bacterial leaf spot of tomato in Tanzania. J. Gen. Plant Pathol. 2012, 78, 277–286. [Google Scholar] [CrossRef]
- Winska, K.; Maczka, W.; Lyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents-myth or real alternative. Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [PubMed]
- Riccioni, L.; Orzali, L.; Romani, M.; Annicchiarico, P.; Pecetti, L. Organic seed treatments with essential oils to control Ascochyta blight in pea. Eur. J. Plant Pathol. 2019, 155, 831–840. [Google Scholar] [CrossRef]
- Scala, V.; Pietricola, C.; Farina, V.; Beccaccioli, M.; Zjalic, S.; Quaranta, F.; Fornara, M.; Zaccaria, M.; Momeni, B.; Reverberi, M.; et al. Tramesan Elicits Durum Wheat Defense against the Septoria Disease Complex. Biomolecules 2020, 10, 608. [Google Scholar] [CrossRef]
- Orzali, L.; Luison, D.; Riccioni, L. Utilizzo di principi attivi di origine naturale per la concia delle sementi e per il controllo delle malattie trasmesse per seme. Dal. SEME 2014, 9, 46–48. [Google Scholar]
- Riccioni, L.; Orzali, L.; Marinelli, E. Seed treatment with essential oils. In Proceedings of the International Conference ‘Future IPM in Europe’ 2013, Riva del Garda, Italy, 19–21 March 2013. [Google Scholar]
- Basim, H.; Turgut, K.; Kaplan, B.; Basim, E.; Turgut, A. (2019). The potential application of Origanum dubium boiss. essential oil as a seed protectant against bean and tomato seed-borne bacterial pathogens. Acta Sci. Pol. Hortoru. 2019, 18, 79–86. [Google Scholar]
- Karabuyuk, F.; Aysan, Y. Aqueous plant extracts as seed treatments on tomato bacterial speck disease. In Proceedings of the V International Symposium on Tomato Diseases: Perspectives and Future Directions in Tomato Protection, Málaga, Spain, 13 June 2016; ISHS International Society for Horticultural Science: Leuven, Belgium, 2016; Volume 1207, pp. 193–196. [Google Scholar]
- Meah, M.B. Biopesticides for crop growth and crop protection. J. Plant Prot. Sci. 2010, 2, 19–32. [Google Scholar]
- Dos Santos e Silva, M.S.B.; Rodrigues, A.A.C.; de Oliveira, A.C.S.; de Jesus Machado Gois de Oliveira, L.; de Jesus Ferreira Costa, N.; Silva, M.R.M.; de Lemos, R.N.S. Health quality and reduction of pathogenic transmission in tomato seeds using plant extracts. Aust. J. Crop Sci. 2019, 13, 635–641. [Google Scholar] [CrossRef]
- Scala, V. (CREA-DC: Research Centre for Plant Protection and Certification, Rome, Italy). Personal communication, 2020.
- Flores, J.B.; García, J.O.; Becheleni, F.R.C.; Espinoza, A.V.; Wong-Corral, F.J.; Rueda-Puente, E.O. Effect of essential oils in the control of the Clavibacter michiganensis subespecie michiganensis in tomato (Lycopersicum esculentum L.) plants. Biotecnia 2018, 20, 96–101. [Google Scholar] [CrossRef]
- Dadasoglu, F.; Aydin, T.; Kotan, R.; Cakir, A.; Ozer, H.; Kordali, S.; Cakmakci, R.; Dikbas, N.; Mete, E. Antibacterial activities of extracts and essential oils of three Origanum species against plant pathogenic bacteria and their potential use as seed disinfectants. J. Plant Pathol. 2011, 93, 271–282. [Google Scholar]
- Vasinauskiene, M.; Radusiene, J.; Zitikaite, I.; Surviliene, E. Antibacterial activities of essential oils from aromatic and medicinal plants against growth of phytopathogenic bacteria. Agron. Res. 2006, 4, 437–440. [Google Scholar]
- Sivropoulou, A.; Papanikolaou, E.; Nikolanou, C.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antimicrobial and cytotoxic activities of Origanum essential oils. J. Agric. Food Chem. 1996, 44, 1202–1205. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Parroni, A.; Bellabarba, A.; Beccaccioli, M.; Scarpari, M.; Reverberi, M.; Infantino, A. Use of the secreted proteome of Trametes versicolor for controlling the cereal pathogen Fusarium langsethiae. Int. J. Mol. Sci. 2019, 20, 4167. [Google Scholar] [CrossRef]
- CLSI, Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 10th ed.; CLSI document M07-A10; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- ISTA. International Rules for Seed Testing. Seed Sci. Technol. 1999, 27, 178. [Google Scholar]
- Oosterhof, J.; Berendsen, S. The development of a specific Real-Time TaqMan for the detection of Clavibacter michiganensis subsp. michiganensis. Phytopathology 2011, 101, S133. [Google Scholar]
- Weller, S.A.; Elphinstone, J.G.; Smith, N.C.; Boonham, N.; Stead, D.E. Detection of Ralstonia solanacearum strains with a quantitative, multiplex, real-time, fluorogenic PCR (TaqMan) assay. Appl. Environ. Microbiol. 2000, 66, 2853–2858. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.Z.; Liu, X.; Ma, Z.H.; Li, B.; Allen, C.; Guo, J.H. A real-time PCR assay for the quantitative detection of Ralstonia solanacearum in the horticultural soil and plant tissues. J. Microbiol. Biotechnol. 2010, 20, 193–201. [Google Scholar] [CrossRef]
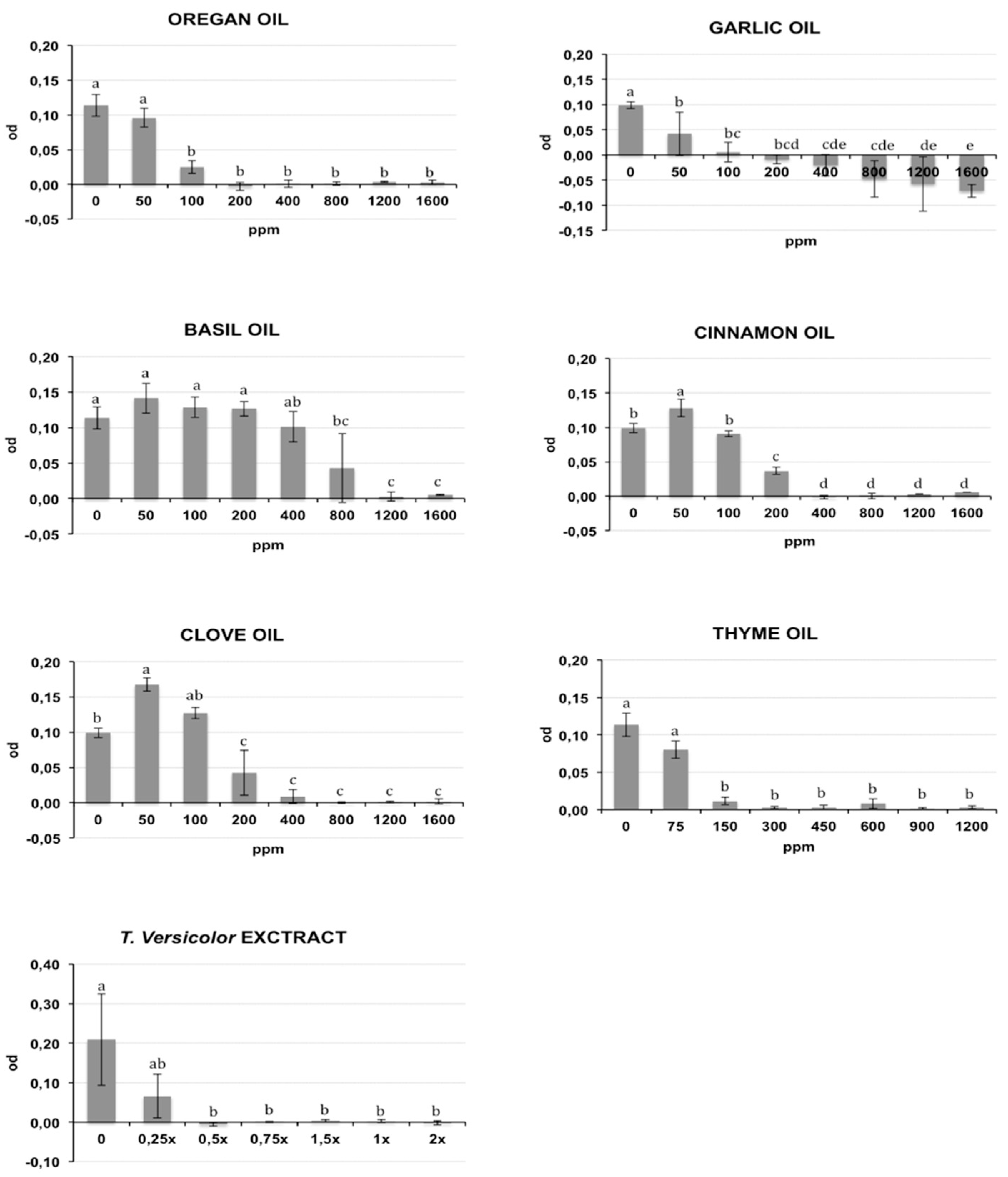
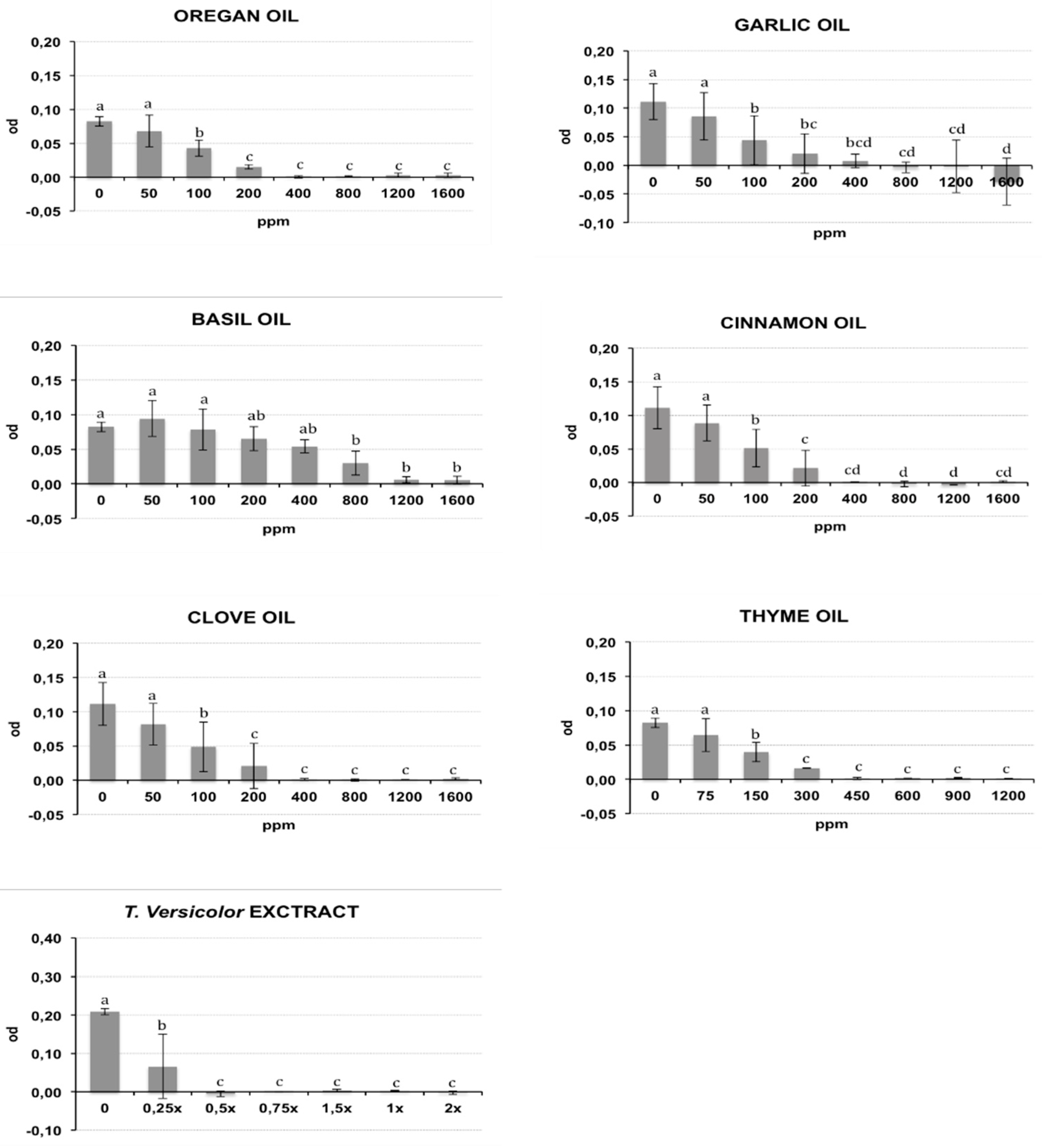
| Compound | MIC90 | MBC | ||
|---|---|---|---|---|
| CMM | RS | CMM | RS | |
| Clove Oil | 400 ppm * | 290 ppm | 1200 ppm | 800 ppm |
| Cinnamon Oil | 320 ppm | 290 ppm | 1200 ppm | 800 ppm |
| Garlic Oil | 130 ppm | 200–400 ppm * | >1600 ppm | 1200 ppm |
| Basil Oil | 1265 ppm | 1270 ppm | >1600 ppm | >1600 ppm |
| Oregan Oil | 155 ppm | 290 ppm | 800 ppm | 800 ppm |
| Thyme Oil | 225 ppm | 360 ppm | 450 ppm | 450 ppm |
| T.versicolor extract | 025×–0.5× * | 0.25×–0.5× * | 1.5× | 0.5× |
| Clove Oil | % | SD | Basil Oil | % | SD | ||
|---|---|---|---|---|---|---|---|
| 0% | 96.3 | 1.06 | a | 0% | 97.8 | 1.06 | a |
| 0.1% (1050 ppm) | 98.0 | 1.41 | a | 0.1% (960 ppm) | 97.8 | 1.77 | a |
| 0.2% (2100 ppm) | 96.0 | 2.83 | a | 0.2% (1920 ppm) | 96.3 | 1.06 | a |
| 0.3% (3150 ppm) | 96.3 | 0.35 | a | 0.3% (2870 ppm) | 96.8 | 0.35 | a |
| 0.4% (4200 ppm) | 86.3 | 1.71 | b | 0.4% (3820 ppm) | 94.3 | 0.35 | a |
| Cinnamon Oil | Thyme Oil | ||||||
| 0% | 97.8 | 1.06 | a | 0% | 97.8 | 1.06 | a |
| 0.1% (1025 ppm) | 98.0 | 0.00 | a | 0.1% (920 ppm) | 94.5 | 0.00 | a |
| 0.2% (2050 ppm) | 96.5 | 2.12 | b | 0.2% (1840 ppm) | 96.8 | 0.35 | a |
| 0.3% (3075 ppm) | 95.3 | 2.47 | b | 0.3% (2750 ppm) | 97.3 | 0.35 | a |
| 0.4% (4100 ppm) | 95.5 | 0.71 | b | 0.4% (3670 ppm) | 96.3 | 2.47 | a |
| Garlic Oil | T.versicolor extract | ||||||
| 0% | 97.8 | 1.06 | a | 0 | 95.3 | 0.33 | a |
| 0.1% (1080 ppm) | 95.5 | 1.41 | a | 0.5× | 94.8 | 1.06 | a |
| 0.2% (2160 ppm) | 96.8 | 0.35 | a | 1× | 95.0 | 0.01 | a |
| 0.3% (3250 ppm) | 97.3 | 2.47 | a | 1.5× | 95.3 | 0.35 | a |
| 0.4% (4330 ppm) | 95.8 | 1.06 | a | ||||
| Oregan Oil | NT | 96 | 2.83 | ||||
| 0% | 97.8 | 1.06 | a | ||||
| 0.1% (940 ppm) | 96.3 | 1.06 | a | ||||
| 0.2% (1880 ppm) | 97.5 | 1.41 | a | ||||
| 0.3% (2820 ppm) | 96.8 | 0.35 | a | ||||
| 0.4% (3760 ppm) | 96.0 | 0.71 | a | ||||
| Samples | Efficiency | R2 | LOD | Analytical Sensitivity |
|---|---|---|---|---|
| DNA of Cmm | 92% | 0.99 | 10 fg | 100 fg |
| DNA of Cmm and tomato | 91% | 0.99 | 100 fg | 1000 fg |
| DNA of Rs | 95% | 0.99 | / | 100 fg |
| DNA of Rs and tomato | 95% | 0.99 | / | 100 fg |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orzali, L.; Valente, M.T.; Scala, V.; Loreti, S.; Pucci, N. Antibacterial Activity of Essential Oils and Trametes versicolor Extract against Clavibacter michiganensis subsp. michiganensis and Ralstonia solanacearum for Seed Treatment and Development of a Rapid In Vivo Assay. Antibiotics 2020, 9, 628. https://doi.org/10.3390/antibiotics9090628
Orzali L, Valente MT, Scala V, Loreti S, Pucci N. Antibacterial Activity of Essential Oils and Trametes versicolor Extract against Clavibacter michiganensis subsp. michiganensis and Ralstonia solanacearum for Seed Treatment and Development of a Rapid In Vivo Assay. Antibiotics. 2020; 9(9):628. https://doi.org/10.3390/antibiotics9090628
Chicago/Turabian StyleOrzali, Laura, Maria Teresa Valente, Valeria Scala, Stefania Loreti, and Nicoletta Pucci. 2020. "Antibacterial Activity of Essential Oils and Trametes versicolor Extract against Clavibacter michiganensis subsp. michiganensis and Ralstonia solanacearum for Seed Treatment and Development of a Rapid In Vivo Assay" Antibiotics 9, no. 9: 628. https://doi.org/10.3390/antibiotics9090628
APA StyleOrzali, L., Valente, M. T., Scala, V., Loreti, S., & Pucci, N. (2020). Antibacterial Activity of Essential Oils and Trametes versicolor Extract against Clavibacter michiganensis subsp. michiganensis and Ralstonia solanacearum for Seed Treatment and Development of a Rapid In Vivo Assay. Antibiotics, 9(9), 628. https://doi.org/10.3390/antibiotics9090628





