Antimicrobial, Antioxidant, and Antiproliferative Effects of Coronilla minima: An Unexplored Botanical Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction
2.3. Estimation of Total Phenolic/Flavonoid Compounds and Antioxidant Activity
2.4. Mass Spectrometry and High Performance Liquid Chromatography (HPLC) Analysis
2.5. Antimicrobial Susceptibility Testing
2.6. Antibacterial Activity Assay
2.7. Antifungal Activity Assay
2.8. Eco-Toxicological Assays
2.9. Cell Culture
2.10. Bioinformatics
2.11. Statistical Analysis
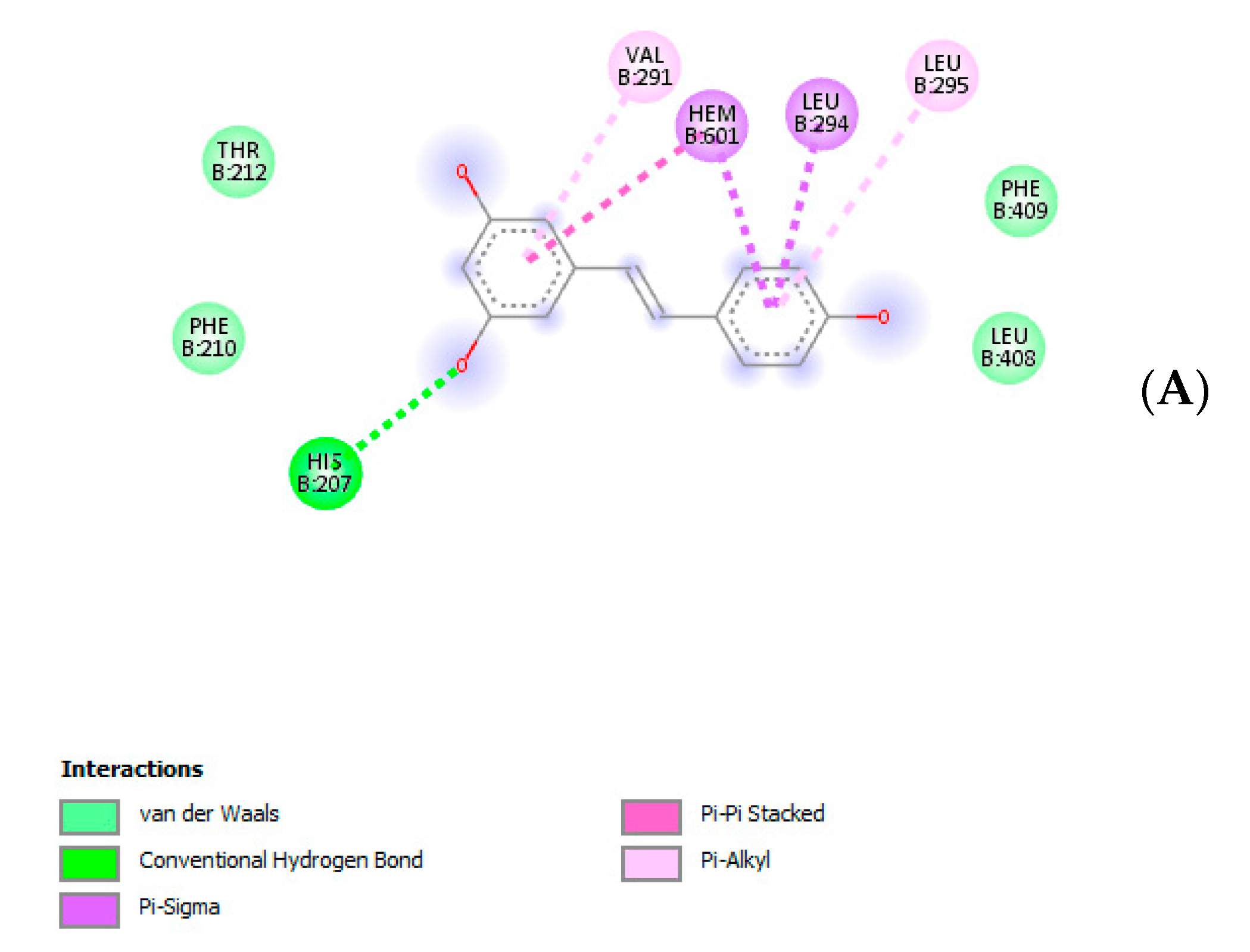
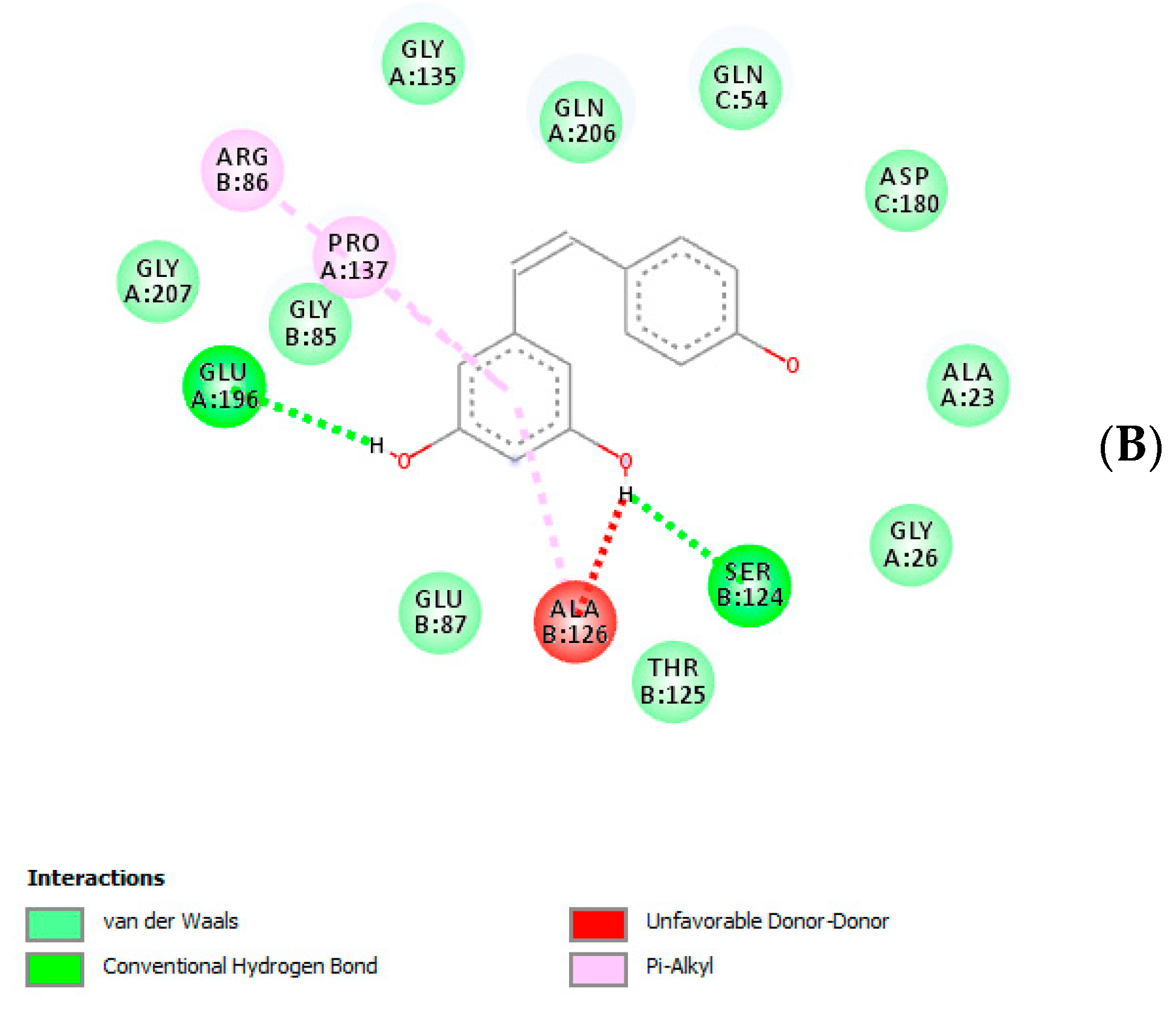
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jones, W.P.; Chin, Y.-W.; Kinghorn, A.D. The Role of Pharmacognosy in Modern Medicine and Pharmacy. Curr. Drug Targets 2006, 7, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Chichiriccò, G.; Ferrante, C.; Menghini, L.; Recinella, L.; Leone, S.; Chiavaroli, A.; Brunetti, L.; Di Simone, S.; Ronci, M.; Piccone, P.; et al. Crocus sativus by-products as sources of bioactive extracts: Pharmacological and toxicological focus on anthers. Food Chem. Toxicol. 2019, 126, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Orlando, G.; Recinella, L.; Chiavaroli, A.; Brunetti, L.; Leone, S.; Carradori, S.; Di Simone, S.C.; Ciferri, M.C.; Zengin, G.; Ak, G.; et al. Water Extract from Inflorescences of Industrial Hemp Futura 75 Variety as a Source of Anti-Inflammatory, Anti-Proliferative and Antimycotic Agents: Results from In Silico, In Vitro and Ex Vivo Studies. Antioxidants 2020, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- The Plant List, Version 1. Available online: http://www.theplantlist.org/ (accessed on 27 February 2020).
- Ozudogru, B.; Akaydın, G.; Erik, S.; Yesilada, E. Inferences from an ethnobotanical field expedition in the selected locations of Sivas and Yozgat provinces (Turkey). J. Ethnopharmacol. 2011, 137, 85–98. [Google Scholar] [CrossRef]
- Slavík, J.; Zácková, P.; Michlová, J.; Opletal, L.; Sovová, M. Phytotherapeutic aspects of diseases of the circulatory system. III. Cardiotonic and cardiotoxic effects of hyrcanoside and deglucohyrcanoside isolated from Coronilla varia L. Ceska Slov. Farm. 1994, 43, 298. [Google Scholar]
- Usta, C.; Yildirim, A.B.; Turker, A.U. Antibacterial and antitumour activities of some plants grown in Turkey. Biotechnol. Biotechnol. Equip. 2014, 28, 306–315. [Google Scholar] [CrossRef]
- Nemati, F.; Dehpouri, A.A.; Eslami, B.; Mahdavi, V.; Mirzanejad, S. Cytotoxic Properties of Some Medicinal Plant Extracts from Mazandaran, Iran. Iran. Red Crescent Med. J. 2013, 15, 8871. [Google Scholar] [CrossRef] [Green Version]
- Renda, G.; Özel, A.; Barut, B.; Korkmaz, B.; Yayli, N. The Volatile Chemical Compositions of the Essential Oil/SPME and Enzyme Inhibitory and Radical Scavenging Activities of Solvent Extracts and the Essential oils from Coronilla orientalis Miller and C. varia L. grows in Turkey. Iran. J. Pharm. Res. 2019, 18, 1831–1842. [Google Scholar]
- Williams, M.; Cassady, J.M. Potential antitumor agents: A cytotoxic cardenolide from Coronilla varia L. J. Pharm. Sci. 1976, 65, 912–914. [Google Scholar] [CrossRef]
- Arnan, X.; Rodrigo, A.; Retana-Alumbreros, J. What are the consequences of ant–seed interactions on the abundance of two dry-fruited shrubs in a Mediterranean scrub? Oecologia 2011, 167, 1027–1039. [Google Scholar] [CrossRef]
- Viano, J.; Masotti, V.; Gaydou, E.M.; Bourreil, P.J.L.; Ghiglione, C.; Giraud, M. Compositional Characteristics of 10 Wild Plant Legumes from Mediterranean French Pastures. J. Agric. Food Chem. 1995, 43, 680–683. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Lamaison, J.; Carnart, A. Teneurs en principaux falvonoïdes des fleurs et des feuilles de Crataegus monogyna Jacq. et de Crataegus laevigata (Poiret) DC. en fonction de la période de végétation. Plantes Médicinales Phytothérapie 1991, 25, 12–16. [Google Scholar]
- Caprioli, G.; Alunno, A.; Beghelli, D.; Bianco, A.; Bramucci, M.; Frezza, C.; Iannarelli, R.; Papa, F.; Quassinti, L.; Sagratini, G.; et al. Polar Constituents and Biological Activity of the Berry-Like Fruits from Hypericum androsaemum L. Front. Plant. Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, A.; Sinan, K.I.; Zengin, G.; Mahomoodally, M.F.; Sadeer, N.B.; Etienne, O.K.; Cziáky, Z.; Jekő, J.; Glamočlija, J.; Sokovic, M.; et al. Identification of Chemical Profiles and Biological Properties of Rhizophora racemosa G. Mey. Extracts Obtained by Different Methods and Solvents. Antioxidants 2020, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Delgado, M.A.; Malovaná, S.; Pérez, J.; Borges, T.; Montelongo, F.G. Separation of phenolic compounds by high-performance liquid chromatography with absorbance and fluorimetric detection. J. Chromatogr. A 2001, 912, 249–257. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standard Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard—Third Edition Clsi Document M27-A4; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Clinical and Laboratory Standard Institute. CLSI Supplement M61: Performance Standards for Antifungal Susceptibility Testing of Filamentous Fungi, 1st ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Clinical and Laboratory Standard Institute. CLSI document M07-A9: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Sabaeifard, P.; Abdi-Ali, A.; Soudi, M.R.; Dinarvand, R. Optimization of tetrazolium salt assay for Pseudomonas aeruginosa biofilm using microtiter plate method. J. Microbiol. Methods 2014, 105, 134–140. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standard Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard—Third Edition Clsi Document M27-A3; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standard Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard—Third Edition Clsi Document M38-A2; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Pagiotti, R.; Angelini, P.; Rubini, A.; Tirillini, B.; Granetti, B.; Venanzoni, R. Identification and characterisation of human pathogenic filamentous fungi and susceptibility to Thymus schimperi essential oil. Mycoses 2010, 54, e364–e376. [Google Scholar] [CrossRef]
- Mahmoodzadeh, H.; Ghasemi, M.; Zanganeh, H. Allelopathic effect of medicinal plant Cannabis sativa L. on Lactuca sativa L. seed germination. Acta Agric. Slov. 2015, 105, 233–239. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B.; et al. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef]
- Özdemir, Z.; Utku, S.; Mathew, B.; Carradori, S.; Orlando, G.; Di Simone, S.; Alagöz, M.A.; Özçelik, A.B.; Uysal, M.; Ferrante, C. Synthesis and biological evaluation of new 3(2H)-pyridazinone derivatives as non-toxic anti-proliferative compounds against human colon carcinoma HCT116 cells. J. Enzym. Inhib. Med. Chem. 2020, 35, 1100–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlando, G.; Chiavaroli, A.; Leone, S.; Brunetti, L.; Politi, M.; Menghini, L.; Recinella, L.; Ferrante, C. Inhibitory Effects Induced by Vicia faba, Uncaria rhyncophylla, and Glycyrrhiza glabra Water Extracts on Oxidative Stress Biomarkers and Dopamine Turnover in HypoE22 Cells and Isolated Rat Striatum Challenged with 6-Hydroxydopamine. Antioxidants 2019, 8, 602. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.D.; Song, J.Y.; Lee, S.J.; Chung, I.M.; Kim, M.J.; Heo, K.; Yu, C.Y. Comparison of resveratrol contents in medicinal plants. Korean J. Medicinal Crop. Sci. 2004, 12, 163–170. [Google Scholar]
- Bertelli, A.A.E. Modulatory effect of resveratrol, a natural phytoalexin, on endothelial adhsion molecules and intracellular signal transduction. Pharmaceutic. Biol. 1998, 36, 44–52. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Brunetti, L.; Orlando, G.; Recinella, L.; Ferrante, C.; Leone, S.; Di Michele, P.; Di Nisio, C.; Vacca, M. Resveratrol inhibits isoprostane production in young and aged rat brain. J. Boil. Regul. Homeost. Agents 2010, 24, 441. [Google Scholar]
- Coker, P.; Radecke, J.; Guy, C.; Camper, N. Potato disc tumor induction assay: A multiple mode of drug action assay. Phytomedicine 2003, 10, 133–138. [Google Scholar] [CrossRef]
- Sinan, K.I.; Chiavaroli, A.; Orlando, G.; Bene, K.; Zengin, G.; Cziáky, Z.; Jekő, J.; Mahomoodally, M.F.; Picot-Allain, M.C.N.; Menghini, L.; et al. Biopotential of Bersama abyssinica Fresen Stem Bark Extracts: UHPLC Profiles, Antioxidant, Enzyme Inhibitory, and Antiproliferative Propensities. Antioxidants 2020, 9, 163. [Google Scholar] [CrossRef] [Green Version]
- Patrignani, P.; Sacco, A.; Sostres, C.; Bruno, A.; Dovizio, M.; Piazuelo, E.; Di Francesco, L.; Contursi, A.; Zucchelli, M.; Schiavone, S.; et al. Low-Dose Aspirin Acetylates Cyclooxygenase-1 in Human Colorectal Mucosa: Implications for the Chemoprevention of Colorectal Cancer. Clin. Pharmacol. Ther. 2017, 102, 52–61. [Google Scholar] [CrossRef]
- Shamsi, F.; Hasan, P.; Queen, A.; Hussain, A.; Khan, P.; Zeya, B.; King, H.M.; Rana, S.; Garrison, J.; Alajmi, M.F.; et al. Synthesis and SAR studies of novel 1,2,4-oxadiazole-sulfonamide based compounds as potential anticancer agents for colorectal cancer therapy. Bioorganic Chem. 2020, 98, 103754. [Google Scholar] [CrossRef]
- Buhrmann, C.; Yazdi, M.; Popper, B.; Shayan, P.; Goel, A.; Aggarwal, B.B.; Shakibaei, M. Evidence that TNF-β induces proliferation in colorectal cancer cells and resveratrol can down-modulate it. Exp. Boil. Med. 2019, 244, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, X.; Lv, J.; Sun, H.; Zhou, F. Resveratrol induces p53 in colorectal cancer through SET7/9. Oncol. Lett. 2019, 17, 3783–3789. [Google Scholar] [CrossRef] [PubMed]
- Elgizawy, H.A.; Ali, A.A.; Hussein, M.A. Resveratrol: Isolation, and Its Nanostructured, Inhibits Cell Proliferation, Induces Cell Apoptosis in Certain Human Cell Lines Carcinoma and Exerts Protective Effect Against Paraquat-Induced Hepatotoxicity. J. Med. Food 2020. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Picot-Allain, M.C.N.; Hosenally, M.; Ugurlu, A.; Mollica, A.; Stefanucci, A.; Llorent-Martínez, E.J.; Baloglu, M.C.; Dall’Acqua, S. Multi-targeted potential of Pittosporum senacia Putt.: HPLC-ESI-MSn analysis, in silico docking, DNA protection, antimicrobial, enzyme inhibition, anti-cancer and apoptotic activity. Comput. Boil. Chem. 2019, 83, 107114. [Google Scholar] [CrossRef] [PubMed]
- Kivrak, E.; Yurt, K.K.; Kaplan, A.A.; Alkan, I.; Altun, G.; Kıvrak, E.G. Effects of electromagnetic fields exposure on the antioxidant defense system. J. Microsc. Ultrastruct. 2017, 5, 167. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Mollica, A.; Stefanucci, A.; Aumeeruddy, M.Z.; Poorneeka, R.; Dall’Acqua, S. Volatile components, pharmacological profile, and computational studies of essential oil from Aegle marmelos (Bael) leaves: A functional approach. Ind. Crop. Prod. 2018, 126, 13–21. [Google Scholar] [CrossRef]
- Shaker, E.S.; Mahmoud, H.; Mnaa, S. Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem. Toxicol. 2010, 48, 803–806. [Google Scholar] [CrossRef]
- Kogiannou, D.A.; Kalogeropoulos, N.; Kefalas, P.; Polissiou, M.G.; Kaliora, A.C. Herbal infusions; their phenolic profile, antioxidant and anti-inflammatory effects in HT29 and PC3 cells. Food Chem. Toxicol. 2013, 61, 152–159. [Google Scholar] [CrossRef]

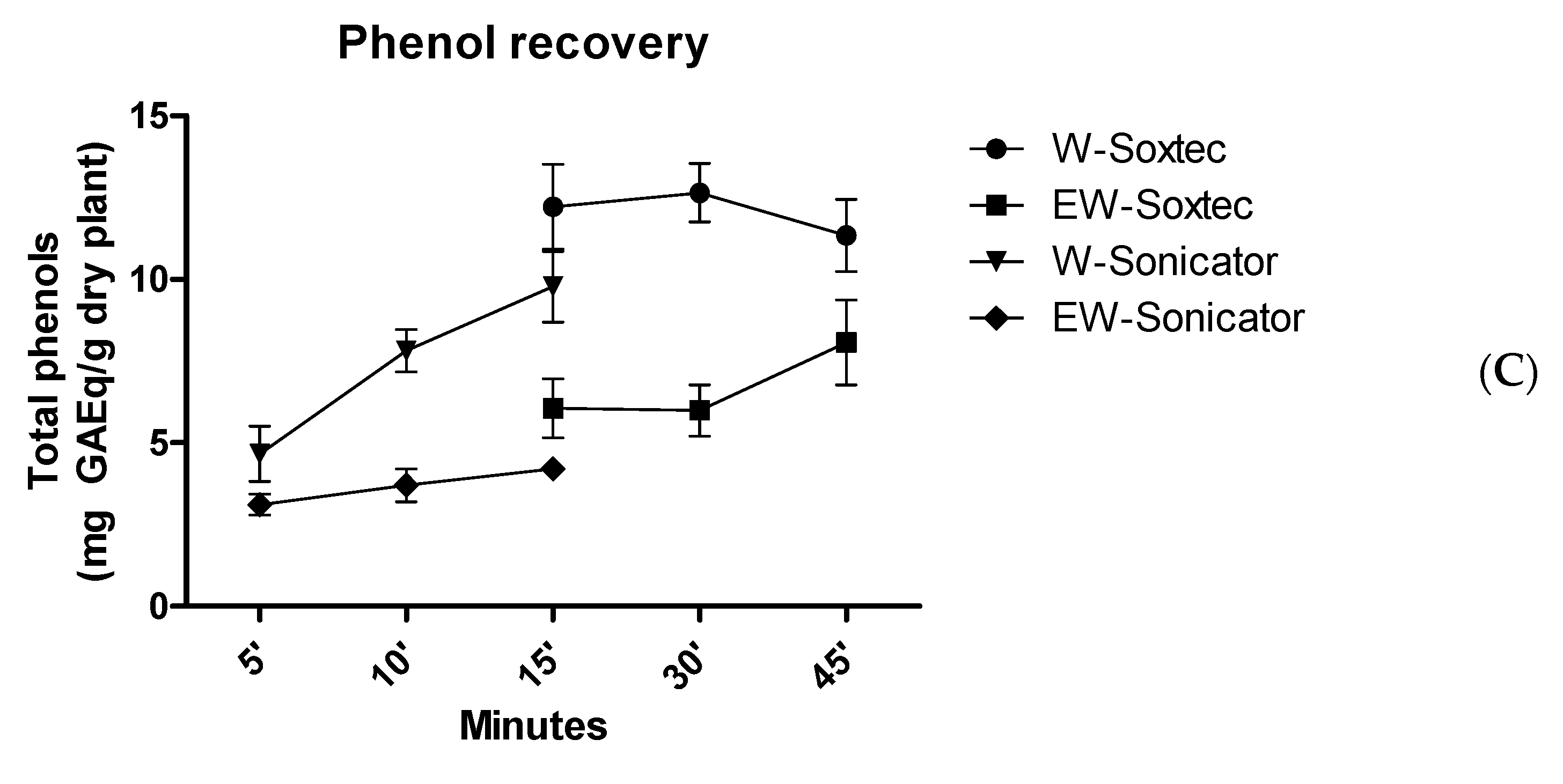
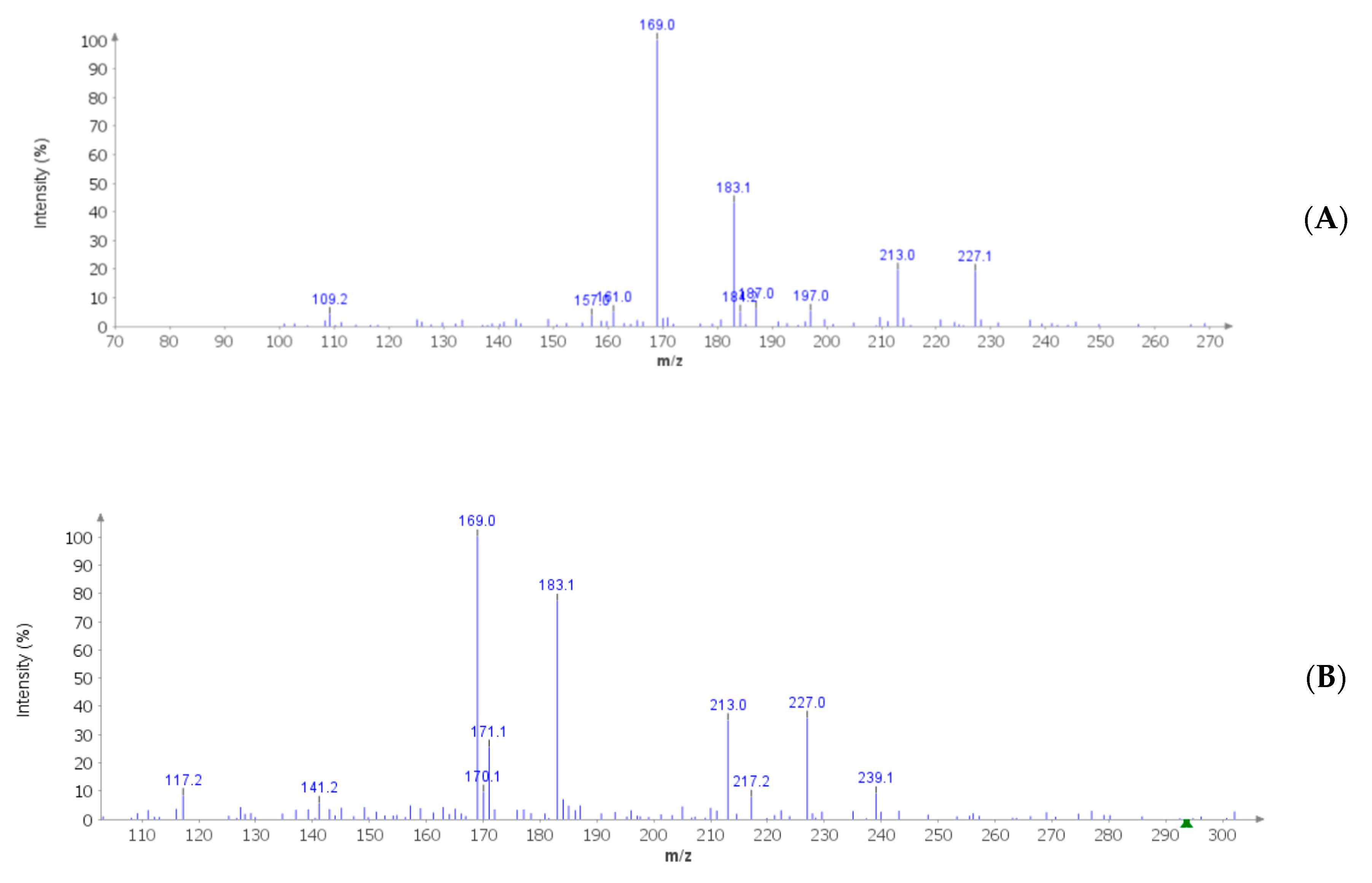


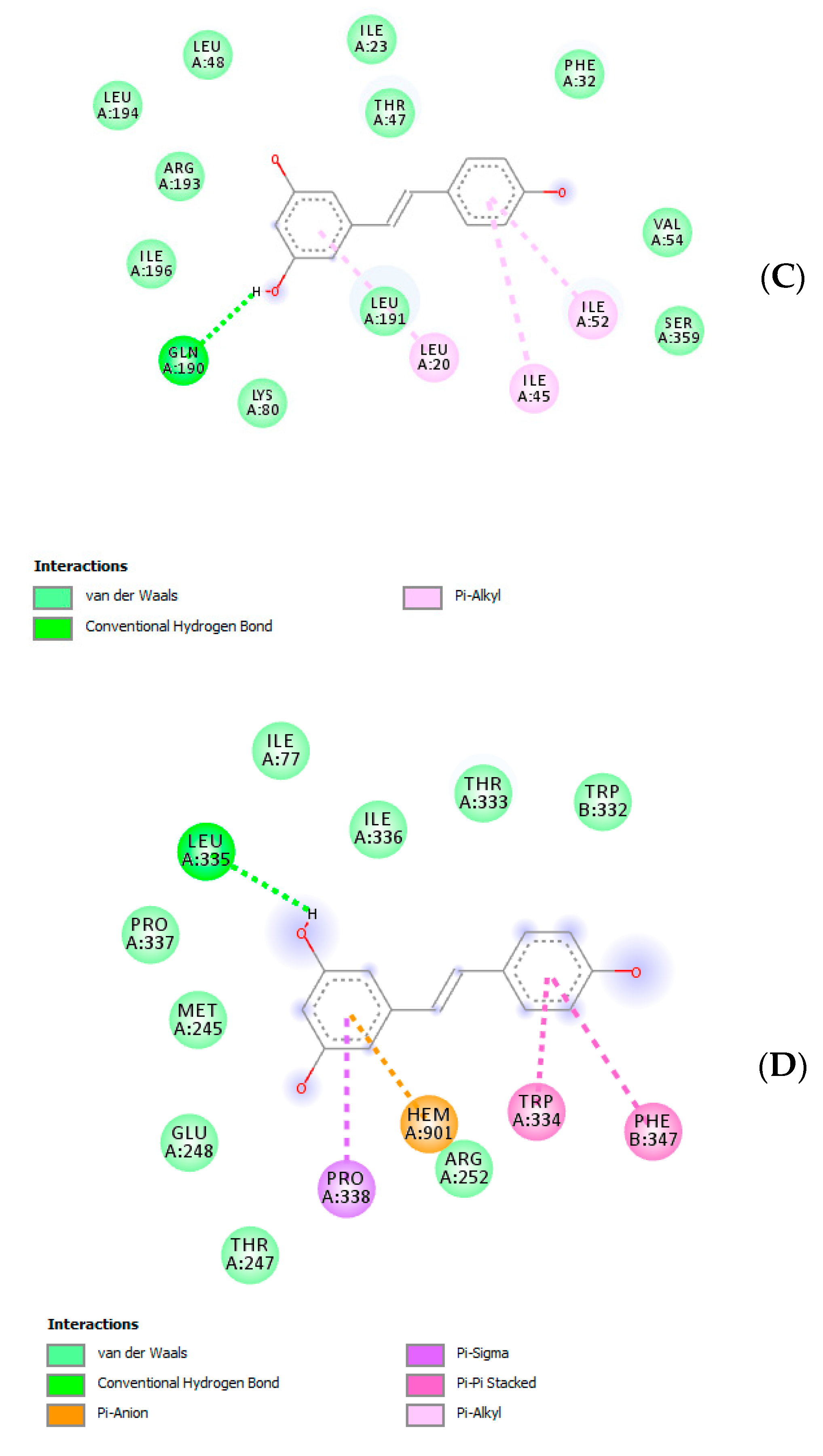

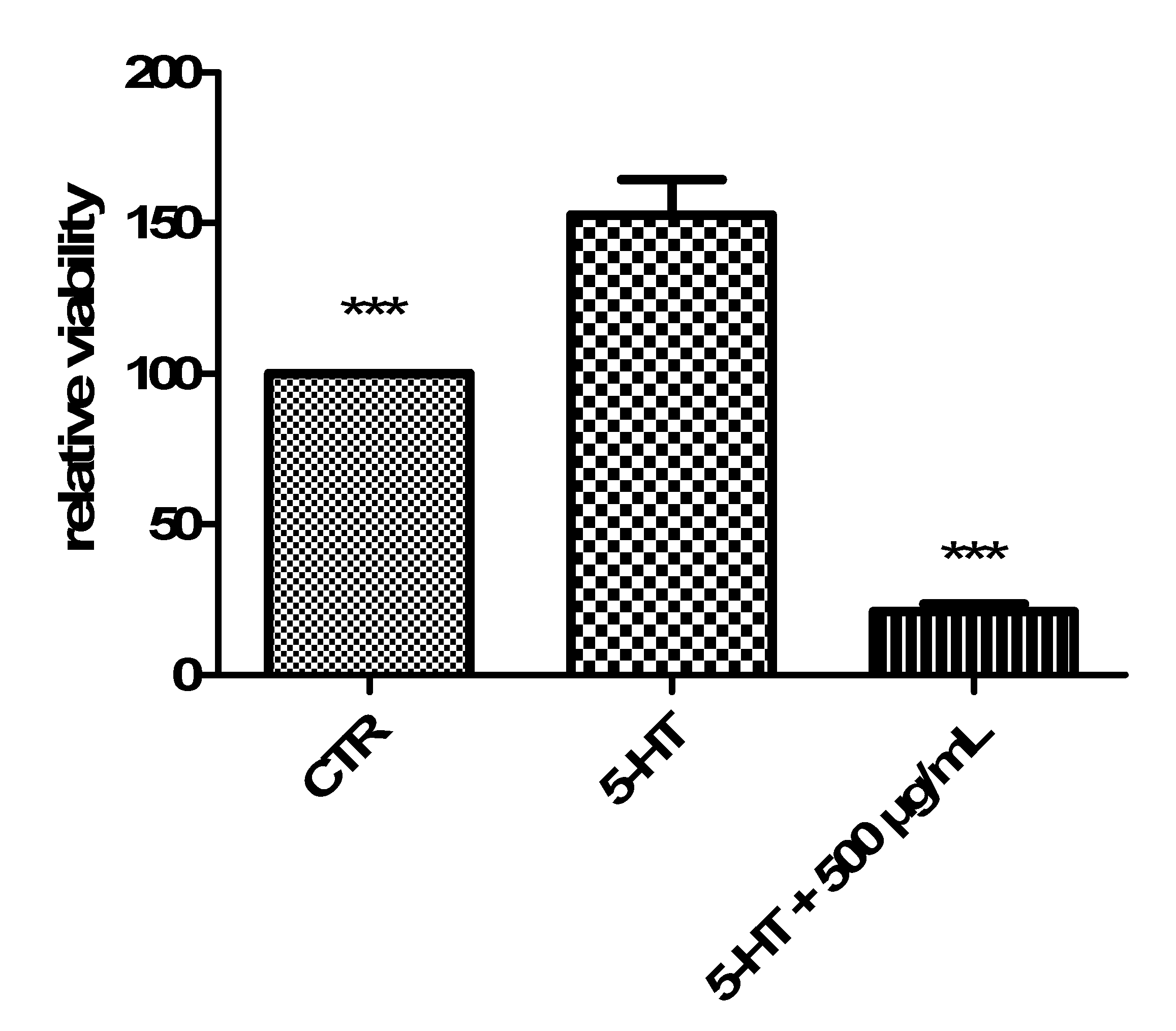

| Soxtec-Assisted Extraction: Extraction Time (Immersion-Rinse) | 45 min–15 min | 30 min–15 min | 15 min–15 min |
|---|---|---|---|
| H2O (200 °C) * | |||
| Et-OH/H2O 50:50 (190 °C) * | |||
| Sonication-assisted extraction: extraction time | 15 min | 10 min | 5 min |
| H2O (60 °C) * | |||
| Et-OH/H2O 50:50 (25 °C) * | |||
| Extracts | Total Phenolic Content (mg GAE/mL ± SD) | Total Flavonoid Content (mg RE/mL ± SD) | DPPH Test IC50 (µg/mL ± SD) | Linoleic Assay IC50 (µg/mL ± SD) |
|---|---|---|---|---|
| Water | 128.23 ± 7.56 | 100.42 ± 6.09 | 26.43 ± 4.20 | 5.44 ± 0.54 |
| EtOH–Water | 61.04 ± 9.18 | 8.95 ± 2.05 | 100.49 ± 7.41 | 54.93 ± 3.99 |
| Solvents | Gallic Acid | Resveratrol |
|---|---|---|
| Water | 722.89 ± 65.06 | 3.18 ± 0.32 |
| Hydroalcoholic solution | 227.25 ± 9.11 | 61.82 ± 6.99 |
| Minimum Inhibitory Concentration (MIC) [µg (Dry Weight) Extract mL−1] * | ||||||
|---|---|---|---|---|---|---|
| Plant Species | Reference Antimycotic Drug | Extract Typology | C. albicans (YEPGA 6183) | C. tropicalis (YEPGA 6184) | A. tubingensis (PeruMycA 21) | A. minutus (PeruMycA 22) |
| Coronilla minima | H2O | 11.34 (9–18) | >18 | >18 | 14.28 (9–18) | |
| H2O:EtOH (1:1) | 7.14 (4.5–9) | >18 | >18 | 11.34 (9–18) | ||
| Fluconazole | - | 2 | 4 | >16 | >16 | |
| Minimum Inhibitory Concentration (MIC) [µg (Dry Weight) extract ml−1] * | ||||||
|---|---|---|---|---|---|---|
| Plant Species | Reference Antibacterial Drug | Extract Typology | B. cereus (ATCC 12826) | S. aureus (ATCC 6538) | E. coli (ATCC 10536) | P. aeruginosa (ATCC 15442) |
| Coronilla minima | H2O | >18 | >18 | >18 | >18 | |
| H2O:EtOH (1:1) | 3.57 (2.25–4.50) | 7.14 (4.5–9) | 7.14 (4.5–9) | 7.14 (4.5–9) | ||
| Ciprofloxacin | - | <0.12 | 0.62 (0.98–0.49) | <0.12 | 1.23 (1.95–0.98) | |
| Assays | Results |
|---|---|
| CUPRAC (mg TE/g) | 62.28 ± 0.22 |
| FRAP (mg TE/g) | 38.88 ± 1.32 |
| DPPH (mg TE/g) | 19.98 ± 0.76 |
| ABTS (mg TE/g) | 61.39 ± 0.30 |
| Phosphomolybdenum (mmol TE/g) | 0.72 ± 0.07 |
| Metal chelating activity (mg EDTAE/g) | 35.83 ± 0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrante, C.; Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Tirillini, B.; Recinella, L.; Chiavaroli, A.; Brunetti, L.; Leone, S.; Di Simone, S.C.; et al. Antimicrobial, Antioxidant, and Antiproliferative Effects of Coronilla minima: An Unexplored Botanical Species. Antibiotics 2020, 9, 611. https://doi.org/10.3390/antibiotics9090611
Ferrante C, Angelini P, Venanzoni R, Angeles Flores G, Tirillini B, Recinella L, Chiavaroli A, Brunetti L, Leone S, Di Simone SC, et al. Antimicrobial, Antioxidant, and Antiproliferative Effects of Coronilla minima: An Unexplored Botanical Species. Antibiotics. 2020; 9(9):611. https://doi.org/10.3390/antibiotics9090611
Chicago/Turabian StyleFerrante, Claudio, Paola Angelini, Roberto Venanzoni, Giancarlo Angeles Flores, Bruno Tirillini, Lucia Recinella, Annalisa Chiavaroli, Luigi Brunetti, Sheila Leone, Simonetta Cristina Di Simone, and et al. 2020. "Antimicrobial, Antioxidant, and Antiproliferative Effects of Coronilla minima: An Unexplored Botanical Species" Antibiotics 9, no. 9: 611. https://doi.org/10.3390/antibiotics9090611
APA StyleFerrante, C., Angelini, P., Venanzoni, R., Angeles Flores, G., Tirillini, B., Recinella, L., Chiavaroli, A., Brunetti, L., Leone, S., Di Simone, S. C., Ciferri, M. C., Zengin, G., Ak, G., Menghini, L., & Orlando, G. (2020). Antimicrobial, Antioxidant, and Antiproliferative Effects of Coronilla minima: An Unexplored Botanical Species. Antibiotics, 9(9), 611. https://doi.org/10.3390/antibiotics9090611
















