Outpatient Antibiotic Dispensing for the Population with Government Health Insurance in Syria in 2018–2019
Abstract
:1. Introduction
2. Methods
2.1. Setting and Data Source
2.2. Study Population
2.3. Data Analysis
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
3.1. Variation of Dispensed Antibiotics According to Patients’ Demographic Characteristics
3.2. Seasonal Variation
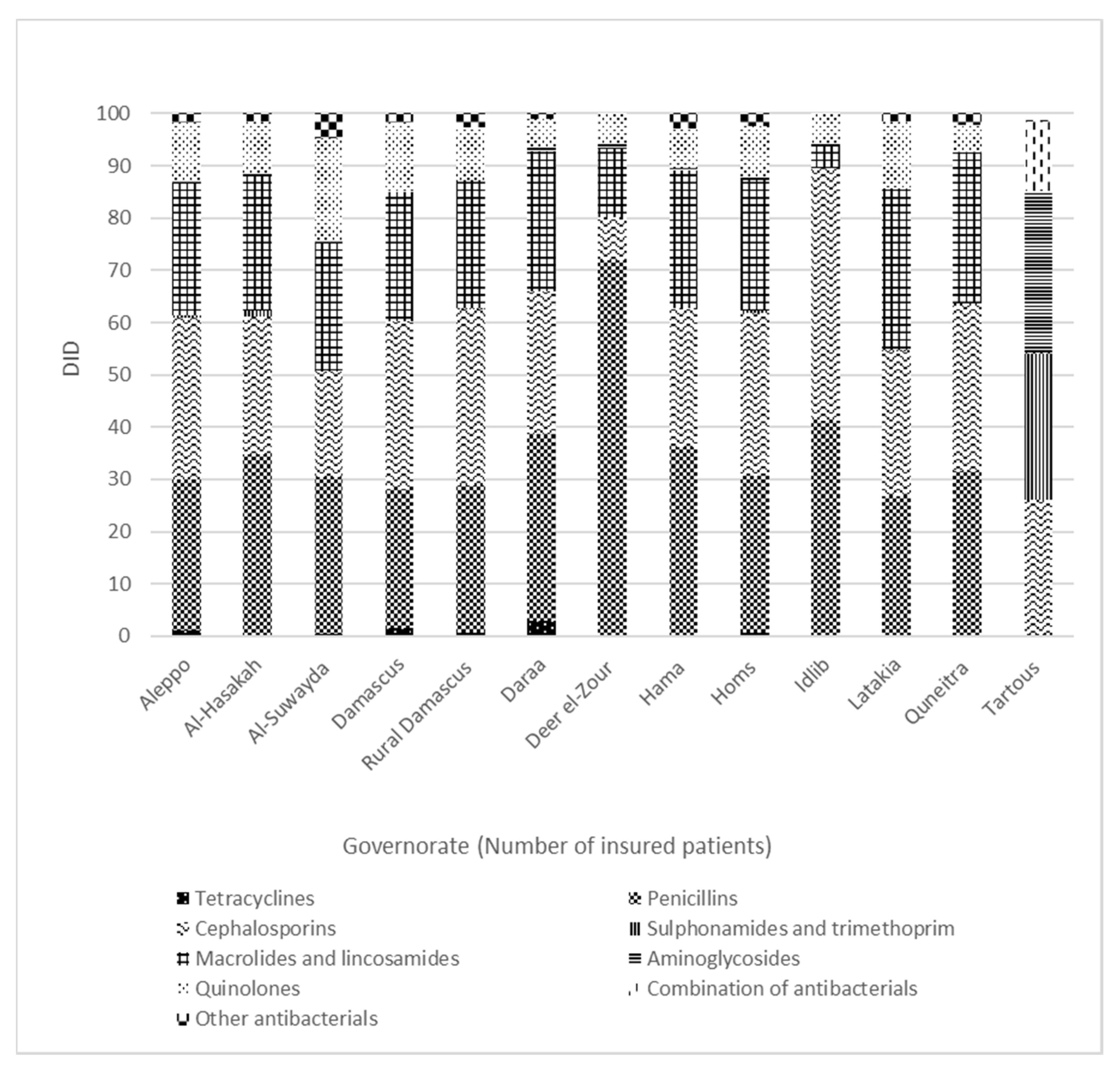
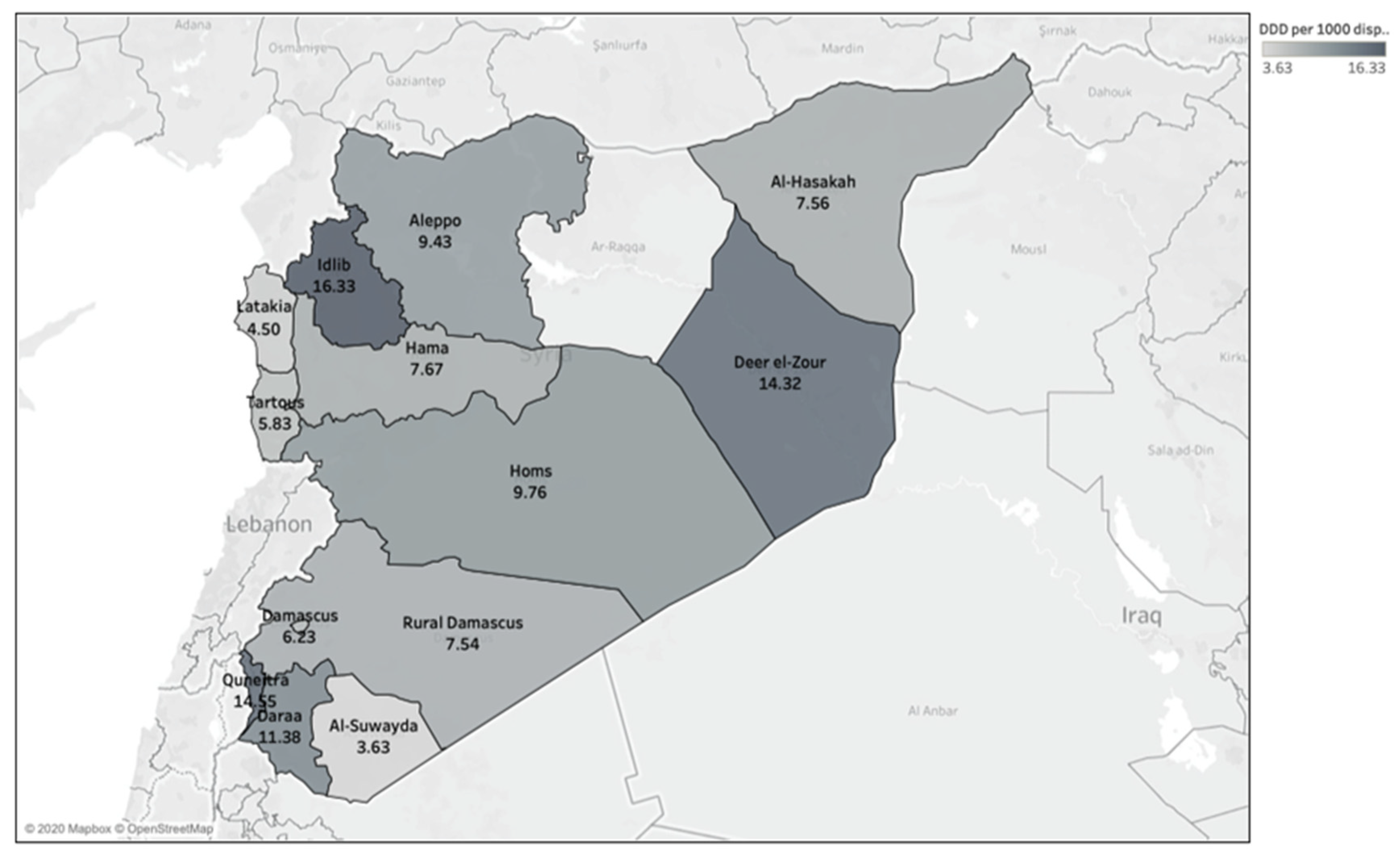
3.3. Regional Variation
4. Discussion
Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Ethics Approval
References
- Aminov, R.I. A brief history of the antibiotic era: Lessons learned and challen ges for the future. Front. Microbiol. 2010, 1, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Global Action Plan on Antimicrobial Resistance 2015. Available online: https://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/ (accessed on 18 May 2020).
- Morgan, D.J.; Okeke, I.N.; Laxminarayan, R.; Perencevich, E.N.; Weisenberg, S. Non-prescription antimicrobial use worldwide: A systematic review. Lancet Infect. Dis. 2011, 11, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Dooling, K.L.; Kandeel, A.; Hicks, L.A.; El-Shoubary, W.; Fawzi, K.; Kandeel, Y.; Etman, A.; Lohiniva, A.L.; Talaat, M. Understanding antibiotic use in Minya District, Egypt: And the factors influencing their practices. Antibiotics 2014, 3, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Haddadin, R.N.; Alsous, M.; Wazaify, M.; Tahaineh, L. Evaluation of antibiotic dispensing practice in community pharmacies in Jordan: A cross sectional study. PLoS ONE 2019, 14, e0216115. [Google Scholar] [CrossRef] [Green Version]
- Syrian Syndicate for Pharmacists. Laws and Orders That Coordinate Pharmacy Career in Syria. Damascus. 1994. Available online: https://www.moph.gov.lb/userfiles/files/Laws%26Regulations/law367-1994.pdf (accessed on 20 April 2020).
- Mansour, O.; Al-Kayali, R. Community Pharmacists’ Role in Controlling Bacterial Antibiotic Resistance in Aleppo, Syria. Iran. J. Pharm. Res. 2017, 16, 1612–1620. [Google Scholar]
- Abbara, A.; Rawson, T.M.; Karah, N.; El-Amin, W.; Hatcher, J.; Tajaldin, B.; Dar, O.; Dewachi, O.; Sitta, G.A.; Uhlin, B.E.; et al. Antimicrobial resistance in the context of the Syrian conflict: Drivers before and after the onset of conflict and key recommendations. Int. J. Infect. Dis. 2018, 73, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Sharara, S.L.; Kanj, S.S. War and infectious diseases: Challenges of the Syrian civil war. PLoS Pathog. 2014, 10, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Syrian Arab Republic, Ministry of Health Statistics. Health Statstics. 2009. Available online: http://www.moh.gov.sy/Default.aspx?tabid=56&language=ar-YE (accessed on 30 May 2020).
- Kherallah, M.; Alahfez, T.; Sahloul, Z.; Eddin, K.D.; Jamil, G. Health care in Syria before and during the crisis. Avicenna J. Med. 2012, 2, 51–53. [Google Scholar] [CrossRef]
- Jakovljevic, M.; Al Ahdab, S.; Jurisevic, M.; Mouselli, S. Antibiotic resistance in Syria: A local problem turns into a global threat. Front. Public Health 2018, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. ANNUAL REPORT 2018: Syrian Arab Republic. 2018. Available online: https://applications.emro.who.int/docs/COPub_SYR_2018_EN_22335.pdf?ua=1&ua=1 (accessed on 30 May 2020).
- Sen, K.; Faisal, W.A. Syria neoliberal reforms in health sector financing: Embedding unequal access? Soc. Med. 2012, 6, 171–182. [Google Scholar]
- Schwefel, D. Towards a National Health Insurance System in Syria: Documents, Materials and Excerpts from Short-Term Consultancy Reports of Detlef Schwefel 2003–2008; Consortium GTZ/EPOS/OPTIONS: Berlin, Germany, 2008. [Google Scholar]
- Mershed, M.; Busse, R.; van Ginneken, E. Healthcare financing in Syria: Satisfaction with the current system and the role of national health insurance—A qualitative study of householders’ views. Int. J. Health Plan. Manag. 2012, 27, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Syrian Insurance Supervisory Commission. Syrian Insurance Sector Report for 2019. Available online: http://www.sisc.sy/Publications/37/32/Ar (accessed on 8 June 2020).
- Wettermark, B.; Godman, B.; Bennie, M. Drug Utilization Research: Methods and Applications; Elseviers, M., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2016; ISBN 9781118949788. [Google Scholar]
- Barah, F.; Morris, J.; Gonçalves, V. Irrational use and poor public beliefs regarding antibiotics in developing countries: A pessimistic example of Syria. Int. J. Clin. Pract. Suppl. 2009, 63, 1263–1264. [Google Scholar] [CrossRef]
- Al-Faham, Z.; Habboub, G.; Takriti, F. The sale of antibiotics without prescription in pharmacies in Damascus, Syria. J. Infect. Dev. Ctries. 2011, 5, 396–399. [Google Scholar] [CrossRef] [Green Version]
- Barah, F.; Gonçalves, V. Antibiotic use and knowledge in the community in Kalamoon, Syrian Arab Republic: A cross-sectional study. East. Mediterr. Health J. 2010, 16, 516–521. [Google Scholar] [CrossRef]
- Syrian Economic Sciences Society. Employment and Livelihood Support in Syria: A Study Conducted for UNDP Syria by the Syrian Economic Sciences Society. 2018. Available online: file:///C:/Users/asus/AppData/Local/Temp/Employment%20and%20Livelihoods%20study_English.pdf (accessed on 10 June 2020).
- WHO Collaborating Centre for Drug Statistics Methodology. Anatomical Therapeutic Chemical (ATC) Classification System: ATC/DDD Index 2020. Available online: http://www.whocc.no/atcddd (accessed on 20 April 2020).
- World Health Organization. WHO Releases the 2019 AWaRe Classification Antibiotics. 2019. Available online: https://www.who.int/medicines/news/2019/WHO_releases2019AWaRe_classification_antibiotics/en/ (accessed on 18 May 2020).
- Bergman, U.; Popa, C.; Tomson, Y.; Wettermark, B.; Einarson, T.R.; Aberg, H.; Sjöqvist, F. Drug utilization 90%—A simple method for assessing the quality of drug prescribing. Eur. J. Clin. Pharmacol. 1998, 54, 113–118. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows; IBM Corp.: Armonk, NY, USA, 2017. [Google Scholar]
- Borg, M.A.; Zarb, P.; Ferech, M.; Goossens, H. Antibiotic consumption in southern and eastern Mediterranean hospitals: Results from the ARMed project. J. Antimicrob. Chemother. 2008, 62, 830–836. [Google Scholar] [CrossRef] [Green Version]
- Chandy, S.J.; Thomas, K.; Mathai, E.; Antonisamy, B.; Holloway, K.A.; Stalsby Lundborg, C. Patterns of antibiotic use in the community and challenges of antibiotic surveillance in a lower-middle-income country setting: A repeated cross-sectional study in Vellore, South India. J. Antimicrob. Chemother. 2013, 68, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Zanichelli, V.; Monnier, A.A.; Gyssens, I.C.; Adriaenssens, N.; Versporten, A.; Pulcini, C.; Le Maréchal, M.; Tebano, G.; Vlahović-Palčevski, V.; Stanić Benić, M.; et al. Variation in antibiotic use among and within different settings: A systematic review. J. Antimicrob. Chemother. 2018, 73, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Al-Niemat, S.I.; Aljbouri, T.M.; Goussous, L.S.; Efaishat, R.A.; Salah, R.K. Antibiotic prescribing patterns in outpatient emergency clinics at queen rania al abdullah ii children’s hospital, Jordan, 2013. Oman Med. J. 2014, 29, 250–254. [Google Scholar] [CrossRef]
- World Health Organization. WHO Report on Surveillance of Antibiotic Consumption: 2016–2018 Early Implementation. 2018. Available online: https://www.who.int/medicines/areas/rational_use/oms-amr-amc-report-2016-2018/en/ (accessed on 5 June 2020).
- European Centre for Disease Prevention and Control. Antimicrobial Consumption Database: (ESAC-Net), 2017–2018. Available online: https://www.ecdc.europa.eu/en/antimicrobial-consumption/database/country-overview (accessed on 5 June 2020).
- Abbara, A.; Rawson, T.M.; Karah, N.; El-Amin, W.; Hatcher, J.; Tajaldin, B.; Dar, O.; Dewachi, O.; Sitta, G.A.; Uhlin, B.E.; et al. A summary and appraisal of existing evidence of antimicrobial resistance in the Syrian conflict. Int. J. Infect. Dis. 2018, 75, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Rann, O.; Sharland, M.; Long, P.; Wong, I.C.K.; Laverty, A.A.; Bottle, A.; Barker, C.I.; Bielicki, J.; Saxena, S. Did the accuracy of oral amoxicillin dosing of children improve after British National Formulary dose revisions in 2014? National cross-sectional survey in England. BMJ Open 2017, 7, e016363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šahman-Zaimović, M.; Vukmirović, S.; Tomić, N.-; Stilinović, N.; Horvat, O.; Tomić, L. Relationship between outpatient antibiotic use and the prevalence of bacterial infections in Montenegro. Vojn. Pregl. 2017, 74, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Aabenhus, R.; Siersma, V.; Hansen, M.P.; Bjerrum, L. Antibiotic prescribing in Danish general practice 2004–13. J. Antimicrob. Chemother. 2016, 71, 2286–2294. [Google Scholar] [CrossRef] [Green Version]
- Norris, P.; Horsburgh, S.; Keown, S.; Arroll, B.; Lovelock, K.; Cumming, J.; Herbison, P.; Crampton, P.; Becket, G. Too much and too little? Prevalence and extent of antibiotic use in a New Zealand region. J. Antimicrob. Chemother. 2011, 66, 1921–1926. [Google Scholar] [CrossRef] [Green Version]
- Hicks, L.A.; Bartoces, M.G.; Roberts, R.M.; Suda, K.J.; Hunkler, R.J.; Taylor, T.H.; Schrag, S.J. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin. Infect. Dis. 2015, 60, 1308–1316. [Google Scholar] [CrossRef] [Green Version]
- Blix, H.S.; Engeland, A.; Litleskare, I.; Rønning, M. Age- and gender-specific antibacterial prescribing in Norway. J. Antimicrob. Chemother. 2007, 59, 971–976. [Google Scholar] [CrossRef] [Green Version]
- Nitzan, O.; Low, M.; Lavi, I.; Hammerman, A.; Klang, S.; Raz, R. Variability in outpatient antimicrobial consumption in Israel. Infection 2010, 38, 12–18. [Google Scholar] [CrossRef]
- Williamson, D.A.; Roos, R.; Verrall, A.; Smith, A.; Thomas, M.G. Trends, demographics and disparities in outpatient antibiotic consumption in New Zealand: A national study. J. Antimicrob. Chemother. 2016, 71, 3593–3598. [Google Scholar] [CrossRef] [Green Version]
- Kandeel, A.; El-Shoubary, W.; Hicks, L.A.; Fattah, M.A.; Dooling, K.L.; Lohiniva, A.L.; Ragab, O.; Galal, R.; Talaat, M. Patient attitudes and beliefs and provider practices regarding antibiotic use for acute respiratory tract infections in Minya, Egypt. Antibiotics 2014, 3, 632–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Versporten, A.; Bolokhovets, G.; Ghazaryan, L.; Abilova, V.; Pyshnik, G.; Spasojevic, T.; Korinteli, I.; Raka, L.; Kambaralieva, B.; Cizmovic, L.; et al. Antibiotic use in eastern Europe: A cross-national database study in coordination with the WHO Regional Office for Europe. Lancet Infect. Dis. 2014, 14, 381–387. [Google Scholar] [CrossRef]
- Coenen, S.; Muller, A.; Adriaenssens, N.; Vankerckhoven, V.; Hendrickx, E.; Goossens, H. European Surveillance of Antimicrobial Consumption (ESAC): Outpatient parenteral antibiotic treatment in Europe. J. Antimicrob. Chemother. 2009, 64, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.A. Outpatient Parenteral Antimicrobial Therapy (OPAT) in the Kingdom of Bahrain: Efficacy, patient satisfaction and cost effectiveness. Infect. Drug Resist. 2013, 7, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Gruenberg, K.; Guglielmo, B.J. Anti-Infective Chemotherapeutic & Antibiotic Agents; McGraw-Hill: New York, NY, USA, 2019; Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=2449§ionid=194442959 (accessed on 13 June 2020).
- European Asylum Support Office. Syria Security Situation. 2019. Available online: https://coi.easo.europa.eu/administration/easo/PLib/11_2019_EASO_COI_Report_Syria_Security_situation.pdf (accessed on 20 June 2020).
- Taleb, Z.B.; Bahelah, R.; Fouad, F.M.; Coutts, A.; Wilcox, M.; Maziak, W. Syria: Health in a country undergoing tragic transition. Int. J. Public Health 2015, 60, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Abbara, A.; Almalla, M.; AlMasri, I.; AlKabbani, H.; Karah, N.; El-Amin, W.; Rajang, L.; Rahhalh, I.; Alabbash, M.; Sahlouli, Z.; et al. The challenges of tuberculosis control in protracted conflict: The case of Syria. Int. J. Infect. Dis. 2020, 90, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Syrian General Association for Insurance. Health Insurance Guide. Available online: https://www.sic-eclaim.com/Assets/SIC/Docs/HealthcareInsuranceGuide.pdf (accessed on 1 June 2020).

| Number | Antibiotic | AWaRe | DID | Proportion of Total DID |
|---|---|---|---|---|
| 1 | Amoxicillin/Clavulanic acid | Access | 5.76 | 28.62% |
| 2 | Clarithromycin | Watch | 4.4 | 21.87% |
| 3 | Cefixime | Watch | 2.66 | 13.05% |
| 4 | Cefuroxime | Watch | 1.85 | 9.17% |
| 5 | Levofloxacin | Watch | 1.09 | 5.4% |
| 6 | Azithromycin | Watch | 0.72 | 3.59% |
| 7 | Cefdinir | Watch | 0.63 | 3.15% |
| 8 | Cefprozil | Watch | 0.55 | 2.71% |
| 9 | Ciprofloxacin | Watch | 0.51 | 2.55% |
| Drug Utilization 90% (DU90%) 1–9 | 18.14 | 90.11% | ||
| Others 10–53 | 1.99 | 9.89% | ||
| Total | 20.13 | 100% |
| Pharmacological Group | DID | Proportion of Total DID |
|---|---|---|
| Cephalosporins (J01D) | 6.14 | 30.49% |
| Penicillins (J01C) | 6.01 | 29.87% |
| Macrolides, lincosamides and streptogramins (J01F) | 5.19 | 25.78% |
| Quinolones (J01M) | 2.08 | 10.31% |
| Other antibacterials (e.g., linezolid, metronidazole and nitrofurantoin) (J01X) | 0.46 | 2.29% |
| Tetracyclines (J01A) | 0.14 | 0.6% |
| Aminoglycosides (J01G) | 0.06 | 0.29% |
| Sulfonamides and trimethoprim (J01E) | 0.04 | 0.21% |
| Combinations of antibacterials (J01R) | 0.01 | 0.06% |
| Total | 20.13 | 100% |
| AWaRe | DID | Proportion of Total DID |
|---|---|---|
| Access | 6.55 | 32.54% |
| Watch | 13.26 | 65.86% |
| Reserve | 0.17 | 0.83% |
| Nonclassified | 0.15 | 0.77% |
| Total | 20.13 | 100% |
| Combination of Antibiotics | AWaRe | DID | Proportion of the Total DID of Antibiotic Combinations |
|---|---|---|---|
| Amoxicillin + clavulanic Acid | Access | 5.76 | 96.51% |
| Ceftriaxone + Sulbactam | Not Recommended | 0.09 | 1.47% |
| Amoxicillin + Flucloxacillin | Not Recommended | 0.06 | 1.08% |
| Sulfamethoxazole + Trimethoprim | Access | 0.04 | 0.7% |
| Spiramycin + Metronidazole | Not Recommended | 0.01 | 0.2% |
| Ampicillin + Cloxacillin | Not Recommended | 0.002 | 0.04% |
| Ampicillin + Sulbactam | Access | 0.0001 | 0.002% |
| Total | 5.96 | 100% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljadeeah, S.; Wirtz, V.J.; Nagel, E. Outpatient Antibiotic Dispensing for the Population with Government Health Insurance in Syria in 2018–2019. Antibiotics 2020, 9, 570. https://doi.org/10.3390/antibiotics9090570
Aljadeeah S, Wirtz VJ, Nagel E. Outpatient Antibiotic Dispensing for the Population with Government Health Insurance in Syria in 2018–2019. Antibiotics. 2020; 9(9):570. https://doi.org/10.3390/antibiotics9090570
Chicago/Turabian StyleAljadeeah, Saleh, Veronika J. Wirtz, and Eckhard Nagel. 2020. "Outpatient Antibiotic Dispensing for the Population with Government Health Insurance in Syria in 2018–2019" Antibiotics 9, no. 9: 570. https://doi.org/10.3390/antibiotics9090570





