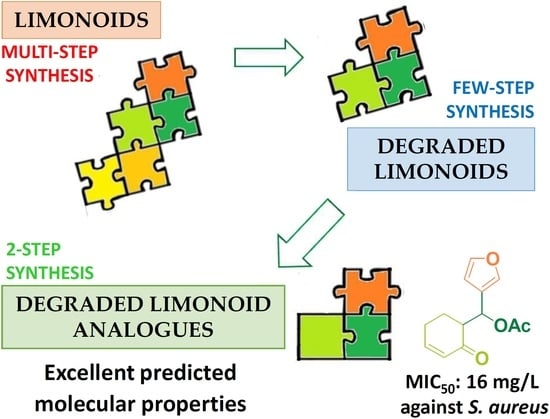
Synthesis of Degraded Limonoid Analogs as New Antibacterial Scaffolds against Staphylococcus aureus
Abstract
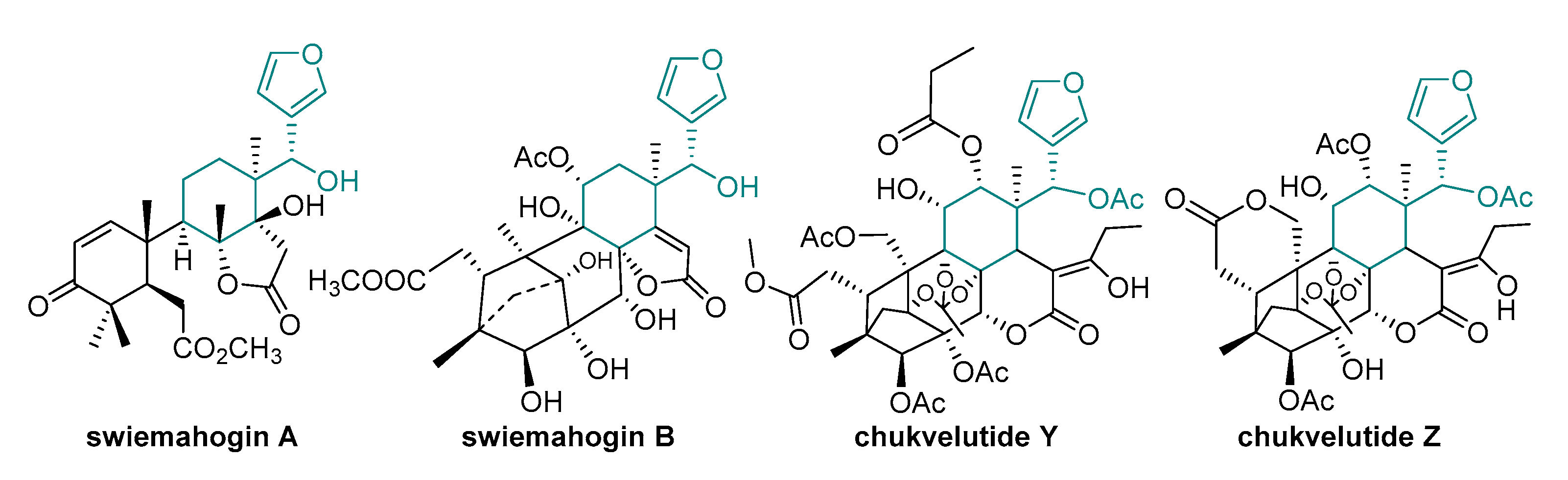
1. Introduction
2. Results
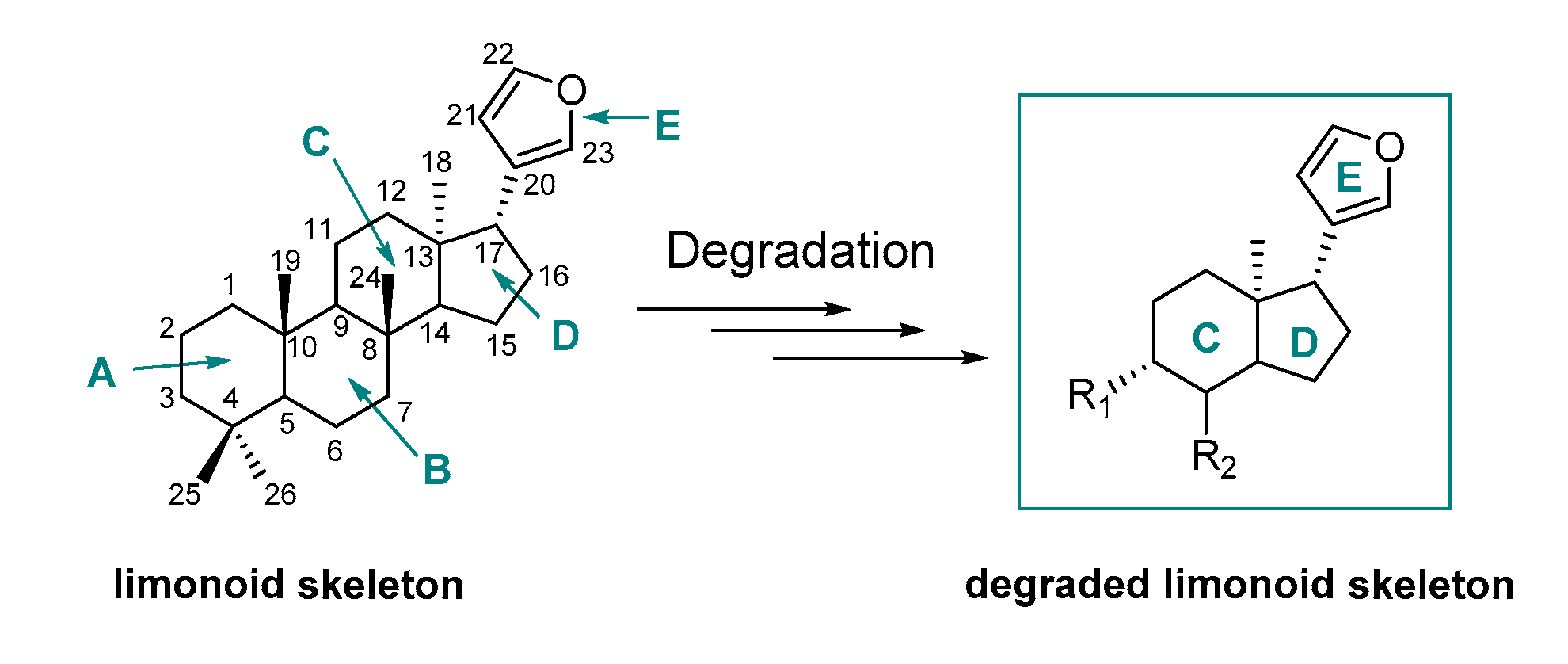
2.1. Design of Model Molecules Based on Degraded Limonoid Skeleton and Molecular Properties Prediction
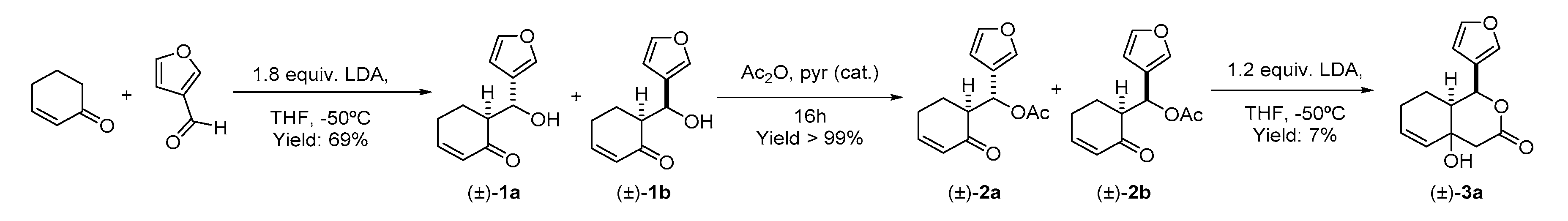
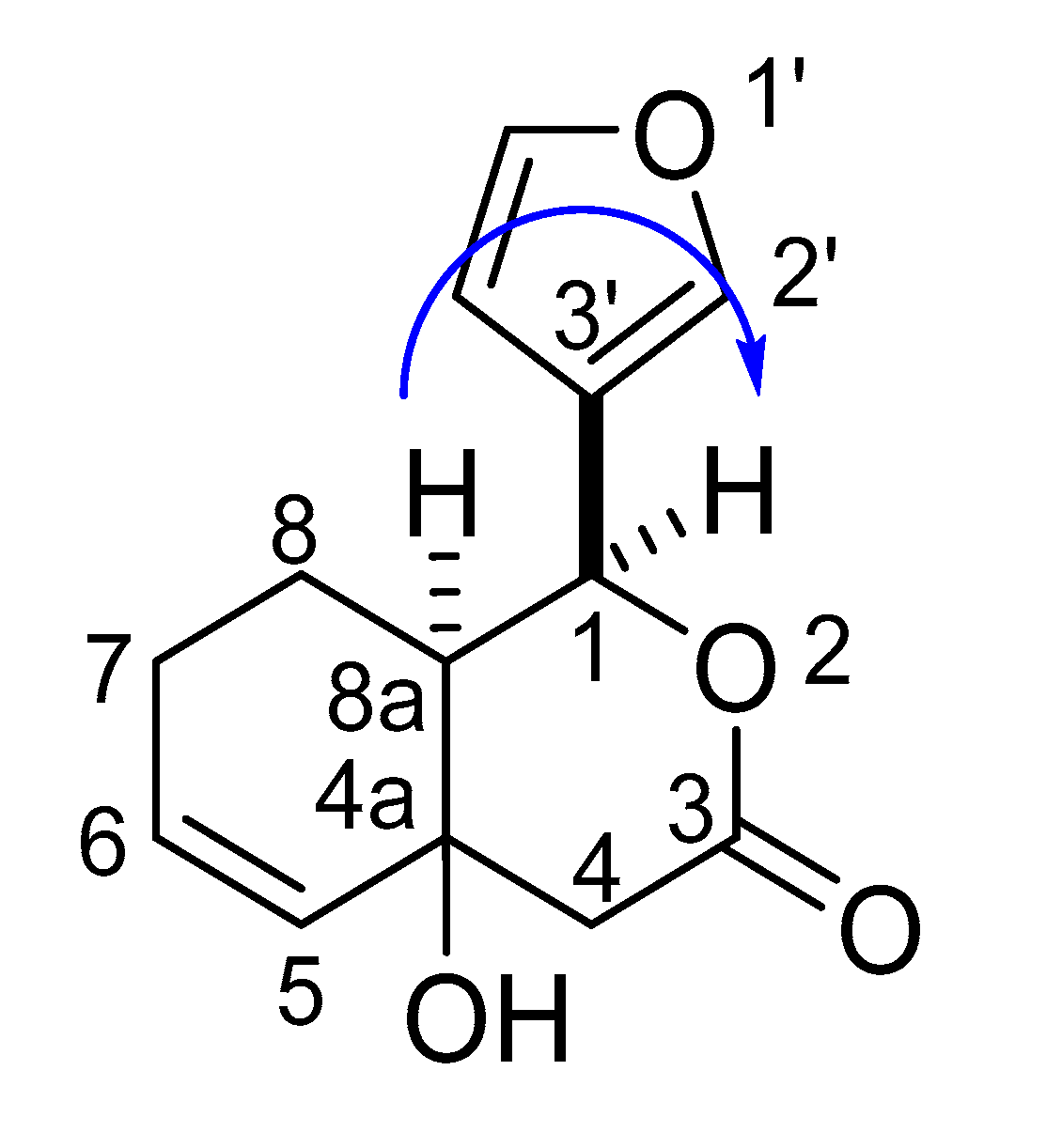
2.2. Synthesis of the Degraded Limonoid Analogs 1–3 and Chemical Characterization
2.3. Antimicrobial Activity of the Degraded Limonoid Analogs 1–3
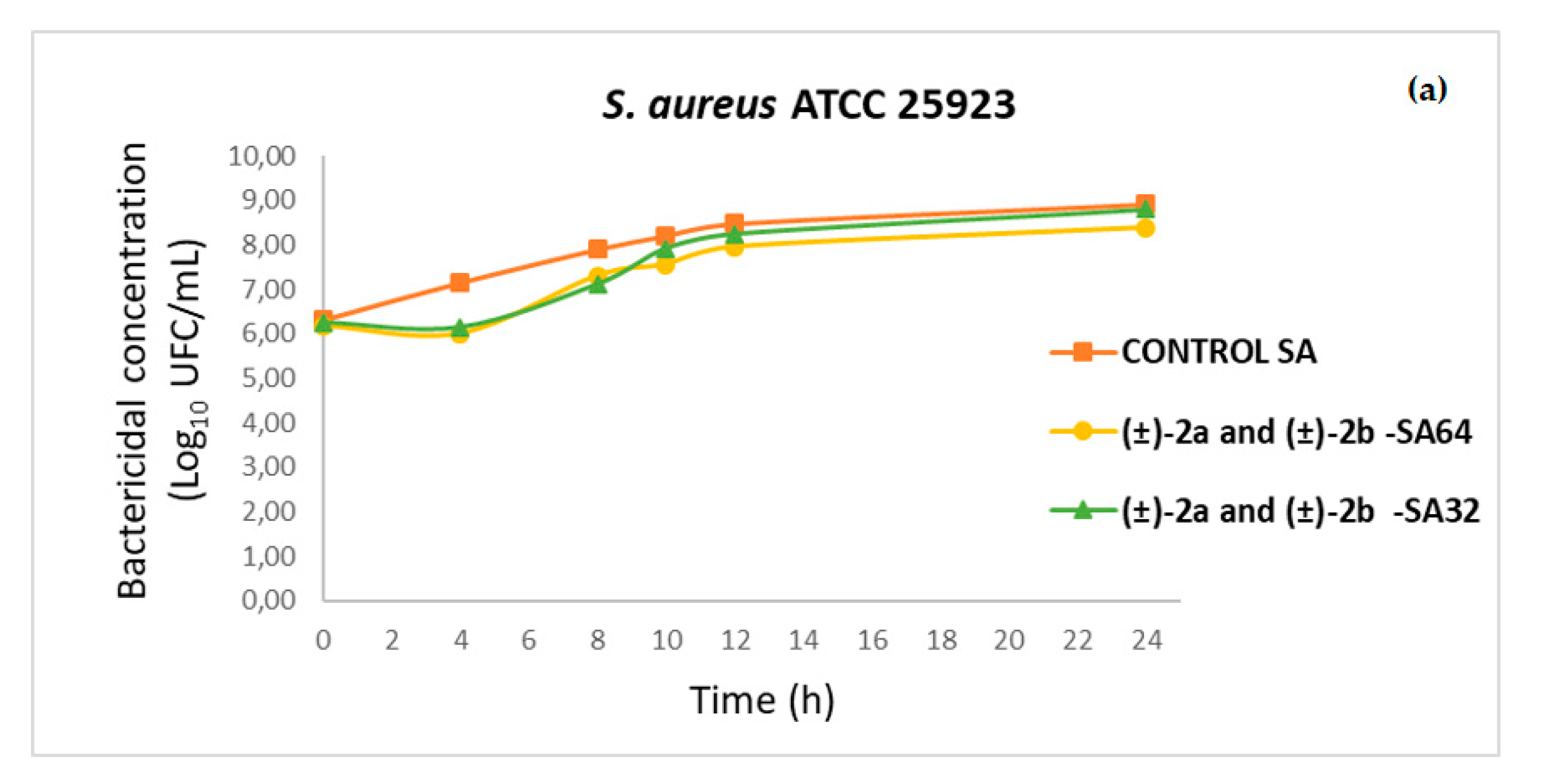
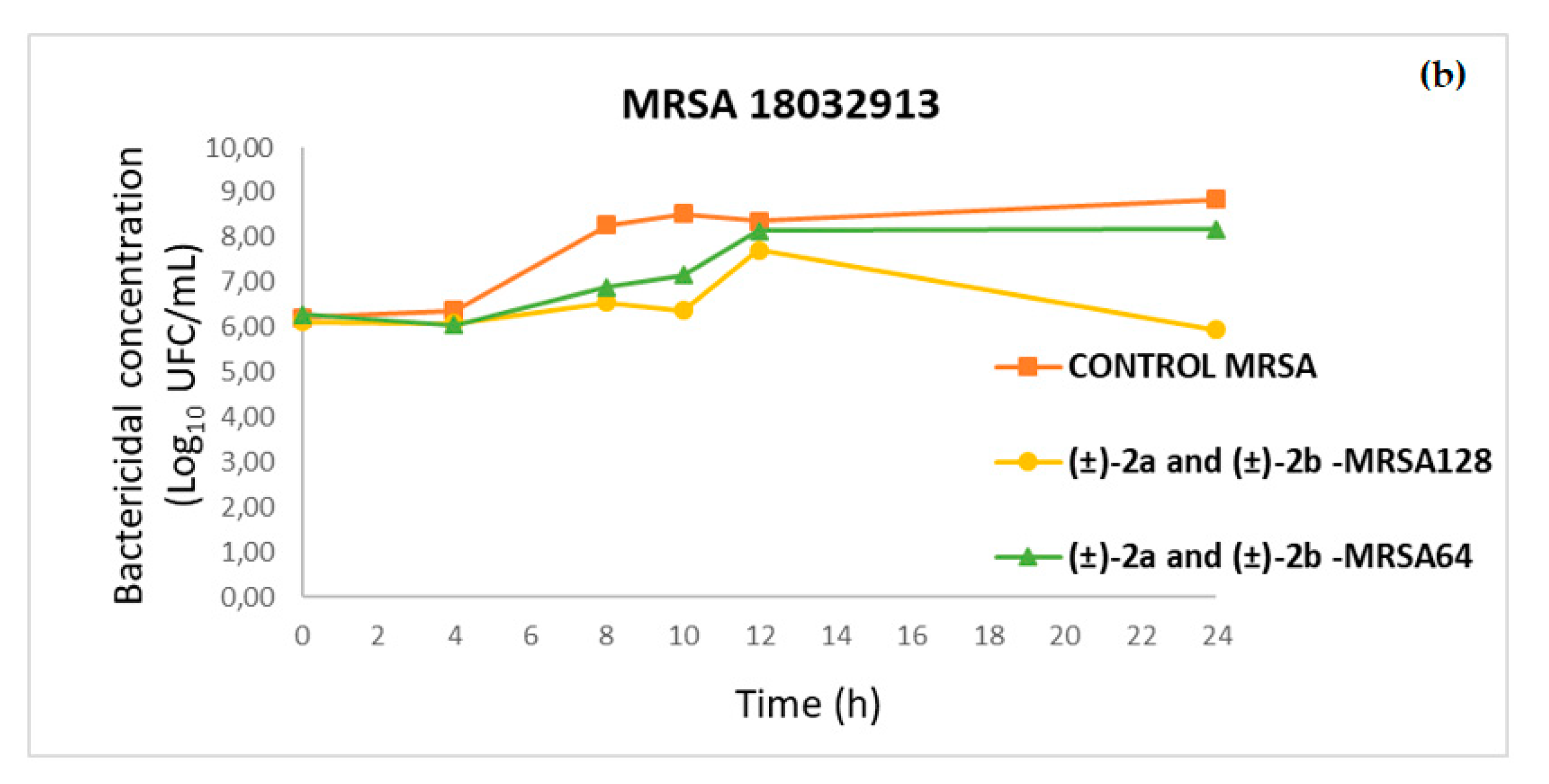
2.4. Time–Kill Curves
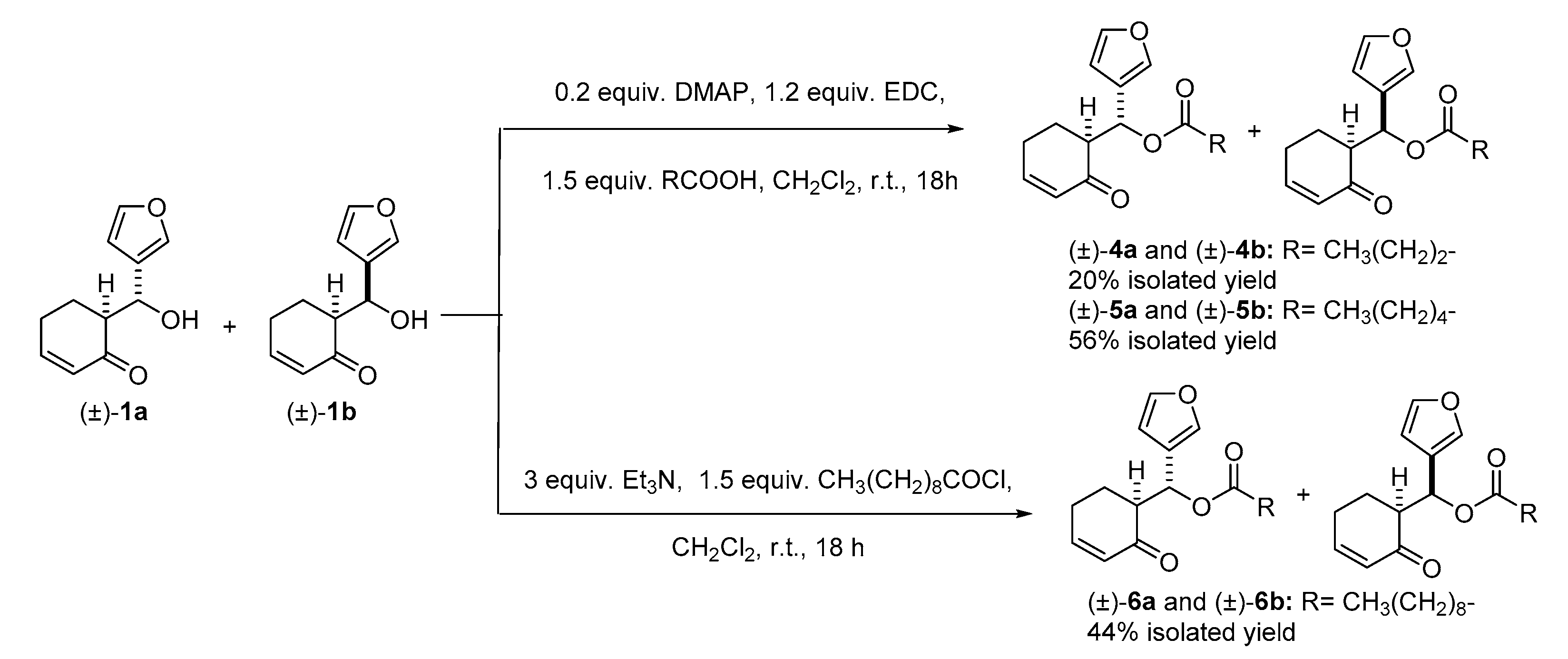
2.5. Synthesis and Antimicrobial Activity of More Lipophilic Degraded-Limonoid Analogs 4–6 and Chemical Characterization
3. Discussion
4. Materials and Methods
4.1. General
4.2. Reaction Procedures
4.2.1. Preparation of 6-(furan-3-yl-(hydroxy)methyl)cyclohex-2-en-1-one (Mixture of Stereoisomers (±)-1a and (±)-1b)
4.2.2. Preparation of furan-3-yl-(2-oxocyclohex-3-en-1-yl)-methyl acetate (Mixture of Stereoisomers (±)-2a and (±)-2b)
4.2.3. Preparation of (1S*,8aS*)-1-(furan-3-yl)-4a-hydroxy-1,4,4a,7,8,8a-hexahydro-3H-isocromen-3-one ((±)-3a)
4.2.4. General Procedure for Steglich Esterification. Preparation of (±)-4a, (±)-4b, (±)-5a and (±)-5b
4.2.5. Preparation of (±)-6a and (±)-6b
4.3. Bacterial Strains
4.4. In Vitro Susceptibility Testing
4.5. Time–Kill Kinetic Assays
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sass, P. Antibiotics: Precious goods in changing times. Methods Mol. Biol. 2017, 1520, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Choo, E.J.; Chambers, H.F. Treatment of methicillin-resistant Staphylococcus aureus bacteremia. Infect. Chemother. 2016, 48, 267–273. [Google Scholar] [CrossRef] [PubMed]
- De Maat, V.; Stege, P.B.; Dedden, M.; Hamer, M.; van Pijkeren, J.-P.; Willems, R.J.L.; van Schaik, W. CRISPR-Cas9-mediated genome editing in vancomycin-resistant Enterococcus faecium. FEMS Microbiol. Lett. 2019, 366, fnz256. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for emerging pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef]
- Lima, M.C.; de Barros, M.; Scatamburlo, T.M.; Polveiro, R.C.; de Castro, L.K.; Guimaraes, S.H.S.; da Costa, S.L.; da Costa, M.M.; Moreira, M.A.S. Profiles of Staphyloccocus aureus isolated from goat persistent mastitis before and after treatment with enrofloxacin. BMC Microbiol. 2020, 20, 127. [Google Scholar] [CrossRef]
- Hoekstra, J.; Rutten, V.P.M.G.; Lam, T.J.G.M.; Van Kessel, K.P.M.; Spaninks, M.P.; Stegeman, J.A.; Benedictus, L.; Koop, G. Activation of a bovine mammary epithelial cell line by ruminant-associated Staphylococcus aureus is lineage dependent. Microorganisms 2019, 7, 688. [Google Scholar] [CrossRef]
- Ross, S.P.; Hoye, T.R. Reactions of hexadehydro-Diels-Alder benzynes with structurally complex multifunctional natural products. Nat. Chem. 2017, 9, 523–530. [Google Scholar] [CrossRef]
- Hong, J. Natural products at the interface of chemistry and biology. Chem. Eur. J. 2014, 20, 10204–10212. [Google Scholar] [CrossRef]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef]
- Genis, D.; Kirpichenok, M.; Kombarov, R. A minimalist fragment approach for the design of natural-product-like synthetic scaffolds. Drug Discov. Today 2012, 17, 1170–1174. [Google Scholar] [CrossRef]
- Pucheault, M. Natural products: Chemical instruments to apprehend biological symphony. Org. Biomol. Chem. 2008, 6, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Mbaveng, A.T.; Sandjo, L.P.; Tankeo, S.B.; Ndifor, A.R.; Pantaleon, A.; Nagdjui, B.T.; Kuete, V. Antibacterial activity of nineteen selected natural products against multi-drug resistant Gram-negative phenotypes. SpringerPlus 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Cappiello, F.; Loffredo, M.R.; Del Plato, C.; Cammarone, S.; Casciaro, B.; Quaglio, D.; Mangoni, M.L.; Botta, B.; Ghirga, F. The revaluation of plant-derived terpenes to fight antibiotic-resistant infections. Antibiotics 2020, 9, 325. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Li, J.; Chen, S.; Liu, Y.; Wu, J.-L.; Zhu, X.-M.; Li, N. New limonoids from the fruits of Melia toosendan and their autophagic activities. Phytochem. Lett. 2020, 35, 15–22. [Google Scholar] [CrossRef]
- Rahman, A.K.M.S.; Chowdhury, A.K.A.; Ali, H.-A.; Raihan, S.Z.; Ali, M.S.; Nahar, L.; Sarker, S.D. Antibacterial activity of two limonoids from Swietenia mahagoni against multiple-drug-resistant (MDR) bacterial strains. J. Nat. Med. 2009, 63, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Naruko, A.; Nakazawa, Y.; Zhao, L.; Hayashi, Y.; Hirama, M. Total Synthesis of limonin. Angew. Chem. Int. Ed. 2015, 54, 8538. [Google Scholar] [CrossRef]
- Noack, F.; Heinze, R.C.; Heretsch, P. Contemporary synthetic strategies towards secosteroids, abeo-steroids, and related triterpenes. Synthesis 2019, 51, 2039–2057. [Google Scholar] [CrossRef]
- Leeson, P.D.; St-Gallay, S.A. The influence of the ‘organizational factor’ on compound quality in drug discovery. Nat. Rev. Drug Discov. 2011, 10, 749–765. [Google Scholar] [CrossRef]
- Leeson, P.D.; Springthorpe, B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat. Rev. Drug Discov. 2007, 6, 881–890. [Google Scholar] [CrossRef]
- Kuentz, M.T.; Arnold, Y. Influence of molecular properties on oral bioavailability of lipophilic drugs—mapping of bulkiness and different measures of polarity. Pharm. Dev. Technol. 2009, 14, 312–320. [Google Scholar] [CrossRef]
- Kuentz, M. The influence of molecular properties on the mean yield pressure of drugs-are issues of compressibility predictable based on chemical structure? J. Drug Deliv. Sci. Technol. 2009, 19, 211–215. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Jin, W.-F.; Wang, Y.-Y.; Wang, G.; Morris-Natschke, S.L.; Liu, J.-S.; Wang, G.-K.; Lee, K.-H. Chemical structures and biological activities of limonoids from the genus Swietenia (Meliaceae). Molecules 2018, 23, 1588. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H. Recent progress in the chemistry and biology of limonoids. RSC Adv. 2017, 7, 35191–35220. [Google Scholar] [CrossRef]
- Gualdani, R.; Cavalluzzi, M.M.; Lentini, G.; Habtemariam, S. The chemistry and pharmacology of citrus limonoids. Molecules 2016, 21, 1530. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Menichini, F. An overview on chemical aspects and potential health benefits of limonoids and their derivatives. Crit. Rev. in Food Sci. Nutr. 2014, 54, 225–250. [Google Scholar] [CrossRef]
- Manners, G.D. Citrus limonoids: Analysis, bioactivity, and biomedical prospects. J. Agric. Food Chem. 2007, 55, 8285–8294. [Google Scholar] [CrossRef]
- ADMETlab: A platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. J. Cheminform. 2018, 10, 29. [CrossRef]
- ADMETlab. Available online: http://admet.scbdd.com/calcpre/calc_rules/# (accessed on 22 June 2020).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliver. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.V.S.; Obach, R.S.; Rotter, C.; Miller, H.R.; Chang, G.; Steyn, S.J.; El-Kattan, A.; Troutman, M.D. Physicochemical space for optimum oral bioavailability: Contribution of human intestinal absorption and first-pass elimination. J. Med. Chem. 2010, 53, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Oprea, T.I. Property distribution of drug-related chemical databases. J. Comput. Aided Mol. Des. 2000, 14, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Mi, C.; Wang, Z.; Zhang, Z.H.; Li, M.Y.; Zuo, H.X.; Wang, J.Y.; Jin, X.; Ma, J. Fraxinellone has anticancer activity in vivo by inhibiting programmed cell death-ligand 1 expression by reducing hypoxia-inducible factor-1α and STAT3. Pharmacol. Res. 2018, 135, 166–180. [Google Scholar] [CrossRef]
- Zhao, W.; Wolfender, J.-L.; Hostettmann, K.; Xu, R.; Qin, G. Antifungal alkaloids and limonoid derivatives from Dictamnus dasycarpus. Phytochemistry 1997, 47, 7–11. [Google Scholar] [CrossRef]
- Yoon, J.S.; Yang, H.; Kim, S.H.; Sung, S.H.; Kim, Y.C. Limonoids from Dictamnus dasycarpus protect against glutamate-induced toxicity in primary cultured rat cortical cells. J. Mol. Neurosci. 2010, 42, 9–16. [Google Scholar] [CrossRef]
- Biavatti, M.W.; Vieira, P.C.; Da Silva, M.F.G.F.; Fernandes, J.B.; Albuquerque, S. Limonoids from the endemic Brazilian species Raulinoa echinata. Z. Naturforsch. 2001, 56, 570–574. [Google Scholar] [CrossRef]
- Lv, M.; Tian, Y.; Zhang, Z.; Liang, J.; Xu, F.; Sun, J. Plant metabolomics driven chemical and biological comparison of the root bark of Dictamnus dasycarpus and Dictamnus angustifolius. RSC Adv. 2015, 5, 15700–15708. [Google Scholar] [CrossRef]
- Kiprop, A.K.; Kiprono, P.C.; Rajab, M.S.; Kosgei, M.K.Z. Limonoids as larvicidal components against mosquito larvae (Aedes aegypti Linn.). Naturforsch. C. J. Biosci. 2007, 62, 826–828. [Google Scholar] [CrossRef]
- Yoon, J.S.; Sung, S.H.; Kim, Y.C. Neuroprotective limonoids of root bark of Dictamnus dasycarpus. J. Nat. Prod. 2008, 71, 208–211. [Google Scholar] [CrossRef]
- Lee, C.S.; Won, C.; Yoo, H.; Yi, E.H.; Cho, Y.; Maeng, J.W.; Sung, S.H.; Ye, S.-K.; Chung, M.-H. Inhibition of double-stranded RNA-induced inducible nitric oxide synthase expression by fraxinellone and sauchinone in murine microglia. Biol. Pharm. Bull. 2009, 32, 1870–1874. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, S.; Yu, Q.; Fang-Yuan, G.; Xue-Feng, W.; Zi-Chun, H.; Ting, C.; Qiang, X. Selective triggering of apoptosis of concanavalin A-activated T cells by fraxinellone for the treatment of T-cell-dependent hepatitis in mice. Biochem. Pharmacol. 2009, 77, 1717–1724. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Wang, X.-N.; Fan, C.-Q.; Yin, S.; Yue, J.-M. Swiemahogins A and B, two novel limonoids from Swietenia mahogany. Tetrahedron Lett. 2007, 48, 7480–7484. [Google Scholar] [CrossRef]
- Yi, L.; Zhang, H.; Tian, X.; Luo, J.; Luo, J.; Kong, L. Four new limonoids from the seeds of Chukrasia tabularis A. Juss. Phytochem. Lett. 2017, 19, 12–17. [Google Scholar] [CrossRef]
- Schuppe, A.W.; Zhao, Y.; Liu, Y.; Newhouse, T.R. Total synthesis of (+)-Granatumine A and related bislactone limonoid alkaloids via a pyran to pyridine interconversion. J. Am. Chem. Soc. 2019, 141, 9191–9196. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Mateos, A.; Grande Benito, M.; Pascual Coca, G.; Rubio González, R.; Tapia Hernández, C. Synthesis of dl-pyroangolensolide. Tetrahedron 1995, 51, 7521–7526. [Google Scholar] [CrossRef]
- Kaur, G.; Kaur, M.; Sharad, L.; Bansal, M. Theoretical molecular predictions and antimicrobial activities of newly synthesized molecular hybrids of norfloxacin and ciprofloxacin. J. Heterocyclic Chem. 2020, 57, 225–237. [Google Scholar] [CrossRef]
- Constantinescu, T.; Lungu, C.N.; Lung, I. Lipophilicity as a central component of drug-like properties of chalchones and flavonoid derivatives. Molecules 2019, 24, 1505. [Google Scholar] [CrossRef]
- Pastuszko, A.; Majchrzak, K.; Czyz, M.; Kupcewicz, B.; Budzisz, E. The synthesis, lipophilicity and cytotoxic effects of new ruthenium(II) arene complexes with chromone derivatives. J. Inorg. Biochem. 2016, 159, 133–141. [Google Scholar] [CrossRef]
- Moreira, J.; Ribeiro, D.; Silva, P.; Nazareth, N.; Monteiro, M.; Palmeira, A.; Saraiva, L.; Pinto, M.; Bousbaa, H.; Cidade, H. New alkoxy flavone derivatives targeting caspases: Synthesis and antitumor activity evaluation. Molecules 2019, 24, 129. [Google Scholar] [CrossRef]
- Tinworth, C.P.; Young, R.J. Facts, patterns, and principles in drug discovery: Appraising the rule of 5 with measured physicochemical data. J. Med. Chem. 2020. Ahead of Print. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline, M26, V. 19, No. 18; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, September 1999. [Google Scholar]
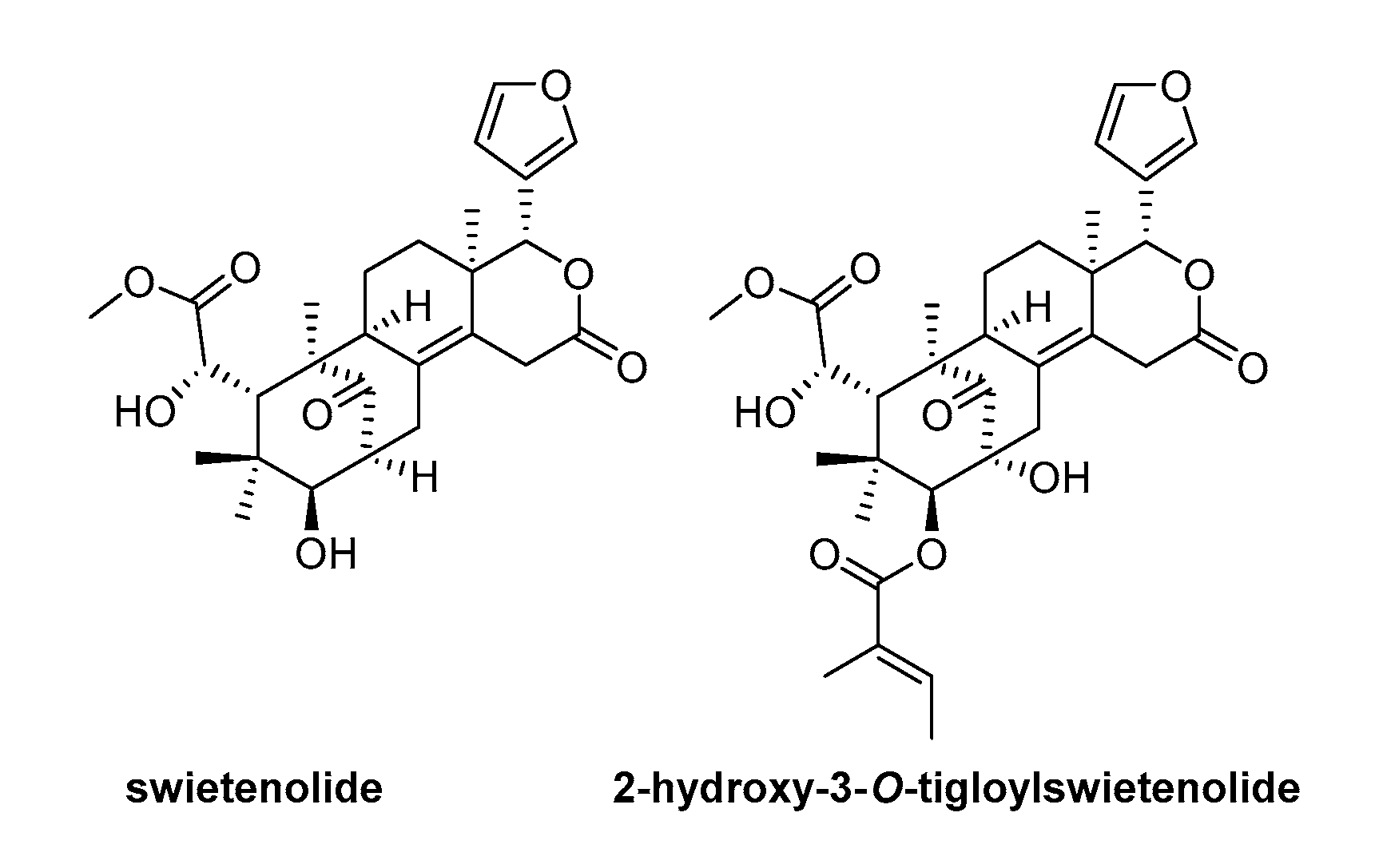

| Molecular Properties | Limonoids | Molecular Properties | Limonoids | ||
|---|---|---|---|---|---|
| Swietenolide | 2-hydroxy-3-O-tigloylswietenolide | Swietenolide | 2-hydroxy-3-O-tigloylswietenolide | ||
| HBD a | 2 | 2 | Nrotb g | 4 | 7 |
| HBA b | 8 | 10 | TPSA h | 123.27 | 149.57 |
| MW c | 486.56 | 584.66 | Log D i | 1.679 | 1.674 |
| Log P d | 3.127 | 3.759 | NR j | 5 | 5 |
| MR e | 122.54 | 147.30 | NRigB k | 35 | 39 |
| TNA f | 69 | 82 | |||
| Matches in Drugability Rules | |||||
| Rules | Lipinski | Ghose | Veber | Varma | Oprea |
| Swietenolide | 100% | 75% | 100% | 80% | 66.7% |
| 2-hydroxy-3-O-tigloylswietenolide | 75% | 25% | 66.7% | 40% | 100% |
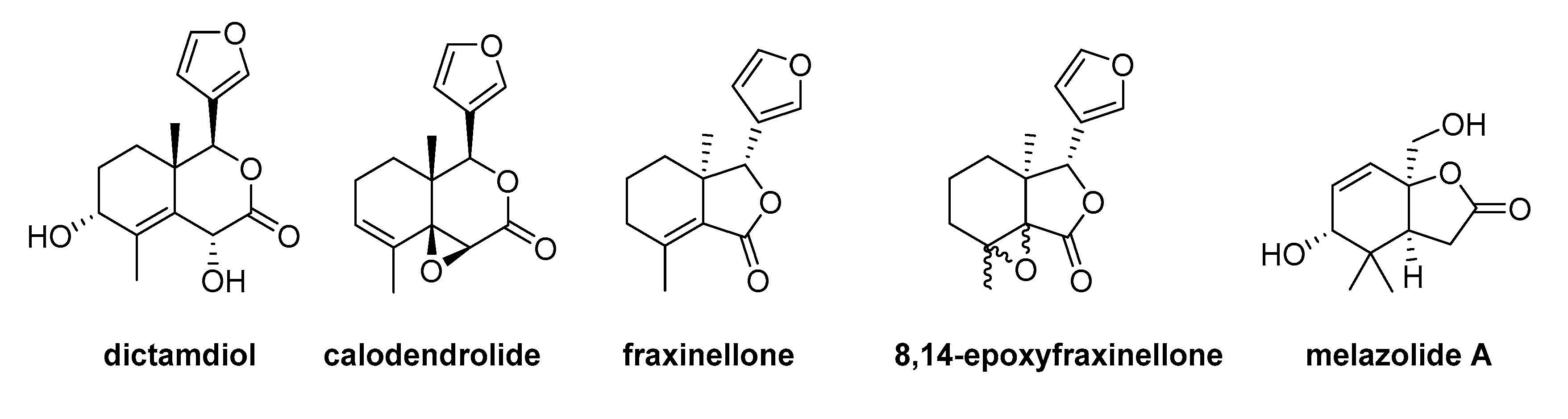
| Molecular Properties | Natural Degraded Limonoids | ||||
|---|---|---|---|---|---|
| Dictamdiol | Calodendrolide | Fraxinellone | 8,14-epoxyfraxinellone | Melazolide A | |
| HBD a | 2 | 0 | 0 | 0 | 2 |
| HBA b | 5 | 4 | 4 | 4 | 4 |
| MW c | 278.304 | 260.289 | 246.262 | 248.278 | 212.245 |
| Log P d | 1.716 | 2.762 | 2.563 | 2.595 | 0.238 |
| MR e | 69.596 | 66.243 | 62.589 | 61.72 | 52.944 |
| TNA f | 38 | 35 | 32 | 34 | 31 |
| Nrotb g | 1 | 1 | 1 | 1 | 1 |
| TPSA h | 79.9 | 51.97 | 56.51 | 51.97 | 66.76 |
| Log D i | 0.978 | 1.513 | 1.383 | 1.374 | 0.189 |
| NR j | 3 | 4 | 3 | 4 | 2 |
| NRigB k | 21 | 21 | 19 | 20 | 15 |
| Rules | Matches | ||||
| Lipinski’s rules | 100% | 100% | 100% | 100% | 100% |
| Ghose’s rules | 100% | 100% | 100% | 100% | 100% |
| Veber’s rules | 100% | 100% | 100% | 100% | 100% |
| Varma’s rules | 100% | 100% | 100% | 100% | 100% |
| Oprea’s rules | 66.7% | 66.7% | 66.7% | 66.7% | 0% |
| Pathogen | Stereoisomers | MIC50 (mg/L) |
|---|---|---|
| S. aureus ATCC 25923 | (±)-1a and (±)-1b | 512 |
| (±)-2a and (±)-2b | 16 | |
| (±)-3a | ND 1 | |
| MRSA 15012406 | (±)-1a and (±)-1b | 512 |
| (±)-2a and (±)-2b | 128 | |
| (±)-3a | ND 1 | |
| MRSA 15019301 | (±)-1a and (±)-1b | 512 |
| (±)-2a and (±)-2b | 64 | |
| (±)-3a | ND 1 | |
| MRSA 17045463 | (±)-1a and (±)-1b | NT 2 |
| (±)-2a and (±)-2b | 128 | |
| (±)-3a | NT 2 | |
| MRSA 18032913 | (±)-1a and (±)-1b | NT 2 |
| (±)-2a and (±)-2b | 64 | |
| (±)-3a | NT 2 |
| Pathogen | L. monocytogenes 17035151 | E. faecalis ATCC 29212 | E. faecalis DapR 1 16028257 | E. faecium VaR 2 16051635 | S. epidermidis LzdR 3 17101107 | S. epidermidis LzdR 3 18031123 |
|---|---|---|---|---|---|---|
| MIC50 (mg/L) | 128 | 256 | 256 | 256 | 64 | 64 |
| Pathogen | Stereoisomers | MIC50 (mg/L) |
|---|---|---|
| S. aureus ATCC 25923 | (±)-4a | 256 |
| (±)-4b | 128 | |
| (±)-5a | 256 | |
| (±)-5b | 128 | |
| (±)-6a | 1024 | |
| (±)-6b | 256 |
| Molecular Properties | Model Compounds | ||||
|---|---|---|---|---|---|
| 1 | 2 | 4 | 5 | 6 | |
| Log P | 1.848 | 2.419 | 3.199 | 3.980 | 5.540 |
| Log D | 0.863 | 1.042 | 1.421 | 1.641 | 2.151 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrera-Suanzes, M.; Prieto, V.; Medina-Olivera, A.J.; Botubol-Ares, J.M.; Galán-Sánchez, F.; Rodríguez-Iglesias, M.A.; Hernández-Galán, R.; Durán-Peña, M.J. Synthesis of Degraded Limonoid Analogs as New Antibacterial Scaffolds against Staphylococcus aureus. Antibiotics 2020, 9, 488. https://doi.org/10.3390/antibiotics9080488
Ferrera-Suanzes M, Prieto V, Medina-Olivera AJ, Botubol-Ares JM, Galán-Sánchez F, Rodríguez-Iglesias MA, Hernández-Galán R, Durán-Peña MJ. Synthesis of Degraded Limonoid Analogs as New Antibacterial Scaffolds against Staphylococcus aureus. Antibiotics. 2020; 9(8):488. https://doi.org/10.3390/antibiotics9080488
Chicago/Turabian StyleFerrera-Suanzes, Marta, Victoria Prieto, Antonio J. Medina-Olivera, José Manuel Botubol-Ares, Fátima Galán-Sánchez, Manuel A. Rodríguez-Iglesias, Rosario Hernández-Galán, and María Jesús Durán-Peña. 2020. "Synthesis of Degraded Limonoid Analogs as New Antibacterial Scaffolds against Staphylococcus aureus" Antibiotics 9, no. 8: 488. https://doi.org/10.3390/antibiotics9080488
APA StyleFerrera-Suanzes, M., Prieto, V., Medina-Olivera, A. J., Botubol-Ares, J. M., Galán-Sánchez, F., Rodríguez-Iglesias, M. A., Hernández-Galán, R., & Durán-Peña, M. J. (2020). Synthesis of Degraded Limonoid Analogs as New Antibacterial Scaffolds against Staphylococcus aureus. Antibiotics, 9(8), 488. https://doi.org/10.3390/antibiotics9080488