Impact of Colistin Dosing on the Incidence of Nephrotoxicity in a Tertiary Care Hospital in Saudi Arabia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
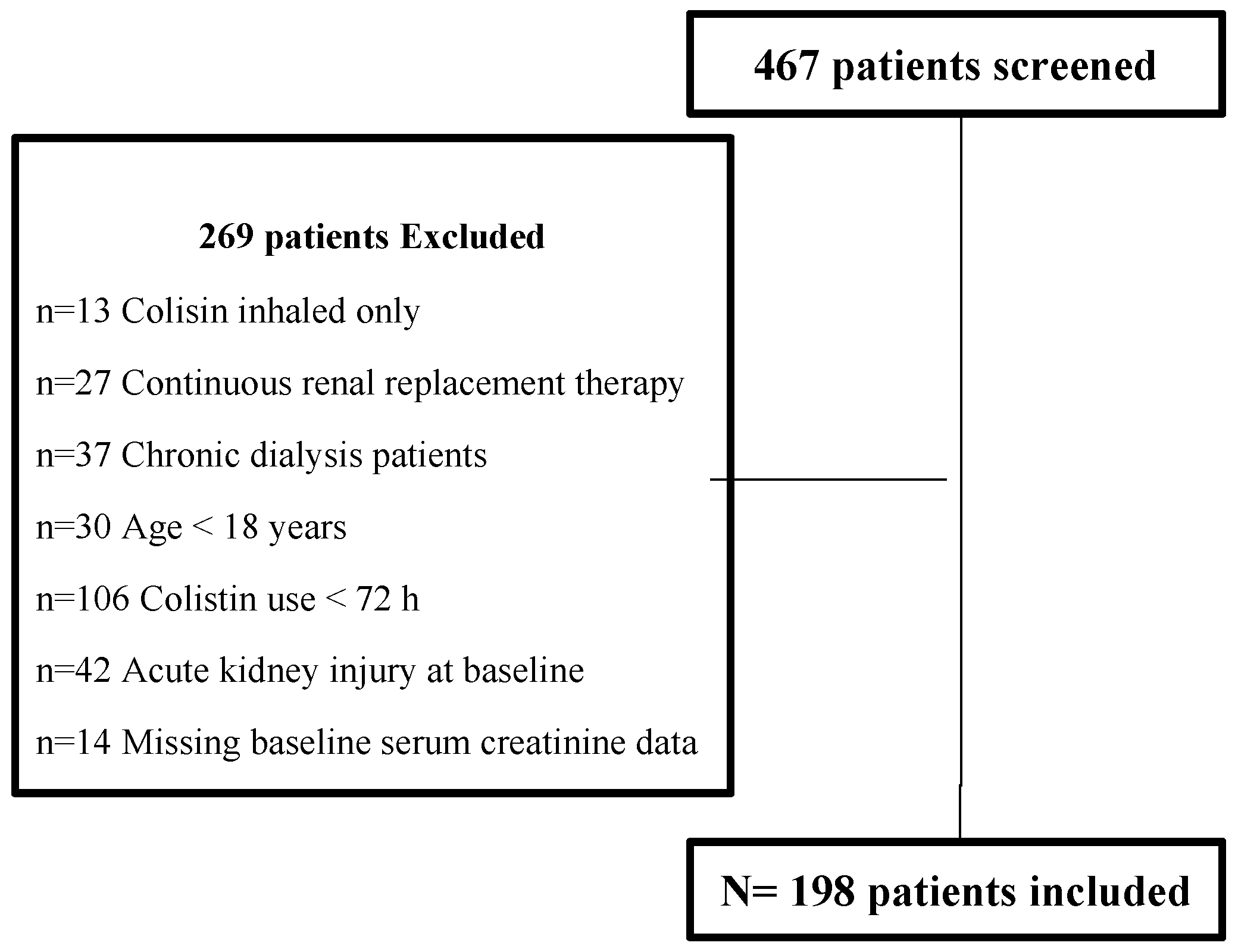
2.2. Study Participants
2.3. Study Outcomes
2.4. Data Collection
2.5. Sample Size
2.6. Statistical Analysis
2.7. Ethics
3. Results
3.1. Patients
3.2. Outcomes
3.2.1. Primary Outcome
3.2.2. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Media Center. Who Publishes a List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/ (accessed on 2 October 2017).
- MacVane, S.H. Antimicrobial Resistance in the Intensive Care Unit: A Focus on Gram-Negative Bacterial Infections. J. Intensive Care Med. 2017, 32, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Lexi-Comp., Inc. Lexi-Drugs®; Lexi-Comp, Inc.: Cleveland, OH, USA, 2017. [Google Scholar]
- Food and Drug Administration. Coly-Mycin® M Parenteral (Colistimethate for Injection, USP). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/050108s026lbl (accessed on 29 July 2020).
- Bergen, P.J.; Li, J.; Nation, R.L. Dosing of colistin-back to basic PK/PD. Curr. Opin. Pharmacol. 2011, 11, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Colomycin Injection. Available online: https://www.medicines.org.uk/emc/medicine/1590 (accessed on 19 August 2017).
- Nation, R.L.; Garonzik, S.; Li, J.; Thamlikitkul, V.; Giamarellos-Bourboulis, E.J.; Paterson, D.L.; Turnidge, J.D.; Forrest, A.; Silveira, F.P. Updated US and European Dose Recommendations for Intravenous Colistin: How Do They Perform? Clin. Infect. Dis. 2016, 62, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Garonzik, S.M.; Li, J.; Thamlikitkul, V.; Paterson, D.L.; Shoham, S.; Jacob, J.; Silveira, F.P.; Forrest, A.; Nation, R.L. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 2011, 55, 3284–3294. [Google Scholar] [CrossRef] [PubMed]
- Dalfino, L.; Puntillo, F.; Mosca, A.; Monno, R.; Spada, M.L.; Coppolecchia, S.; Giuseppe, M.; Bruno, F.; Brienza, N. High-dose, extended-interval colistin administration in critically ill patients: Is this the right dosing strategy? A preliminary study. Clin. Infect. Dis. 2012, 54, 1720–1726. [Google Scholar] [CrossRef]
- Elefritz, J.L.; Bauer, K.A.; Jones, C.; Mangino, J.E.; Porter, K.; Murphy, C.V. Efficacy and Safety of a Colistin Loading Dose, High-Dose Maintenance Regimen in Critically Ill. Patients With Multidrug-Resistant Gram-Negative Pneumonia. J. Intensive Care Med. 2017, 32, 487–493. [Google Scholar] [CrossRef]
- Akajagbor, D.S.; Wilson, S.L.; Shere-Wolfe, K.D.; Dakum, P.; Charurat, M.E.; Gilliam, B.L. Higher incidence of acute kidney injury with intravenous colistimethate sodium compared with polymyxin B in critically ill patients at a tertiary care medical center. Clin. Infect. Dis. 2013, 57, 1300–1303. [Google Scholar] [CrossRef]
- Gauthier, T.P.; Wolowich, W.R.; Reddy, A.; Cano, E.; Abbo, L.; Smith, L.B. Incidence and predictors of nephrotoxicity associated with intravenous colistin in overweight and obese patients. Antimicrob. Agents Chemother. 2012, 56, 2392–2396. [Google Scholar] [CrossRef]
- Pogue, J.M.; Lee, J.; Marchaim, D.; Yee, V.; Zhao, J.J.; Chopra, T.; Lephart, P.; Kaye, K.S. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin. Infect. Dis. 2011, 53, 879–884. [Google Scholar] [CrossRef]
- Rocco, M.; Montini, L.; Alessandri, E.; Venditti, M.; Laderchi, A.; De Pascale, G.; Raponi, G.; Vitale, M.; Pietropaoli, P.; Antonelli, M. Risk factors for acute kidney injury in critically ill patients receiving high intravenous doses of colistin methanesulfonate and/or other nephrotoxic antibiotics: A retrospective cohort study. Crit. Care 2013, 17, R174. [Google Scholar] [CrossRef]
- Kwon, J.A.; Lee, J.E.; Huh, W.; Peck, K.R.; Kim, Y.G.; Kim, D.J.; Oh, H.Y. Predictors of acute kidney injury associated with intravenous colistin treatment. Int. J. Antimicrob. Agent 2010, 35, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Tuon, F.F.; Rigatto, M.H.; Lopes, C.K.; Kamei, L.K.; Rocha, J.L.; Zavascki, A. Risk factors for acute kidney injury in patients treated with polymyxin B or colistin methanesulfonate sodium. Int. J. Antimicrob. Agents 2014, 43, 349–452. [Google Scholar] [CrossRef] [PubMed]
- Hartzell, J.D.; Neff, R.; Ake, J.; Howard, R.; Olson, S.; Paolino, K.; Vishnepolsky, M.; Weintrob, A.; Wortmann, G. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin. Infect. Dis. 2009, 48, 1724–1728. [Google Scholar] [CrossRef] [PubMed]
- Spapen, H.; Jacobs, R.; Van Gorp, V.; Troubleyn, J.; Honore, P.M. Renal and neurological side effects of colistin in critically ill patients. Ann. Intensive Care 2011, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012, 2 (Suppl. 1), 1–138. [Google Scholar]
- Wolfensberger, A.; Kuster, S.P.; Marchesi, M.; Zbinden, R.; Hombach, M. The effect of varying multidrug-resistence (MDR) definitions on rates of MDR gram-negative rods. Antimicrob. Resist. Infect. Control 2019, 8, 193. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Gibson, G.A.; Bauer, S.R.; Neuner, E.A.; Bass, S.N.; Lam, S.W. Influence of Colistin Dose on Global Cure in Patients with Bacteremia Due to Carbapenem-Resistant Gram-Negative Bacilli. Antimicrob. Agents Chemother. 2015, 60, 431–436. [Google Scholar] [CrossRef]
- OpenEpi. Sample Size for Frequency in a Population. Available online: http://www.openepi.com/SampleSize/SSPropor.htm (accessed on 12 May 2016).
- Sterne, J.A.C.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009, 338, b2393. [Google Scholar] [CrossRef]
- Pedersen, A.B.; Mikkelsen, E.M.; Cronin-Fenton, D.; Kristensen, N.R.; Pham, T.M.; Pedersen, L.; Petersen, I. Missing data and multiple imputation in clinical epidemiological research. Clin. Epidemiol. 2017, 9, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Groenwold, R.H.H.; White, I.R.; Donders, A.R.T.; Carpenter, J.R.; Altman, D.G.; Moons, K.G.M. Missing covariate data in clinical research: When and when not to use the missing-indicator method for analysis. Can. Med. Assoc. J. J. Med. Can. 2012, 184, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. European Medicines Agency Completes Review of Polymyxin-Based Medicines. Recommendations Issued for Safe Use in Patients with Serious Infections Resistant to Standard Antibiotics. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2014/10/WC500176334 (accessed on 21 July 2017).
- Food and Drug Administration. Coly-Mycin® M Parenteral (Colistimethate for Injection, USP). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/050108s033lbl (accessed on 29 July 2020).
- Tsuji, B.T.; Pogue, J.M.; Zavascki, A.P.; Paul, M.; Daikos, G.L.; Forrest, A.; Giacobbe, D.R.; Viscoli, C.; Giamarellou, H.; Karaiskos, I.; et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 2019, 39, 10–39. [Google Scholar] [PubMed]
- Chien, H.T.; Lin, Y.C.; Sheu, C.C.; Hsieh, K.P.; Chang, J.S. Is colistin-associated acute kidney injury clinically important in adults? A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2020, 55, 105889. [Google Scholar] [CrossRef]
- Phe, K.; Lee, Y.; McDaneld, P.M.; Prasad, N.; Yin, T.; Figueroa, D.A.; Musick, W.L.; Cottreau, J.M.; Hu, M.; Tam, V.H. In vitro assessment and multicenter cohort study of comparative nephrotoxicity rates associated with colistimethate versus polymyxin B therapy. Antimicrob. Agents Chemother. 2014, 58, 2740–2746. [Google Scholar] [CrossRef]
- Shields, R.K.; Anand, R.; Clarke, L.G.; Paronish, J.A.; Weirich, M.; Perone, H.; Kieserman, J.; Freedy, H.; Andrzejewski, C.; Bonilla, H. Defining the incidence and risk factors of colistin-induced acute kidney injury by KDIGO criteria. PLoS ONE 2017, 12, e0173286. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Rellos, K.; Triarides, N.A.; Falagas, M.E. Colistin loading dose: Evaluation of the published pharmacokinetic and clinical data. Int J. Antimicrob. Agents 2016, 48, 475–484. [Google Scholar] [CrossRef]
- Omrani, A.S.; Alfahad, W.A.; Shoukri, M.M.; Baadani, A.M.; Aldalbahi, S.; Almitwazi, A.A.; Albarrak, A.M. High dose intravenous colistin methanesulfonate therapy is associated with high rates of nephrotoxicity; a prospective cohort study from Saudi Arabia. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 3. [Google Scholar] [CrossRef]
- Sorli, L.; Luque, S.; Grau, S.; Berenguer, N.; Segura, C.; Montero, M.M.; Alvarez-Lerma, F.; Knobel, H.; Benito, N.; Horcajada, J.P. Trough colistin plasma level is an independent risk factor for nephrotoxicity: A prospective observational cohort study. BMC Infect. Dis. 2013, 13, 380. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; di Masi, A.; Leboffe, L.; Del Bono, V.; Rossi, M.; Cappiello, D.; Coppo, E.; Marchese, A.; Casulli, A.; Signori, A.; et al. Hypoalbuminemia as a predictor of acute kidney injury during colistin treatment. Sci. Rep. 2018, 8, 11968. [Google Scholar] [CrossRef]
- Yapa, S.; Li, J.; Patel, K.; Wilson, J.W.; Dooley, M.J.; George, J.; Clark, D.; Poole, S.; Williams, E.; Porter, C.J.; et al. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: Targeting advantage of inhalational administration. Antimicrob. Agents Chemother. 2014, 58, 2570–2579. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratala, J.; et al. Executive Summary: Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, 575–582. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing.Eucast Warnings Concerning Antimicrobial Susceptibility Testing Products or Procedures. 20 June 2017. Available online: http://www.eucast.org/ast_of_bacteria/warnings/ (accessed on 10 January 2018).
| Baseline Characteristics | AKI 1 (n = 88) | No AKI 1 (n = 110) | p-Value 2 |
|---|---|---|---|
| Age (years) | 59.5 ± 17.7 | 52.62 ± 20.11 | 0.011 |
| Sex (male) | 50 (56.8) | 72 (65.5) | 0.214 |
| Body mass index (kg/m2) | 28 (24–34.2) | 25.1 (21.5–28.7) | 0.003 |
| Baseline CrCL3 (mL/min) | 99.79 ± 45.44 | 115.53 ± 45.91 | 0.017 |
| ICU admission | 65 (73.9) | 66 (60) | 0.04 |
| ICU stay prior to colistin therapy (days) | 3 (0–11) | 6 (0–16) | 0.079 |
| Charlson Comorbidity Index (CCI) | 5.5 (3–7) | 3 (0–6) | <0.001 |
| APACHE II score 4 | 22.93 ± 7.19 | 19.72 ± 6.91 | 0.013 |
| Anaemia | 84 (95.45) | 102 (92.73) | 0.424 |
| Serum albumin concentration (g/L) 5 | 24.53 ± 4.9 | 27.85 ± 5.19 | <0.001 |
| Colistin | |||
| Dosing body weight, DBW (kg) 6 | 58.64 ± 12.89 | 60.9 ± 13.45 | 0.392 |
| Duration of colistin therapy (days) | 11 (6–16.5) | 12 (8–16) | 0.203 |
| Colistin dose (mg/kg/day) 7 | 3.29 ± 1 | 3.24 ± 0.97 | 0.702 |
| Causative organism | 0.182 | ||
| Acinetobacter baumannii | 28 (31.82) | 43 (39.09) | - |
| Klebsiella pneumoniae | 17 (19.32) | 9 (8.2) | - |
| Pseudomonas aeruginosa | 7 (8) | 17 (15.5) | - |
| Site of infection | 0.111 | ||
| Respiratory | 36 (40.9) | 58 (52.7) | - |
| Blood | 25 (28.4) | 19 (17.3) | - |
| Urine | 13 (14.8) | 22 (20) | - |
| Other 8 | 14 (15.9) | 11 (10) | - |
| Antibiotic therapy | 0.667 | ||
| Combined use of antimicrobial agents | 76 (86.4) | 94 (85.5) | - |
| Combined systemic, nebulizer use of colistin and other antibiotics | 9 (10.2) | 14 (12.7) | - |
| Other 9 | 3 (3.4) | 2 (1.82) | |
| Combined use with nephrotoxic agents | 87 (98.9) | 106 (96.4) | 0.265 |
| ACEIs 10/ARBs 11 | 22 (25) | 21 (19.1) | 0.316 |
| Intravenous contrast media | 39 (44.3) | 43 (39.1) | 0.458 |
| Diuretics | 67 (60.9) | 72 (81.8) | 0.001 |
| Vasopressors | 59 (67.1) | 46 (41.8) | 0.001 |
| Aminoglycosides | 15 (17.1) | 11 (10) | 0.145 |
| Amphotericin | 10 (11.4) | 2 (1.82) | 0.005 |
| Vancomycin | 78 (88.6) | 86 (78.2) | 0.053 |
| Acyclovir | 11 (12.5) | 13 (11.8) | 0.884 |
| Non-steroidal anti-inflammatory drugs | 2 (2.3) | 4 (3.6) | 0.578 |
| Variables | Univariate Analysis Odd Ratio (OR), 95% CI, p-Value (p) | Multivariate Analysis OR, 95% CI, p-Value |
|---|---|---|
| Colistin dose (mg/kg/day) | 1.09, 95% CI (0.83–1.46), p = 0.52 | OR: 1.57, 95% CI (1.08–2.30), p = 0.02 1 |
| Age (years) | 1.02, 95% CI (1.003–1.035), p = 0.01 | 0.98, 95% CI (0.96–1.01), p = 0.26 |
| Sex (female) | 1.44, 95% CI (0.81–2.56), p = 0.215 | NA 2 |
| BMI (kg/m2) | 1.07, 95% CI (1.03–1.12), p < 0.001 | 1.06, 95% CI (1.01–1.11), p = 0.02 |
| Creatinine clearance (m//min) | 0.99, 95% CI (0.986–0.998), p = 0.02 | 0.99, 95% CI (0.98–1), p = 0.09 |
| Admission to ICU | 1.88, 95% CI (1.02–3.47), p = 0.042 | 0.25, 95% CI (0.03–1.90), p = 0.18 |
| Hospitalization in ICU prior to colistin use (days) | 0.99, 95% CI (0.99–1.004), p = 0.24 | NA 2 |
| Charlson Comorbidity Index | 1.20, 95% CI (1.09–1.33), p = <0.001 | 1.16, 95% CI (1–1.34), p = 0.04 |
| APCHAE-II score | 1.04, 95% CI (1.02–1.07), p = <0.001 | 1.03, 95% CI (0.97–1.10), p = 0.31 |
| Serum albumin (g/L) | 0.87, 95% CI (0.81–0.93), p = <0.001 | 0.89, 95% CI (0.83–0.96), p = 0.004 |
| Use of nephrotoxic agent | 3.28, 95% CI (0.36–29.91), p = 0.29 | NA 3 |
| Diuretic use | 2.89, 95% CI (1.49–5.60), p = 0.002 | 1.01, 95% CI (0.43–2.36), p = 0.99 |
| Vasopressor use | 2.83, 95% CI (1.58–5.08), p < 0.001 | 2.1, 95% CI (0.90–4.91), p = 0.09 |
| Secondary End Points | AKI 88 (44.4) n (%) | No AKI 110 (55.5) n (%) | p-Value |
|---|---|---|---|
| AKI classifications 1 | |||
| Stage I | 29 (33) | - | - |
| Stage II | 39 (44.3) | - | - |
| Stage III | 20 (22.7) | - | - |
| Clinical cure 2 | 5 (5.7) | 13 (11.8) | 0.136 |
| Mortality at 30 days | 42 (47.7) | 24 (21.8) | <0.001 |
| Dosing appropriateness | |||
| FDA 3 | 63 (72) | 74 (67.3) | 0.513 |
| EMA | |||
| Loading dose | None | None | - |
| Maintenance dose | 5 (5.7) | 10 (9.1) | 0.368 |
| Garonzik et al. [8] | |||
| Loading dose | None | None | - |
| Maintenance dose | 5 (5.7) | 0 (0) | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almutairy, R.; Aljrarri, W.; Noor, A.; Elsamadisi, P.; Shamas, N.; Qureshi, M.; Ismail, S. Impact of Colistin Dosing on the Incidence of Nephrotoxicity in a Tertiary Care Hospital in Saudi Arabia. Antibiotics 2020, 9, 485. https://doi.org/10.3390/antibiotics9080485
Almutairy R, Aljrarri W, Noor A, Elsamadisi P, Shamas N, Qureshi M, Ismail S. Impact of Colistin Dosing on the Incidence of Nephrotoxicity in a Tertiary Care Hospital in Saudi Arabia. Antibiotics. 2020; 9(8):485. https://doi.org/10.3390/antibiotics9080485
Chicago/Turabian StyleAlmutairy, Reem, Waad Aljrarri, Afnan Noor, Pansy Elsamadisi, Nour Shamas, Mohammad Qureshi, and Sherine Ismail. 2020. "Impact of Colistin Dosing on the Incidence of Nephrotoxicity in a Tertiary Care Hospital in Saudi Arabia" Antibiotics 9, no. 8: 485. https://doi.org/10.3390/antibiotics9080485
APA StyleAlmutairy, R., Aljrarri, W., Noor, A., Elsamadisi, P., Shamas, N., Qureshi, M., & Ismail, S. (2020). Impact of Colistin Dosing on the Incidence of Nephrotoxicity in a Tertiary Care Hospital in Saudi Arabia. Antibiotics, 9(8), 485. https://doi.org/10.3390/antibiotics9080485






