Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance
Abstract
1. Introduction
2. Isolation and Characterization of Bioactive Compounds
3. Screening of PDSs for Drug Discovery
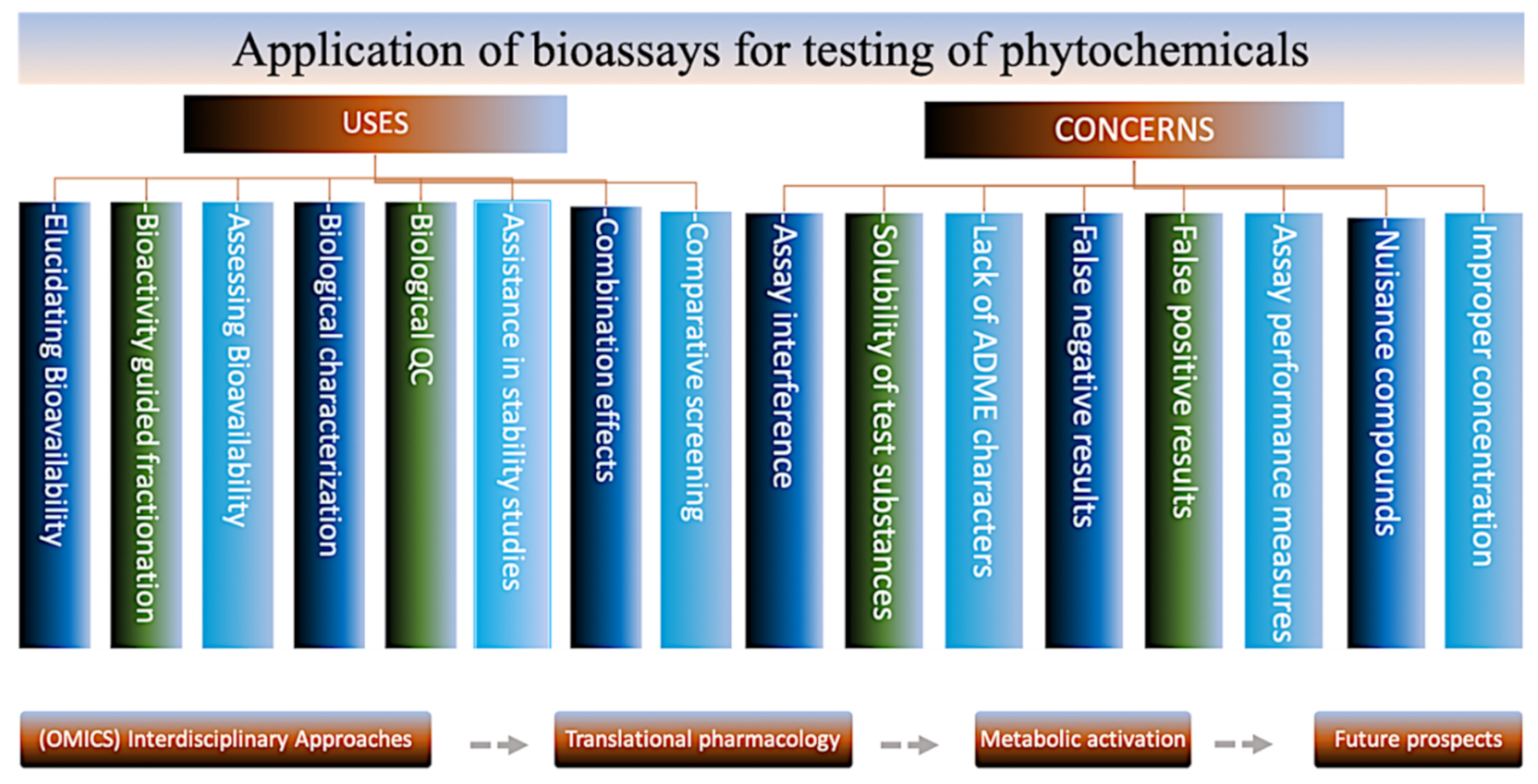
4. Bioassays for Phytochemical Testing
4.1. In Vitro Assays
4.2. In Situ Assay with Isolated Tissues or Organs
4.3. In Vivo Assays Based on Model Organisms
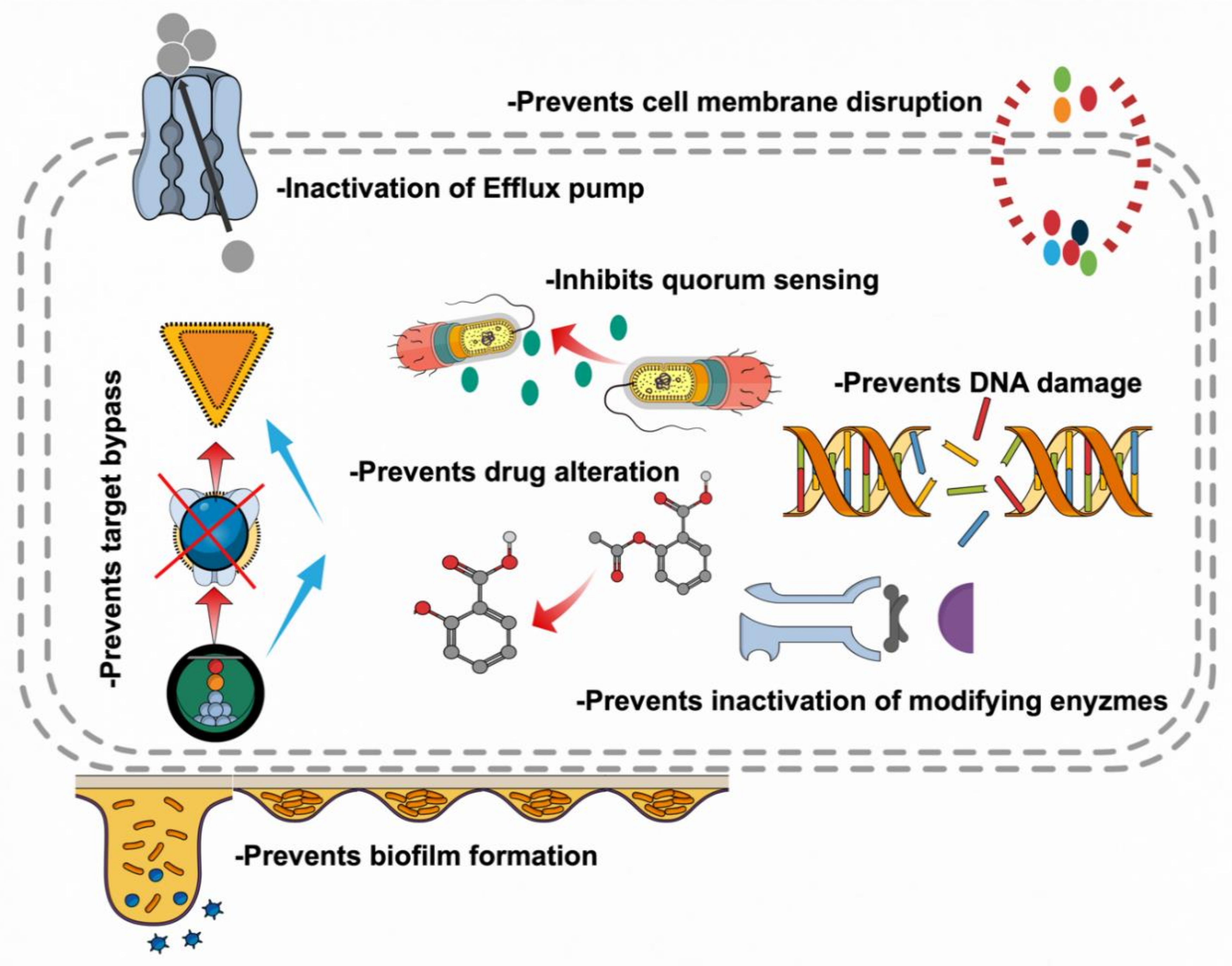
5. Mechanistic Insights of Research on Botanicals
5.1. Inhibition of Cell Wall Synthesis
5.2. Inhibition of Bacterial Physiology
5.3. Modulation of Antibiotic Susceptibility
5.4. Biofilms Inhibition
5.5. Attenuating Bacterial Virulence
6. Hurdles to Overcome
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. World Health Organization Model List of Essential Medicines; amended August 2017; 20th List; WHO: Geneva, Switzerland, March 2017. [Google Scholar]
- Dunphy, L.J.; Yen, P.; Papin, J.A. Integrated experimental and computational analyses reveal differential metabolic functionality in antibiotic-resistant Pseudomonas aeruginosa. Cell Syst. 2019, 8, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, A.; Magana, M.; Bologa, C.G.; Oprea, T.I.; Paulsen, I.T.; Tegos, G.P. Defining the microbial effluxome in the content of the host-microbiome interaction. Front. Pharm. 2015, 6, 31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lister, P.D.; Wolter, D.J.; Hanson, N.D. Antibacterial-resistant Pseudomonas aeruginosa: Clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 2009, 22, 582–610. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Beceiro, A.; Tomas, M.; Bou, G. Antimicrobial resistance and virulence: A successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013, 26, 185–230. [Google Scholar] [CrossRef]
- Beighton, D. Can the ecology of the dental biofilm be beneficially altered? Adv. Dent. Res. 2009, 21, 69–73. [Google Scholar] [CrossRef]
- Bunce, J.T.; Hellyer, P. Antibiotic resistance and antibiotic prescribing by dentists in England 2007–2016. Br. Dent. J. 2018, 225, 81–84. [Google Scholar] [CrossRef]
- Guerrini, L.; Monaco, A.; Pietropaoli, D.; Ortu, E.; Giannoni, M.; Marci, M.C. Antibiotics in dentistry: A narrative review of literature and guidelines considering antibiotic resistance. Open Dent. J. 2019, 13, 383–398. [Google Scholar] [CrossRef]
- Bansal, R.; Jain, A.; Goyal, M.; Singh, T.; Sood, H.; Malviya, H.S. Antibiotic abuse during endodontic treatment: A contributing factor to antibiotic resistance. J. Fam. Med. Prim. Care 2019, 8, 3518–3524. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Sartelli, M.; Haque, S.Z. Dental infection and resistance-global health consequences. Dent. J. 2019, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Zhou, Y.; Li, Z.; Huang, T.; Xiao, Y.; Cheng, L.; Peng, X.; Zhang, L.; Ren, B. Application of antibiotics/antimicrobial agents on dental caries. Biomed. Res. Int. 2020, 2020, 5658212. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Powers, J.H.; Brass, E.P.; Miller, L.G.; Edwards, J.E.J. Trends in antimicrobial drug development: Implications for the future. Clin. Infect. Dis. 2004, 38, 1279–1286. [Google Scholar] [CrossRef]
- Yother, J. Capsules of Streptococcus pneumoniae and other bacteria: Paradigms for polysaccharide biosynthesis and regulation. Annu. Rev. Microbiol. 2011, 65, 563–581. [Google Scholar] [CrossRef]
- Lewis, K.; Ausubel, F.M. Prospects for plant-derived antibacterials. Nat. Biotechnol. 2006, 24, 1504–1507. [Google Scholar] [CrossRef]
- Kolli, M.E.; Laouer, H.; Kolli, H.E.; Akkal, S.; Sahli, F. Chemical analysis, antimicrobial and anti-oxidative properties of Daucus gracilis essential oil and its mechanism of action. Asian Pac. J. Trop. Biomed. 2016, 6, 8–15. [Google Scholar] [CrossRef]
- Gemeda, N.; Tadele, A.; Lemma, H.; Girma, B.; Addis, G.; Tesfaye, B.; Abebe, A.; Gemechu, W.; Yirsaw, K.; Teka, F.; et al. Development, characterization, and evaluation of novel broad-spectrum antimicrobial topical formulations from Cymbopogon martini (Roxb.) W. watson essential oil. Evid. Based Complement. Altern. Med. 2018, 2018, 9812093. [Google Scholar] [CrossRef]
- Appendino, G.; Scafati, O.T.; Romano, A.; Pollastro, F.; Avonto, C.; Rubiolo, P. Genepolide, a sesterpene gamma-lactone with a novel carbon skeleton from mountain wormwood (Artemisia umbelliformis). J. Nat. Prod. 2009, 72, 340–344. [Google Scholar] [CrossRef]
- Conrad, J.; Dinchev, D.; Klaiber, I.; Mika, S.; Kostova, I.; Kraus, W. A novel furostanol saponin from Tribulus terrestris of bulgarian origin. Fitoterapia 2004, 75, 117–122. [Google Scholar] [CrossRef]
- Deng, X.H.; Fang, F.F.; Zheng, C.J.; Wu, Y.; Qin, L.P. Monoterpenoids from the whole herb of Veronicastrum axillare. Pharm. Biol. 2014, 52, 661–663. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, L.; Zhang, L.; Li, N.; Liu, J.Y.; Cai, P.L.; Yang, S.L. New triterpenoid saponins from Patrinia scabiosaefolia. Carbohydr. Res. 2011, 346, 2881–2885. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, Y.X.; Kang, L.P.; Zhang, T.; Yu, H.S.; Zhao, Y.; Xiong, C.Q.; Ma, B.P. Two novel furostanol saponins from the tubers of Ophiopogon japonicus. J. Asian Nat. Prod. Res. 2013, 15, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hsieh, D.; Yang, Y.L.; Xu, Z.; Peto, C.; Jablons, D.M.; You, L. Coumestrol from the national cancer institute’s natural product library is a novel inhibitor of protein kinase CK2. BMC Pharm. Toxicol. 2013, 14, 36. [Google Scholar] [CrossRef]
- Ramasamy, D.; Saraswathy, A. Vitiquinolone—A quinolone alkaloid from Hibiscus vitifolius linn. Food Chem. 2014, 145, 970–975. [Google Scholar] [CrossRef]
- Su, Y.F.; Zhang, Z.X.; Guo, C.Y.; Guo, D.A. A nobel cyanogenic glycoside from Semiaquilegia adoxoides. J. Asian Nat. Prod. Res. 2005, 7, 171–174. [Google Scholar] [CrossRef]
- Wiedenfeld, H.; Dumaa, M.; Malinowski, M.; Furmanowa, M.; Narantuya, S. Phytochemical and analytical studies of extracts from Rhodiola rosea and Rhodiola quadrifida. Pharmazie 2007, 62, 308–311. [Google Scholar]
- Agarwal, A.; D’Souza, P.; Johnson, T.S.; Dethe, S.M.; Chandrasekaran, C. Use of in vitro bioassays for assessing botanicals. Curr. Opin. Biotechnol. 2014, 25, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Pathmasiri, W.; Seedi, H.R.E.; Han, X.; Janson, J.C.; Huss, U.; Bohlin, L. Aryl ketones from Acronychia pedunculata with cyclooxygenase-2 inhibitory effects. Chem. Biodivers. 2005, 2, 463–469. [Google Scholar] [CrossRef]
- Xiao, Z.Y.; Mu, Q.; Shiu, W.K.; Zeng, Y.H.; Gibbons, S. Polyisoprenylated benzoylphloroglucinol derivatives from Hypericum sampsonii. J. Nat. Prod. 2007, 70, 1779–1782. [Google Scholar] [CrossRef]
- Zhang, H.J.; Tamez, P.A.; Vu, D.H.; Ghee, T.T.; Nguyen, V.H.; Le, T.X.; Le, M.H.; Nguyen, M.C.; Do, T.T.; Soejarto, D.D.; et al. Antimalarial compounds from Rhaphidophora decursiva. J. Nat. Prod. 2001, 64, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Blunder, M.; Fakhrudin, N.; Liu, X.; Noha, S.M.; Malainer, C.; Kramer, M.P.; Cocic, A.; Kunert, O.; Schinkovitz, A.; et al. Polyacetylenes from notopterygium incisum--new selective partial agonists of peroxisome proliferator-activated receptor-gamma. PLoS ONE 2013, 8, e61755. [Google Scholar] [CrossRef] [PubMed]
- Fakhrudin, N.; Waltenberger, B.; Cabaravdic, M.; Atanasov, A.G.; Malainer, C.; Schachner, D.; Heiss, E.H.; Liu, R.; Noha, S.M.; Grzywacz, A.M.; et al. Identification of plumericin as a potent new inhibitor of the NF-kappaB pathway with anti-inflammatory activity in vitro and in vivo. Br. J. Pharm. 2014, 171, 1676–1686. [Google Scholar] [CrossRef] [PubMed]
- Mabona, U.; Viljoen, A.; Shikanga, E.; Marston, A.; Vuuren, S.V. Antimicrobial activity of southern African medicinal plants with dermatological relevance: From an ethnopharmacological screening approach, to combination studies and the isolation of a bioactive compound. J. Ethnopharmacol. 2013, 148, 45–55. [Google Scholar] [CrossRef]
- Nievergelt, A.; Huonker, P.; Schoop, R.; Altmann, K.H.; Gertsch, J. Identification of serotonin 5-HT1A receptor partial agonists in ginger. Bioorg. Med. Chem. 2010, 18, 3345–3351. [Google Scholar] [CrossRef]
- Junio, H.A.; Cordero, A.A.S.; Ettefagh, K.A.; Burns, J.T.; Micko, K.T.; Graf, T.N.; Richter, S.J.; Cannon, R.E.; Oberlies, N.H.; Cech, N.B. Synergy-directed fractionation of botanical medicines: A case study with goldenseal (Hydrastis canadensis). J. Nat. Prod. 2011, 74, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Ndhlala, A.R.; Aderogba, M.A.; Ncube, B.; Staden, J.V. Anti-oxidative and cholinesterase inhibitory effects of leaf extracts and their isolated compounds from two closely related Croton species. Molecules 2013, 18, 1916–1932. [Google Scholar] [CrossRef] [PubMed]
- Nitteranon, V.; Zhang, G.; Darien, B.J.; Parkin, K. Isolation and synergism of in vitro anti-inflammatory and quinone reductase (QR) inducing agents from the fruits of Morinda citrifolia (noni). Food Res. Int. 2011, 44, 2271–2277. [Google Scholar] [CrossRef]
- Tafesh, A.; Najami, N.; Jadoun, J.; Halahlih, F.; Riepl, H.; Azaizeh, H. Synergistic antibacterial effects of polyphenolic compounds from olive mill wastewater. Evid. Based Complement. Alternat. Med. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, S.; Li, F.; Zhang, B.; Qu, Y.; Sun, T.; Luo, T.; Li, D. Investigation of antioxidant interactions between Radix astragali and Cimicifuga foetida and identification of synergistic antioxidant compounds. PLoS ONE 2014, 9, e87221. [Google Scholar] [CrossRef]
- Goretta, L.A.; Leveques, A.; Giuffrida, F.; Michailidis, F.R.; Viton, F.; Barron, D.; Paton, M.D.; Manzano, S.G.; Buelga, C.S.; Williamson, G.; et al. Elucidation of (-)-epicatechin metabolites after ingestion of chocolate by healthy humans. Free. Radic. Biol. Med. 2012, 53, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Akao, T.; Yoshino, T.; Kobashi, K.; Hattori, M. Evaluation of salicin as an antipyretic prodrug that does not cause gastric injury. Planta Med. 2002, 68, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Berman, S.; Humbert, O.; Lampe, J.W. In vitro incubation of human feces with daidzein and antibiotics suggests interindividual differences in the bacteria responsible for equol production. J. Nutr. 2004, 134, 596–599. [Google Scholar] [CrossRef]
- Chen, J.; Ma, X.; Gao, K.; Wang, Y.; Zhao, H.; Wu, H.; Wang, J.; Xie, H.; OuYang, Y.; Luo, L.; et al. The active ingredients of Jiang-Zhi-Ning: Study of the nelumbo nucifera alkaloids and their main bioactive metabolites. Molecules 2012, 17, 9855–9867. [Google Scholar] [CrossRef]
- Guerrero, L.; Margalef, M.; Pons, Z.; Quinones, M.; Arola, L.; Arnal, A.A.; Muguerza, B. Serum metabolites of proanthocyanidin-administered rats decrease lipid synthesis in HepG2 cells. J. Nutr. Biochem. 2013, 24, 2092–2099. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Chen, M.; Wang, K.; Sun, Z.; Li, Z.; Wu, B.; Huang, C. Systematic screening and characterization of the major bioactive components of poria cocos and their metabolites in rats by LC-ESI-MS(n). Biomed. Chromatogr. 2012, 26, 1109–1117. [Google Scholar] [CrossRef]
- Wan, J.Y.; Liu, P.; Wang, H.Y.; Qi, L.W.; Wang, C.Z.; Li, P.; Yuan, C.S. Biotransformation and metabolic profile of American ginseng saponins with human intestinal microflora by liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2013, 1286, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Kang, J.; Chen, J.R.; Nagarajan, S.; Badger, T.M.; Wu, X. Phenolic acids are in vivo atheroprotective compounds appearing in the serum of rats after blueberry consumption. J. Agric. Food Chem. 2011, 59, 10381–10387. [Google Scholar] [CrossRef]
- Tawfike, A.F.; Viegelmann, C.; Ebel, R.E. Metabolomics and dereplication strategies in natural products. Methods Mol. Biol. 2013, 1055, 227–244. [Google Scholar] [PubMed]
- Wolfender, J.L.; Marti, G.; Thomas, A.; Bertrand, S. Current approaches and challenges for the metabolite profiling of complex natural extracts. J. Chromatogr. A 2015, 1382, 136–164. [Google Scholar] [CrossRef]
- Gülcemal, D.; Masullo, M.; Napolitano, A.; Karayıldırım, T.; Bedir, E.; Çalışkan, Ö.A.; Piacente, S. Oleanane glycosides from Astragalus tauricolus: Isolation and structural elucidation based on a preliminary liquid chromatography-electrospray ionization tandem mass spectrometry profiling. Phytochemistry 2013, 86, 184–194. [Google Scholar] [CrossRef]
- Hou, C.C.; Chen, C.H.; Yang, N.S.; Chen, Y.P.; Lo, C.P.; Wang, S.Y.; Tien, Y.J.; Tsai, P.W.; Shyur, L.F. Comparative metabolomics approach coupled with cell- and gene-based assays for species classification and anti-inflammatory bioactivity validation of echinacea plants. J. Nutr. Biochem. 2010, 21, 1045–1059. [Google Scholar] [CrossRef]
- Inui, T.; Wang, Y.; Pro, S.M.; Franzblau, S.G.; Pauli, G.F. Unbiased evaluation of bioactive secondary metabolites in complex matrices. Fitoterapia 2012, 83, 1218–1225. [Google Scholar] [CrossRef]
- Keerthi, D.; Geethu, C.; Nair, R.A.; Pillai, P. Metabolic profiling of zingiber zerumbet following pythium myriotylum infection: Investigations on the defensive role of the principal secondary metabolite, zerumbone. Appl. Biochem. Biotechnol. 2014, 172, 2593–2603. [Google Scholar] [CrossRef]
- Mao, Q.; Yang, J.; Cui, X.M.; Li, J.J.; Qi, Y.T.; Zhang, P.H.; Wang, Q. Target separation of a new anti-tumor saponin and metabolic profiling of leaves of panax notoginseng by liquid chromatography with eletrospray ionization quadrupole time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2012, 59, 67–77. [Google Scholar] [CrossRef]
- Modarai, M.; Yang, M.; Suter, A.; Kortenkamp, A.; Heinrich, M. Metabolomic profiling of liquid echinacea medicinal products with in vitro inhibitory effects on cytochrome P450 3A4 (CYP3A4). Planta Med. 2010, 76, 378–385. [Google Scholar] [CrossRef]
- Sandasi, M.; Kamatou, G.P.; Viljoen, A.M. An untargeted metabolomic approach in the chemotaxonomic assessment of two salvia species as a potential source of alpha-bisabolol. Phytochemistry 2012, 84, 94–101. [Google Scholar] [CrossRef]
- Gyllenhaal, C.; Kadushin, M.R.; Southavong, B.; Sydara, K.; Bouamanivong, S.; Xaiveu, M.; Xuan, L.T.; Hiep, N.T.; Hung, N.V.; Loc, P.K.; et al. Ethnobotanical approach versus random approach in the search for new bioactive compounds: Support of a hypothesis. Pharm. Biol. 2012, 50, 30–41. [Google Scholar] [CrossRef]
- Khafagi, I.K.; Dewedar, A. The efficiency of random versus ethno-directed research in the evaluation of Sinai medicinal plants for bioactive compounds. J. Ethnopharmacol. 2000, 71, 365–376. [Google Scholar] [CrossRef]
- Nielsen, A.L.; Kristensen, L.H.; Stephansen, K.B.; Kristensen, J.B.; Helgstrand, C.; Lees, M.; Cloos, P.; Helin, K.; Gajhede, M.; Olsen, L. Identification of catechols as histone-lysine demethylase inhibitors. FEBS Lett. 2012, 586, 1190–1194. [Google Scholar] [CrossRef][Green Version]
- Oliveira, D.R.; Leitão, G.G.; Coelho, T.S.; Silva, P.E.A.; Lourenço, M.C.S.; Leitão, S.G. Ethnopharmacological versus random plant selection methods for the evaluation of the antimycobacterial activity. Rev. Bras. Farm. 2011, 21, 793–806. [Google Scholar] [CrossRef]
- Shaneyfelt, M.E.; Burke, A.D.; Graff, J.W.; Jutila, M.A.; Hardy, M.E. Natural products that reduce rotavirus infectivity identified by a cell-based moderate-throughput screening assay. Virol. J. 2006, 3, 68. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Wenzig, E.M.P.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Ekuadzi, E.; Dickson, R.; Fleischer, T.; Annan, K.; Pistorius, D.; Oberer, L.; Gibbons, S. Flavonoid glycosides from the stem bark of margaritaria discoidea demonstrate antibacterial and free radical scavenging activities. Phytother. Res. 2014, 28, 784–787. [Google Scholar] [CrossRef]
- Noreen, Y.; Seedi, H.E.; Perera, P.; Bohlin, L. Two new isoflavones from ceiba pentandra and their effect on cyclooxygenase-catalyzed prostaglandin biosynthesis. J. Nat. Prod. 1998, 61, 8–12. [Google Scholar] [CrossRef]
- Siriwatanametanon, N.; Heinrich, M. The Thai medicinal plant gynura pseudochina var. hispida: Chemical composition and in vitro NF-kappaB inhibitory activity. Nat. Prod. Commun. 2011, 6, 627–630. [Google Scholar] [CrossRef]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar]
- Heinrich, M. Ethnopharmacology in the 21st century—Grand challenges. Front. Pharm. 2010, 1, 8. [Google Scholar] [CrossRef]
- Leonti, M. The future is written: Impact of scripts on the cognition, selection, knowledge and transmission of medicinal plant use and its implications for ethnobotany and ethnopharmacology. J. Ethnopharmacol. 2011, 134, 542–555. [Google Scholar] [CrossRef]
- Ahamad, S.; Rahman, S.; Khan, F.I.; Dwivedi, N.; Ali, S.; Kim, J.; Imtaiyaz Hassan, M. QSAR based therapeutic management of M. tuberculosis. Arch. Pharm. Res. 2017, 40, 676–694. [Google Scholar] [CrossRef]
- Gavernet, L.; Talevi, A.; Castro, E.A.; Blanch, L.E.B. A Combined virtual screening 2D and 3D QSAR methodology for the selection of new anticonvulsant candidates from a natural product library. QSAR Comb. Sci. 2008, 27, 1120–1129. [Google Scholar] [CrossRef]
- Gaulton, A.; Bellis, L.J.; Bento, A.P.; Chambers, J.; Davies, M.; Hersey, A.; Light, Y.; McGlinchey, S.; Michalovich, D.; Lazikani, B.A.; et al. ChEMBL: A large-scale bioactivity database for drug discovery. Nucleic Acids Res. 2012, 40. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cheng, T.; Wang, Y.; Bryant, S.H. PubChem as a public resource for drug discovery. Drug Discov. Today 2010, 15, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Fakhrudin, N.; Ladurner, A.; Atanasov, A.G.; Heiss, E.H.; Baumgartner, L.; Markt, P.; Schuster, D.; Ellmerer, E.P.; Wolber, G.; Rollinger, J.M.; et al. Computer-aided discovery, validation, and mechanistic characterization of novel neolignan activators of peroxisome proliferator-activated receptor gamma. Mol. Pharm. 2010, 77, 559–566. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, X.; Chen, L.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. Molecular determinants of magnolol targeting both RXRalpha and PPARgamma. PLoS ONE 2011, 6, e28253. [Google Scholar]
- Waszkowycz, B.; Clark, D.E.; Gancia, E. Outstanding challenges in protein–ligand docking and structure-based virtual screening. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 229–259. [Google Scholar] [CrossRef]
- Balamurugan, K.; Nishanthini, A.; Mohan, V.R. GC–MS analysis of Polycarpaea corymbosa (L.) lam whole plant. Asian Pac. J. Trop. Biomed. 2012, 2, S1289–S1292. [Google Scholar] [CrossRef]
- Doshi, G.M.; Nalawade, V.V.; Mukadam, A.S.; Chaskar, P.K.; Zine, S.P.; Somani, R.R.; Une, H.D. Structural elucidation of chemical constituents from Benincasa hispida seeds and carissa congesta roots by gas chromatography: Mass spectroscopy. Pharmacogn. Res. 2015, 7, 282–293. [Google Scholar] [CrossRef]
- Dubey, D.; Patnaik, R.; Ghosh, G.; Padhy, R.N. In vitro antibacterial activity, gas chromatography-mass spectrometry analysis of Woodfordia fruticosa kurz. leaf extract and host toxicity testing with in vitro cultured lymphocytes from human umbilical cord blood. Osong Public Health Res. Perspect. 2014, 5, 298–312. [Google Scholar] [CrossRef]
- Mickymaray, S.; Aboody, M.S.A.; Rath, P.K.; Annamalai, P.; Nooruddin, T. Screening and antibacterial efficacy of selected Indian medicinal plants. Asian Pac. J. Trop. Biomed. 2016, 6, 185–191. [Google Scholar] [CrossRef]
- Sabatini, S.; Piccioni, M.; Felicetti, T.; De Marco, S.; Manfroni, G.; Pagiotti, R.; Nocchetti, M.; Cecchetti, V.; Pietrella, D. Investigation on the effect of known potent S. aureus NorA efflux pump inhibitors on the staphylococcal biofilm formation. RSC Adv. 2017, 7, 37007–37014. [Google Scholar] [CrossRef]
- Mishra, M.P.; Rath, S.; Swain, S.S.; Ghosh, G.; Das, D.; Padhy, R.N. In vitro antibacterial activity of crude extracts of 9 selected medicinal plants against UTI causing MDR bacteria. J. King Saud Univ. Sci. 2017, 29, 84–95. [Google Scholar] [CrossRef]
- Cesa, S.; Sisto, F.; Zengin, G.; Scaccabarozzi, D.; Kokolakis, A.K.; Scaltrito, M.M.; Grande, R.; Locatelli, M.; Cacciagrano, F.; Angiolella, L. Phytochemical analyses and pharmacological screening of neem oil. S. Afr. J. Bot. 2019, 120, 331–337. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations against gram-positive and gram-negative bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L.; et al. Chromatographic Analyses, in vitro biological activities, and cytotoxicity of cannabis sativa L. essential oil: A multidisciplinary study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef]
- Ruiz, G.; Turner, T.; Nelson, E.; Sparks, L.; Langland, J. Bacterial development of resistance to botanical antimicrobials. J. Evol. Health 2017, 2, 3. [Google Scholar] [CrossRef][Green Version]
- Roy, S.K.; Pahwa, S.; Nandanwar, H.; Jachak, S.M. Phenylpropanoids of Alpinia galanga as efflux pump inhibitors in mycobacterium smegmatis mc(2) 155. Fitoterapia 2012, 83, 1248–1255. [Google Scholar] [CrossRef]
- Dwivedi, G.R.; Upadhyay, H.C.; Yadav, D.K.; Singh, V.; Srivastava, S.K.; Khan, F.; Darmwal, N.S.; Darokar, M.P. 4-Hydroxy-alpha-tetralone and its derivative as drug resistance reversal agents in multi drug resistant Escherichia coli. Chem. Biol. Drug Des. 2014, 83, 482–492. [Google Scholar] [CrossRef]
- Aghayan, S.S.; Mogadam, H.K.; Fazli, M.; Sarokhalil, D.D.; Khoramrooz, S.S.; Jabalameli, F.; Yaslianifard, S.; Mirzaii, M. The effects of berberine and palmatine on efflux pumps inhibition with different gene patterns in Pseudomonas aeruginosa isolated from burn infections. Avicenna J. Med. Biotechnol. 2017, 9, 2–7. [Google Scholar]
- Maisuria, V.B.; Hosseinidoust, Z.; Tufenkji, N. Polyphenolic extract from maple syrup potentiates antibiotic susceptibility and reduces biofilm formation of Pathogenic bacteria. Appl. Environ. Microbiol. 2015, 81, 3782–3792. [Google Scholar] [CrossRef]
- Dwivedi, G.R.; Tyagi, R.; Gupta, S.; Tripathi, S.; Pati, S.; Srivastava, S.K.; Darokar, M.P.; Sharma, A. Antibiotics potentiating potential of catharanthine against superbug Pseudomonas aeruginosa. J. Biomol. Struct. Dyn. 2018, 36, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Siriyong, T.; Srimanote, P.; Chusri, S.; Yingyongnarongkul, B.E.; Suaisom, C.; Tipmanee, V.; Voravuthikunchai, S.P. Conessine as a novel inhibitor of multidrug efflux pump systems in Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2017, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kakarla, P.; Floyd, J.; Mukherjee, M.; Devireddy, A.R.; Inupakutika, M.A.; Ranweera, I.; Kc, R.; Shrestha, U.; Cheeti, U.R.; Willmon, T.M.; et al. Inhibition of the multidrug efflux pump LmrS from Staphylococcus aureus by cumin spice Cuminum cyminum. Arch. Microbiol. 2017, 199, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Chovanova, R.; Mezovska, J.; Vaverkova, S.; Mikulasova, M. The inhibition the Tet(K) efflux pump of tetracycline resistant Staphylococcus epidermidis by essential oils from three salvia species. Lett. Appl. Microbiol. 2015, 61, 58–62. [Google Scholar] [CrossRef]
- Limaverde, P.W.; Campina, F.F.; Cunha, F.A.B.D.; Crispim, F.D.; Figueredo, F.G.; Lima, L.F.; Datiane, M.O.-T.C.; Matos, Y.D.; Braga, M.F.B.M.; Menezes, I.R.A.; et al. Inhibition of the TetK efflux-pump by the essential oil of chenopodium ambrosioides L. and alpha-terpinene against Staphylococcus aureus IS-58. Food Chem. Toxicol. 2017, 109, 957–961. [Google Scholar] [CrossRef]
- Bag, A.; Chattopadhyay, R.R. Efflux-pump inhibitory activity of a gallotannin from terminalia chebula fruit against multidrug-resistant uropathogenic Escherichia coli. Nat. Prod. Res. 2014, 28, 1280–1283. [Google Scholar] [CrossRef]
- Holler, J.G.; Christensen, S.B.; Slotved, H.C.; Rasmussen, H.B.; Guzman, A.; Olsen, C.E.; Petersen, B.; Molgaard, P. Novel inhibitory activity of the Staphylococcus aureus NorA efflux pump by a kaempferol rhamnoside isolated from persea lingue nees. J. Antimicrob. Chemother. 2012, 67, 1138–1144. [Google Scholar] [CrossRef]
- Maurya, A.; Dwivedi, G.R.; Darokar, M.P.; Srivastava, S.K. Antibacterial and synergy of clavine alkaloid lysergol and its derivatives against nalidixic acid-resistant Escherichia coli. Chem. Biol. Drug Des. 2013, 81, 484–490. [Google Scholar] [CrossRef]
- Shiu, W.K.; Malkinson, J.P.; Rahman, M.M.; Curry, J.; Stapleton, P.; Gunaratnam, M.; Neidle, S.; Mushtaq, S.; Warner, M.; Livermore, D.M.; et al. A new plant-derived antibacterial is an inhibitor of efflux pumps in Staphylococcus aureus. Int. J. Antimicrob. Agents 2013, 42, 513–518. [Google Scholar] [CrossRef]
- Bame, J.R.; Graf, T.N.; Junio, H.A.; Bussey, R.O.; Jarmusch, S.A.; Elimat, T.E.; Falkinham, J.O.; Oberlies, N.H.; Cech, R.A.; Cech, N.B. Sarothrin from Alkanna orientalis is an antimicrobial agent and efflux pump inhibitor. Planta Med. 2013, 79, 327–329. [Google Scholar] [CrossRef]
- Mocan, A.; Babota, M.; Pop, A.; Fizesan, I.; Diuzheva, A.; Locatelli, M.; Carradori, S.; Campestre, C.; Menghini, L.; Sisea, C.R.; et al. Chemical constituents and biologic activities of sage species: A comparison between Salvia officinalis L., S. glutinosa L. and S. transsylvanica (Schur ex Griseb. & Schenk) schur. Antioxidants 2020, 9, 480. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.; Zhao, B.; Tang, M.; Dong, H.; Zhang, L.; Lv, B.; Wei, L. Ex vivo and in situ approaches used to study intestinal absorption. J. Pharm. Toxicol. Methods 2013, 68, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A. In vivo/ex vivo and in situ assays used in cancer research: A brief review. Toxicol. Pathol. 2009, 37, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.R.; Lund, G.; Sapelnikova, S.; Tyrrell, D.L.; Kneteman, N.M. Chimeric rodents with humanized liver: Bridging the preclinical/clinical trial gap in ADME/toxicity studies. Xenobiotica 2014, 44, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Zambrowicz, B.P.; Sands, A.T. Knockouts model the 100 best-selling drugs--will they model the next 100? Nat. Rev. Drug Discov. 2003, 2, 38–51. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, G.; Ding, X.; Lu, C. Preclinical experimental models of drug metabolism and disposition in drug discovery and development. Acta Pharm. Sin. B 2012, 2, 549–561. [Google Scholar] [CrossRef]
- Pulak, R. Techniques for analysis, sorting, and dispensing of C. elegans on the COPAS flow-sorting system. Methods Mol. Biol. 2006, 351, 275–286. [Google Scholar]
- O’Reilly, L.P.; Luke, C.J.; Perlmutter, D.H.; Silverman, G.A.; Pak, S.C.C. elegans in high-throughput drug discovery. Adv. Drug Deliv. Rev. 2014, 69–70, 247–253. [Google Scholar]
- Wood, A.J.; Lo, T.W.; Zeitler, B.; Pickle, C.S.; Ralston, E.J.; Lee, A.H.; Amora, R.; Miller, J.C.; Leung, E.; Meng, X.; et al. Targeted genome editing across species using ZFNs and TALENs. Science 2011, 333, 307. [Google Scholar] [CrossRef]
- Cutuli, M.A.; Petronio, G.P.; Vergalito, F.; Magnifico, I.; Pietrangelo, L.; Venditti, N.; Marco, R.G. Galleria mellonella as a consolidated in vivo model hosts: New developments in antibacterial strategies and novel drug testing. Virulence 2019, 10, 527–541. [Google Scholar] [CrossRef]
- Humann, J.; Lenz, L.L. Bacterial peptidoglycan degrading enzymes and their impact on host muropeptide detection. J. Innate Immun. 2009, 1, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Upadhyaya, I.; Johny, A.K.; Venkitanarayanan, K. Combating pathogenic microorganisms using plant-derived antimicrobials: A minireview of the mechanistic basis. Biomed. Res. Int. 2014, 2014, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Marais, J.P.; Khoo, C.; LaPlante, K.; Vejborg, R.M.; Givskov, M.; Nielsen, T.T.; Seeram, N.P.; Rowley, D.C. Cranberry (Vaccinium macrocarpon) oligosaccharides decrease biofilm formation by uropathogenic Escherichia coli. J. Funct. Foods 2015, 17, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Ulrey, R.K.; Barksdale, S.M.; Zhou, W.; Hoek, M.L.V. Cranberry proanthocyanidins have anti-biofilm properties against Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2014, 14, 499. [Google Scholar] [CrossRef] [PubMed]
- Wojnicz, D.; Goska, D.T.; Korzekwa, K.; Kicia, M.; Hendrich, A.B. Study of the impact of cranberry extract on the virulence factors and biofilm formation by Enterococcus faecalis strains isolated from urinary tract infections. Int. J. Food Sci. Nutr. 2016, 67, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Simoes, L.C.; Lemos, M.; Pereira, A.M.; Abreu, A.C.; Saavedra, M.J.; Simoes, M. Persister cells in a biofilm treated with a biocide. Biofouling 2011, 27, 403–411. [Google Scholar] [CrossRef]
- Suarez, M.; Haenni, M.; Canarelli, S.; Fisch, F.; Chodanowski, P.; Servis, C.; Michielin, O.; Freitag, R.; Moreillon, P.; Mermod, N. Structure-function characterization and optimization of a plant-derived antibacterial peptide. Antimicrob. Agents Chemother. 2005, 49, 3847–3857. [Google Scholar] [CrossRef]
- Giacinto, B.; Cosentino, M.; Sakurada, T. Aromatherapy: Basic Mechanisms And Evidence Based Clinical Use; CRC Press Taylor Francis Group: Boca Raton, FL, USA, 2016; p. 174. [Google Scholar]
- Willers, C.; Wentzel, J.F.; Plessis, L.H.D.; Gouws, C.; Hamman, J.H. Efflux as a mechanism of antimicrobial drug resistance in clinical relevant microorganisms: The role of efflux inhibitors. Expert Opin. Targets 2017, 21, 23–36. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Rad, J.S.; Salehi, B.; Varoni, E.M.; Sharopov, F.; Yousaf, Z.; Ayatollahi, S.A.; Kobarfard, F.; Rad, M.S.; Afdjei, M.H.; Iriti, M. Plants of the melaleuca genus as antimicrobial agents: From farm to pharmacy. Phytother. Res. 2017, 31, 1475–1494. [Google Scholar]
- Li, X.Z.; Plesiat, P.; Nikaido, H. The challenge of efflux-mediated antibiotic resistance in gram-negative bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Li, Y.; Guan, J.; Zhao, J.; Cui, J.; Wang, R.; Liu, Y. Effects of efflux pump inhibitors on colistin resistance in multidrug-resistant gram-negative bacteria. Antimicrob. Agents Chemother. 2016, 60, 3215–3218. [Google Scholar] [CrossRef] [PubMed]
- Selim, S.; Alfy, S.; Ruwaili, M.A.; Abdo, A.; Jaouni, S.A. Susceptibility of imipenem-resistant Pseudomonas aeruginosa to flavonoid glycosides of date palm (Phoenix dactylifera L.) tamar growing in Al madinah, saudi arabia. Afr. J. Biotechnol. 2012, 11, 416–422. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Eumkeb, G.; Sakdarat, S.; Siriwong, S. Reversing beta-lactam antibiotic resistance of Staphylococcus aureus with galangin from Alpinia officinarum hance and synergism with ceftazidime. Phytomedicine 2010, 18, 40–45. [Google Scholar] [CrossRef]
- Eumkeb, G.; Siriwong, S.; Phitaktim, S.; Rojtinnakorn, N.; Sakdarat, S. Synergistic activity and mode of action of flavonoids isolated from smaller galangal and amoxicillin combinations against amoxicillin-resistant Escherichia coli. J. Appl. Microbiol. 2012, 112, 55–64. [Google Scholar] [CrossRef]
- Davidson, P.M.; Naidu, A.S. Phyto-phenols. In Natural Food Antimicrobial Systems; Naidu, A.S., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 278–307. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl. Environ. Microbiol. 2004, 70, 5750–5755. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006, 108, 1–9. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Inhibition of membrane bound ATPases of Escherichia coli and Listeria monocytogenes by plant oil aromatics. Int. J. Food Microbiol. 2006, 111, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Ozfenerci, M.; Calıskan, U.K. Tea tree oil and its use in aromatherapy. Curr. Pers. Maps 2018, 2, 90–102. [Google Scholar]
- Lorenzi, V.; Muselli, A.; Bernardini, A.F.; Berti, L.; Pages, J.M.; Amaral, L.; Bolla, J.M. Geraniol restores antibiotic activities against multidrug-resistant isolates from gram-negative species. Antimicrob. Agents Chemother. 2009, 53, 2209–2211. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Khan, M.S.; Owais, M.; Ahmad, I. Effect of certain bioactive plant extracts on clinical isolates of beta-lactamase producing methicillin resistant Staphylococcus aureus. J. Basic. Microbiol. 2005, 45, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Takaishi, Y.; Shibata, H.; Higuti, T. ILSMRs (intensifier of beta-lactam-susceptibility in methicillin-resistant Staphylococcus aureus) from tara [caesalpinia spinosa (Molina) kuntze]. Phytomedicine 2006, 13, 209–212. [Google Scholar] [CrossRef]
- Rodrigues, F.F.; Costa, J.G.; Coutinho, H.D. Synergy effects of the antibiotics gentamicin and the essential oil of croton zehntneri. Phytomedicine 2009, 16, 1052–1055. [Google Scholar] [CrossRef]
- Grande, M.J.; Lopez, R.L.; Abriouel, H.; Valdivia, E.; Omar, N.B.; Maqueda, M.; Canamero, M.M.; Galvez, A. Treatment of vegetable sauces with enterocin AS-48 alone or in combination with phenolic compounds to inhibit proliferation of Staphylococcus aureus. J. Food Prot. 2007, 70, 405–411. [Google Scholar] [CrossRef]
- Chan, B.C.; Ip, M.; Lau, C.B.; Lui, S.L.; Jolivalt, C.; Elbaz, C.G.; Litaudon, M.; Reiner, N.E.; Gong, H.; See, R.H.; et al. Synergistic effects of baicalein with ciprofloxacin against NorA over-expressed methicillin-resistant Staphylococcus aureus (MRSA) and inhibition of MRSA pyruvate kinase. J. Ethnopharmacol. 2011, 137, 767–773. [Google Scholar] [CrossRef]
- Nikaido, H. Porins and specific diffusion channels in bacterial outer membranes. J. Biol. Chem. 1994, 269, 3905–3908. [Google Scholar]
- Pieboji, J.G.; Baurin, S.; Frere, J.M.; Ngassam, P.; Ngameni, B.; Azebaze, A.; Pegnyemb, D.E.; Watchueng, J.; Goffin, C.; Galleni, M. Screening of some medicinal plants from cameroon for beta-lactamase inhibitory activity. Phytother. Res. 2007, 21, 284–287. [Google Scholar] [CrossRef]
- Zhao, W.H.; Hu, Z.Q.; Hara, Y.; Shimamura, T. Inhibition of penicillinase by epigallocatechin gallate resulting in restoration of antibacterial activity of penicillin against penicillinase-producing Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 2266–2268. [Google Scholar] [CrossRef]
- Johny, A.K.; Hoagland, T.; Venkitanarayanan, K. Effect of subinhibitory concentrations of plant-derived molecules in increasing the sensitivity of multidrug-resistant Salmonella enterica serovar typhimurium DT104 to antibiotics. Foodborne Pathog. Dis. 2010, 7, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Moussaoui, F.; Alaoui, T. Evaluation of antibacterial activity and synergistic effect between antibiotic and the essential oils of some medicinal plants. Asian Pac. J. Trop. Biomed. 2016, 6, 32–37. [Google Scholar] [CrossRef]
- Senatore, F.; Rigano, D.; Fusco, R.D.; Bruno, M. Composition of the essential oil from flowerheads of Chrysanthemum coronarium L. (Asteraceae) growing wild in southern italy. Flavour. Fragr. 2004, 19, 149–152. [Google Scholar] [CrossRef]
- Davey, M.E.; O’Toole, G.A. Microbial biofilms: From ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 2000, 64, 847–867. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, R.A.N.; Frank, J.F. Biofilm formation and control in food processing facilities. Compr. Rev. Food Sci. Food Saf. 2003, 2, 22–32. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Langsrud, S.; Sidhu, M.S.; Heir, E.; Holck, A.L. Bacterial disinfectant resistance—A challenge for the food industry. Int. Biodeterior. Biodegrad. 2003, 51, 283–290. [Google Scholar] [CrossRef]
- Simoes, M.; Bennett, R.N.; Rosa, E.A. Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat. Prod. Rep. 2009, 26, 746–757. [Google Scholar] [CrossRef]
- Simões, M.; Simões, L.C.; Machado, I.; Pereira, M.O.; Vieira, M.J. Control of flow-generated biofilms with surfactants: Evidence of resistance and recovery. Food Bioprod. Proces. 2006, 84, 338–345. [Google Scholar] [CrossRef]
- Upadhyay, A.; Upadhyaya, I.; Johny, A.K.; Venkitanarayanan, K. Antibiofilm effect of plant derived antimicrobials on Listeria monocytogenes. Food Microbiol. 2013, 36, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Marino, A.; Blanco, A.R.; Cellini, L.; Di Giulio, M.; Pizzimenti, F.; Roccaro, A.S.; Bisignano, G. In vitro activity of carvacrol against staphylococcal preformed biofilm by liquid and vapour contact. J. Med. Microbiol. 2009, 58, 791–797. [Google Scholar] [CrossRef]
- Nostro, A.; Scaffaro, R.; D’Arrigo, M.; Botta, L.; Filocamo, A.; Marino, A.; Bisignano, G. Study on carvacrol and cinnamaldehyde polymeric films: Mechanical properties, release kinetics and antibacterial and antibiofilm activities. Appl. Microbiol. Biotechnol. 2012, 96, 1029–1038. [Google Scholar] [CrossRef]
- Nostro, A.; Sudano Roccaro, A.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef]
- Walencka, E.; Rozalska, S.; Wysokinska, H.; Rozalski, M.; Kuzma, L.; Rozalska, B. Salvipisone and aethiopinone from salvia sclarea hairy roots modulate staphylococcal antibiotic resistance and express anti-biofilm activity. Planta Med. 2007, 73, 545–551. [Google Scholar] [CrossRef]
- Bakri, A.G.A.; Othman, G.; Afifi, F.U. Determination of the antibiofilm, antiadhesive, and anti-MRSA activities of seven salvia species. Pharm. Mag. 2010, 6, 264–270. [Google Scholar] [CrossRef]
- Wu, H.; Lee, B.; Yang, L.; Wang, H.; Givskov, M.; Molin, S.; Hoiby, N.; Song, Z. Effects of ginseng on Pseudomonas aeruginosa motility and biofilm formation. FEMS Immunol. Med. Microbiol. 2011, 62, 49–56. [Google Scholar] [CrossRef]
- Cheng, K.; Anderson, D.; Dan, A. Inhibiting biofilm formation of Pseudomonas aeruginosa: A two-pronged attack. Young Sci. J. 2009, 2, 8–13. [Google Scholar]
- Grudniak, A.M.; Kurek, A.; Szarlak, J.; Wolska, K.I. Oleanolic and ursolic acids influence affect the expression of the cysteine regulon and the stress response in Escherichia coli. Curr. Microbiol. 2011, 62, 1331–1336. [Google Scholar] [CrossRef]
- Ren, D.; Zuo, R.; Barrios, A.F.G.; Bedzyk, L.A.; Eldridge, G.R.; Pasmore, M.E.; Wood, T.K. Differential gene expression for investigation of Escherichia coli biofilm inhibition by plant extract ursolic acid. Appl. Environ. Microbiol. 2005, 71, 4022–4034. [Google Scholar] [CrossRef] [PubMed]
- Amalaradjou, M.A.; Narayanan, A.; Venkitanarayanan, K. Trans-cinnamaldehyde decreases attachment and invasion of uropathogenic Escherichia coli in urinary tract epithelial cells by modulating virulence gene expression. J. Urol. 2011, 185, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Goh, E.B.; Yim, G.; Tsui, W.; McClure, J.; Surette, M.G.; Davies, J. Transcriptional modulation of bacterial gene expression by subinhibitory concentrations of antibiotics. Proc. Natl. Acad. Sci USA 2002, 99, 17025–17030. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Feng, H.; Lu, J.; Xiang, H.; Wang, D.; Dong, J.; Wang, J.; Wang, X.; Liu, J.; Deng, X. Eugenol reduces the expression of virulence-related exoproteins in Staphylococcus aureus. Appl. Environ. Microbiol. 2010, 76, 5846–5851. [Google Scholar] [CrossRef] [PubMed]
- Tsui, W.H.; Yim, G.; Wang, H.H.; McClure, J.E.; Surette, M.G.; Davies, J. Dual effects of MLS antibiotics: Transcriptional modulation and interactions on the ribosome. Chem. Biol. 2004, 11, 1307–1316. [Google Scholar] [CrossRef]
- Upadhyay, A.; Johny, A.K.; Amalaradjou, M.A.; Baskaran, S.A.; Kim, K.S.; Venkitanarayanan, K. Plant-derived antimicrobials reduce Listeria monocytogenes virulence factors in vitro, and down-regulate expression of virulence genes. Int. J. Food Microbiol. 2012, 157, 88–94. [Google Scholar] [CrossRef]
- Yim, G.; McClure, J.; Surette, M.G.; Davies, J.E. Modulation of salmonella gene expression by subinhibitory concentrations of quinolones. J. Antibiot 2011, 64, 73–78. [Google Scholar] [CrossRef]
- Brackman, G.; Defoirdt, T.; Miyamoto, C.; Bossier, P.; Calenbergh, S.V.; Nelis, H.; Coenye, T. Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol. 2008, 8, 149. [Google Scholar] [CrossRef]
- Zou, Y.; Woo, J.; Ahn, J. Cellular and molecular responses of salmonella typhimurium to antimicrobial-induced stresses during the planktonic-to-biofilm transition. Lett. Appl. Microbiol. 2012, 55, 274–282. [Google Scholar] [CrossRef]
- Jakobsen, T.H.; Gennip, M.V.; Phipps, R.K.; Shanmugham, M.S.; Christensen, L.D.; Alhede, M.; Skindersoe, M.E.; Rasmussen, T.B.; Friedrich, K.; Uthe, F.; et al. Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob. Agents Chemother. 2012, 56, 2314–2325. [Google Scholar] [CrossRef]
- Amalaradjou, M.A.; Narayanan, A.; Baskaran, S.A.; Venkitanarayanan, K. Antibiofilm effect of trans-cinnamaldehyde on uropathogenic Escherichia coli. J. Urol. 2010, 184, 358–363. [Google Scholar] [CrossRef]
- Dalleau, S.; Cateau, E.; Berges, T.; Berjeaud, J.M.; Imbert, C. In vitro activity of terpenes against candida biofilms. Int. J. Antimicrob. Agents 2008, 31, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Ahmad, I. Biofilm inhibition by cymbopogon citratus and syzygium aromaticum essential oils in the strains of Candida albicans. J. Ethnopharmacol. 2012, 140, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.R.; Roller, S.; Murray, D.B.; Naidu, A.S. Antimicrobial action of carvacrol at different stages of dual-species biofilm development by Staphylococcus aureus and Salmonella enterica serovar typhimurium. Appl. Environ. Microbiol. 2005, 71, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.C.; Ferreira, R.B. Intercellular communication in bacteria. Crit. Rev. Microbiol. 2009, 35, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Novick, R.P.; Geisinger, E. Quorum sensing in staphylococci. Annu. Rev. Genet. 2008, 42, 541–564. [Google Scholar] [CrossRef]
- Quinn, T.; O’Mahony, R.; Baird, A.W.; Drudy, D.; Whyte, P.; Fanning, S. Multi-drug resistance in Salmonella enterica: Efflux mechanisms and their relationships with the development of chromosomal resistance gene clusters. Curr. Drug Targets 2006, 7, 849–860. [Google Scholar] [CrossRef]
- Adonizio, A.L.; Downum, K.; Bennett, B.C.; Mathee, K. Anti-quorum sensing activity of medicinal plants in southern florida. J. Ethnopharmacol. 2006, 105, 427–435. [Google Scholar] [CrossRef]
- Bauer, W.D.; Mathesius, U. Plant responses to bacterial quorum sensing signals. Curr. Opin. Plant Biol. 2004, 7, 429–433. [Google Scholar] [CrossRef]
- Teplitski, M.; Robinson, J.B.; Bauer, W.D. Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol. Plant. Microbe Interact. 2000, 13, 637–648. [Google Scholar] [CrossRef]
- Bodini, S.F.; Manfredini, S.; Epp, M.; Valentini, S.; Santori, F. Quorum sensing inhibition activity of garlic extract and p-coumaric acid. Lett. Appl. Microbiol. 2009, 49, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Cech, N.B.; Junio, H.A.; Ackermann, L.W.; Kavanaugh, J.S.; Horswill, A.R. Quorum quenching and antimicrobial activity of goldenseal (Hydrastis canadensis) against methicillin-resistant Staphylococcus aureus (MRSA). Planta Med. 2012, 78, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Talekar, S.J.; Chochua, S.; Nelson, K.; Klugman, K.P.; Quave, C.L.; Vidal, J.E. 220D-F2 from rubus ulmifolius kills Streptococcus pneumoniae planktonic cells and pneumococcal biofilms. PLoS ONE 2014, 9, e97314. [Google Scholar] [CrossRef] [PubMed]
- Bazargani, M.M.; Rohloff, J. Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control. 2016, 61, 156–164. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C. The mechanism of action of a citrus oil blend against Enterococcus faecium and Enterococcus faecalis. J. Appl. Microbiol. 2009, 106, 1343–1349. [Google Scholar] [CrossRef]
- Huskins, W.C.; Huckabee, C.M.; O’Grady, N.P.; Murray, P.; Kopetskie, H.; Zimmer, L.; Walker, M.E.; Cochran, R.L.S.; Jernigan, J.A.; Samore, M.; et al. Intervention to reduce transmission of resistant bacteria in intensive care. N. Engl. J. Med. 2011, 364, 1407–1418. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Youns, M.; Readi, M.Z.E.; Ashour, M.L.; Nibret, E.; Sporer, F.; Herrmann, F.; Reichling, J.; Wink, M. Biological activity of the essential oil of Kadsura longipedunculata (Schisandraceae) and its major components. J. Pharm. Pharm. 2010, 62, 1037–1044. [Google Scholar] [CrossRef]
- Zomorodian, K.; Saharkhiz, M.J.; Rahimi, M.J.; Bandegi, A.; Shekarkhar, G.; Bandegani, A.; Pakshir, K.; Bazargani, A. Chemical composition and antimicrobial activities of the essential oils from three ecotypes of Zataria multiflora. Pharm. Mag. 2011, 7, 53–59. [Google Scholar]
- Hobby, G.H.; Quave, C.L.; Nelson, K.; Compadre, C.M.; Beenken, K.E.; Smeltzer, M.S. Quercus cerris extracts limit Staphylococcus aureus biofilm formation. J. Ethnopharmacol. 2012, 144, 812–815. [Google Scholar] [CrossRef]
- Ranfaing, J.; Remy, C.D.; Lavigne, J.P.; Sotto, A. Propolis potentiates the effect of cranberry (Vaccinium macrocarpon) in reducing the motility and the biofilm formation of uropathogenic Escherichia coli. PLoS ONE 2018, 13, e0202609. [Google Scholar] [CrossRef]
- Hyams, C.; Camberlein, E.; Cohen, J.M.; Bax, K.; Brown, J.S. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect. Immun. 2010, 78, 704–715. [Google Scholar] [CrossRef]
- Sanders, M.E.; Norcross, E.W.; Robertson, Z.M.; Moore, Q.C.; Fratkin, J.; Marquart, M.E. The Streptococcus pneumoniae capsule is required for full virulence in pneumococcal endophthalmitis. Invest. Ophthalmol. Vis. Sci. 2011, 52, 865–872. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, K.; Lee, J.C. Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 2004, 17, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Potera, C. Forging a link between biofilms and disease. Science 1999, 283, 1837–1839. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.M.; Roberts, I.S. Capsular polysaccharides and their role in virulence. Contrib. Microbiol. 2005, 12, 55–66. [Google Scholar]
- Johny, A.K.; Mattson, T.; Baskaran, S.A.; Amalaradjou, M.A.; Babapoor, S.; March, B.; Valipe, S.; Darre, M.; Hoagland, T.; Schreiber, D.; et al. Reduction of salmonella enterica Serovar enteritidis colonization in 20-day-old broiler chickens by the plant-derived compounds trans-cinnamaldehyde and eugenol. Appl. Environ. Microbiol. 2012, 78, 2981–2987. [Google Scholar] [CrossRef]
- Derakhshan, S.; Sattari, M.; Bigdeli, M. Effect of subinhibitory concentrations of cumin (Cuminum cyminum L.) seed essential oil and alcoholic extract on the morphology, capsule expression and urease activity of klebsiella pneumoniae. Int. J. Antimicrob. Agents 2008, 32, 432–436. [Google Scholar] [CrossRef]
- Shah, J. The salicylic acid loop in plant defense. Curr. Opin. Plant. Biol. 2003, 6, 365–371. [Google Scholar] [CrossRef]
- Huang, C.T.; Stewart, P.S. Reduction of polysaccharide production in Pseudomonas aeruginosa biofilms by bismuth dimercaprol (BisBAL) treatment. J. Antimicrob. Chemother. 1999, 44, 601–605. [Google Scholar] [CrossRef]
- Mehreen, A.; Waheed, M.; Liaqat, I.; Arshad, N. Phytochemical, antimicrobial, and toxicological evaluation of traditional herbs used to treat sore throat. Biomed. Res. Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Popova, I.E.; Hall, C.; Kubatova, A. Determination of lignans in flaxseed using liquid chromatography with time-of-flight mass spectrometry. J. Chromatogr. A 2009, 1216, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.R.L.; Butler, M.S.; Phetsang, W.; Cooper, M.A.; Blaskovich, M.A.T. Fluorescent antibiotics: New research tools to fight antibiotic resistance. Trends Biotechnol. 2018, 36, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Carradori, S.; Chimenti, P.; Fazzari, M.; Granese, A.; Angiolella, L. Antimicrobial activity, synergism and inhibition of germ tube formation by crocus sativus-derived compounds against Candida spp. J. Enzym. Inhib. Med. Chem. 2016, 31, 189–193. [Google Scholar] [CrossRef]
| Plant Name | Plant Derivatives | Bacterial sp. | * MIC Value | References |
|---|---|---|---|---|
| Anogeissusa cuminata | terpenoids, flavonoids, saponins, tannins, alkaloids | S. aureus | 0.29 mg/mL | [83] |
| A. baumannii | 1.51 mg/mL | |||
| C. freundii | 3.41 mg/mL | |||
| E. coli | 3.41 mg/mL | |||
| K. oxytoca | 1.51 mg/mL | |||
| K. pneumoniae | 0.67 mg/mL | |||
| P. aeruginosa | 0.67 mg/mL | |||
| Azadirachta indica | β-sitosterol, flavonoids | S. aureus | 3.41 mg/mL | [83] [84] |
| A. baumannii | 4.27 mg/mL | |||
| C. freundii | 3.41 mg/mL | |||
| E. coli | 4.27 mg/mL | |||
| K. oxytoca | 9.63 mg/mL | |||
| K. pneumoniae | 4.27 mg/mL | |||
| P. aeruginosa H. pylori | 9.63 mg/mL 128 µg/mL | |||
| Bauhinia variegata | terpenoids, flavonoids, tannins, saponins, glucoside, | S. aureus | 3.41 mg/mL | [83] |
| A. baumannii | 9.63 mg/mL | |||
| C. freundii | 9.63 mg/mL | |||
| E. coli | 4.27 mg/mL | |||
| K. oxytoca | 9.63 mg/mL | |||
| K. pneumoniae | 4.27 mg/mL | |||
| P. aeruginosa | 3.41 mg/mL | |||
| Boerhaavia diffusa | β-sitosterol, flavonoids | S. aureus | 4.27 mg/mL | [83] |
| A. baumannii | 9.63 mg/mL | |||
| C. freundii | 9.63 mg/mL | |||
| E. coli | NA | |||
| K. oxytoca | 9.63 mg/mL | |||
| K. pneumoniae | 9.63 mg/mL | |||
| P. aeruginosa | 9.63 mg/mL | |||
| Punica granatum | flavonoids, ellagitannin, punicalagin, ellagic acid | S. aureus | 0.29 mg/mL | [83] |
| A. baumannii | 3.41 mg/mL | |||
| C. freundii | 0.67 mg/mL | |||
| E. coli | 0.67 mg/mL | |||
| K. oxytoca | 3.41 mg/mL | |||
| K. pneumoniae | 3.41 mg/mL | |||
| P. aeruginosa | 0.67 mg/mL | |||
| Soymida febrifuga | methyl angolensate, luteolin 7-O-glucoside, flavonoid, sitosterol, myricetin | S. aureus | 067 mg/mL | [83] |
| A. baumannii | 1.51 mg/mL | |||
| C. freundii | 3.41 mg/mL | |||
| E. coli | 4.27 mg/mL | |||
| K. oxytoca | 3.41 mg/mL | |||
| K. pneumoniae | 3.41 mg/mL | |||
| P. aeruginosa | 4.27 mg/mL | |||
| Terminalia chebula | flavonoids and flavins, terpenoids, steroids, alkaloids, tannins and their derivatives, glycosides | S. aureus | 1.51 mg/mL | [83] |
| A. baumannii | 9.63 mg/mL | |||
| C. freundii | NA | |||
| E. coli | 9.63 mg/mL | |||
| K. oxytoca | 9.63 mg/mL | |||
| K. pneumoniae | 9.63 mg/mL | |||
| P. aeruginosa | 9.63 mg/mL | |||
| Tinospora cordifolia | alkaloids terpenoids, lactones, glycosides, steroids, phenolics | S. aureus | 4.27 mg/mL | [83] |
| A. baumannii | NA | |||
| C. freundii | 9.63 mg/mL | |||
| E. coli | 4.27 mg/mL | |||
| K. oxytoca | 9.63 mg/mL | |||
| K. pneumoniae | 9.63 mg/mL | |||
| P. aeruginosa | 4.27 mg/mL | |||
| Tribulus terrestris | flavonoids, flavonol glycosides, steroidal saponins and alkaloids | S. aureus | 3.41 mg/mL | [83] |
| A. baumannii | 9.63 mg/mL | |||
| C. freundii | 9.63 mg/mL | |||
| E. coli | 4.27 mg/mL | |||
| K. oxytoca | 4.27 mg/mL | |||
| K. pneumoniae | 9.63 mg/mL | |||
| P. aeruginosa | 3.41 mg/mL | |||
| Petroselinum crispum EO (Essential oil) | phenolic, flavonoids, coumarins | B. cereus | 22.68 µL/mL | [85] |
| S. aureus | 10.80 µL/mL | |||
| P. aeruginosa | 47.62 µL/mL | |||
| E. coli | 10.80 µL/mL | |||
| S. typhimurium | 47.62 µL/mL | |||
| Levisticum officinale EO | terpenoids, n-butylidene phthalide n-butyl-phthalide, sedanonic anhydride, d-terpineol, l, l phenolic, | B. cereus | 47.62 µL/mL | [85] |
| S. aureus | 2.45 µL/mL | |||
| P. aeruginosa | 22.68 µL/mL | |||
| E. coli | 10.80 µL/mL | |||
| S. typhimurium | 47.62 µL/mL | |||
| Occimomum basilicum EO | rosmarinic acid, phenol and terpenoid | B. cereus | 10.80 µL/mL | [85] |
| S. aureus | 2.45 µL/mL | |||
| P. aeruginosa | 22.68 µL/mL | |||
| E. coli | 10.80 µL/mL | |||
| S. typhimurium | 22.68 µL/mL | |||
| Thymus vulgare EO | p-cymene, γ-terpinene, thymol | B. cereus | 0.56 µL/mL | [85] |
| S. aureus | 0.06 µL/mL | |||
| P. aeruginosa | 0.56 µL/mL | |||
| E. coli | 0.27 µL/mL | |||
| S. typhimurium | 0.56 µL/mL | |||
| Cannabis sativa L., EO | phenol, flavonoid | S. aureus | 8 mg/mL | [86] |
| Acrosta phylosuvaursi | ellagic and gallic acid tannins, flavonoids, phenol | S. aureus | 90 µg/mL | [87] |
| Coptis chinensis | isoquinoline, alkaloids | 121 µg/mL | ||
| Eucalyptus globulus | 1,8-cineole, α-pinene, p-cymene | 118 µg/mL | ||
| Larreatri dentata | alkaloids, lignans, flavonoid, terpenoids | 60 µg/mL | ||
| Alpinia galanga | α-pinene, myrcene, limonene | Mycobacterium smegmatis mc2 155 | 3.12–25 mg/L | [88] |
| Ammannia spp. | dioxyflavanol, quercetin and kaempferol | E. coli | 125 µg/mL | [89] |
| Berberis vulgaris | isoquinoline, alkaloids, | P. aeruginosa | 250–1000 µg/mL | [90] |
| Acer saccharum | flavonoids, tannins | E. coli | 5 and 10 mg/mL | [91] |
| P. aeruginosa | ||||
| P. mirabilis | ||||
| Catharanthus roseus | limonene, terpenoid | P. aeruginosa | 25 mg/L | [92] |
| Holarrhena antidysenterica | triterpenoids, sitosterol, phytosterol | P. aeruginosa | 20 mg/L | [93] |
| Cuminum cyminum | alkaloid, phenols, flavonoid, glycoside, saponin, tannin and steroid | S. aureus | 5 mg/mL | [94] |
| Salvia fruticosa | flavonoids, phenolics and rosemarinic acid | S. epidermidis | 5 µl/mL | [95] |
| Chenopodium Ambrosioides | α-terpinene, ascaridole | S. aureus | 170.6 µl/mL | [96] |
| Terminalia chebola | ellagic acid, gallic acid | E. coli | 12.1–97.5 µg/mL | [97] |
| Persea lingue | flavonoids, | S. aureus | 1.56 mg/L | [98] |
| Ipomoea muricata | ipomine, ipalbine, ipalbidine and ipalbinium | E. coli | 10 µg/mL | [99] |
| Hypericum olympicum | essential oils, α-pinene, β-ocimene, β-caryophyllene, germacrene-D | S. aureus | 50 µM | [100] |
| Alkanna orientalis | β-eudesmol, α-eudesmol and γ-eudesmol | S. aureus | 100 µM | [101] |
| Eucalyptus tereticornis | saponins, tannins, steroids flavonoids, cardiac glycosides | E. coli | 25 and 50 µg/mL | [89] |
| Salvia officinalis | phenols, terpenoids | E. cloacae, E. coli, S. typhimurium, P. aeruginosa, B. cereus | 0.01 mg/mL, 0.045 mg/mL, 0.045 mg/mL, 0.045 mg/mL, 0.09 mg/mL | [102] |
| Phytochemicals | Extract | Mode of Action | Antimicrobial Resistant Microbes | References |
|---|---|---|---|---|
| Flavonoids | Vaccinium macrocarpon Alt (cranberry) | Modifies biofilm formation | Enterococcus faecalis, E. coli, Pseudomonas aeruginosa | [114,115,116] |
| Myricetin, robinetin, epigallocatechin | Blocks bacterial DNA synthesis | E. coli | [117] | |
| Quercetin | Inhibit ATPase activity, GrYB protein, elevates extracellular phosphatase and β-galactosidase | E. coli, S. aureus | [118] | |
| Plant-derived peptides | Moringa oleifera | Membrane disruption | E. coli, S. aureus, P. aeruginosa, S. Typhimurium | [119] |
| Essential oils (EOs) | Petroselinum crispum EO, Levisticum officinale EO, Ocimum basilicum EO, Thymus vulgaris EO Cannabis sativa L., EO | Increase cell permeability, leakage of cell constituents, alteration of bacterial cell wall and membrane disturbance, ATP loss, inhibit protein synthesis, lead to pH disturbance, intracytoplasmic damage, DNA damage, inhibit quorum sensing Inhibits biofilm formation | Bacillus cereus, Staphylococcus aureus, P. aeruginosa, E. coli, S. Typhimurium S. aureus | [120,121] [102] |
| Tea tree oil (TTO) | terpenes, monoterpenes, sesquiterpenes | disrupts membrane permeability, damages cell membrane, obstructs cell growth, cause cell death | E. coli, S. aureus, C. albicans | [122,123] |
| Natural Efflux pump inhibitors (EPIs) | reserpine, gallotannin, piperine, curcumin, berberine, chalcones, carnosic acid | Inhibit various efflux pump in bacteria (EtBr EP, MexAB-OprM) | MDR Uropathogenic E. coli, MDR, P. aeruginosa (clinical isolates) | [82,90,97,121,124,125] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlSheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Tasleem Jan, A.; Haq, Q.M.R. Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics 2020, 9, 480. https://doi.org/10.3390/antibiotics9080480
AlSheikh HMA, Sultan I, Kumar V, Rather IA, Al-Sheikh H, Tasleem Jan A, Haq QMR. Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics. 2020; 9(8):480. https://doi.org/10.3390/antibiotics9080480
Chicago/Turabian StyleAlSheikh, Hana Mohammed Al, Insha Sultan, Vijay Kumar, Irfan A. Rather, Hashem Al-Sheikh, Arif Tasleem Jan, and Qazi Mohd Rizwanul Haq. 2020. "Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance" Antibiotics 9, no. 8: 480. https://doi.org/10.3390/antibiotics9080480
APA StyleAlSheikh, H. M. A., Sultan, I., Kumar, V., Rather, I. A., Al-Sheikh, H., Tasleem Jan, A., & Haq, Q. M. R. (2020). Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics, 9(8), 480. https://doi.org/10.3390/antibiotics9080480







