Antimicrobial and Antifungal Activity of Rare Substituted 1,2,3-Thiaselenazoles and Corresponding Matched Pair 1,2,3-Dithiazoles
Abstract
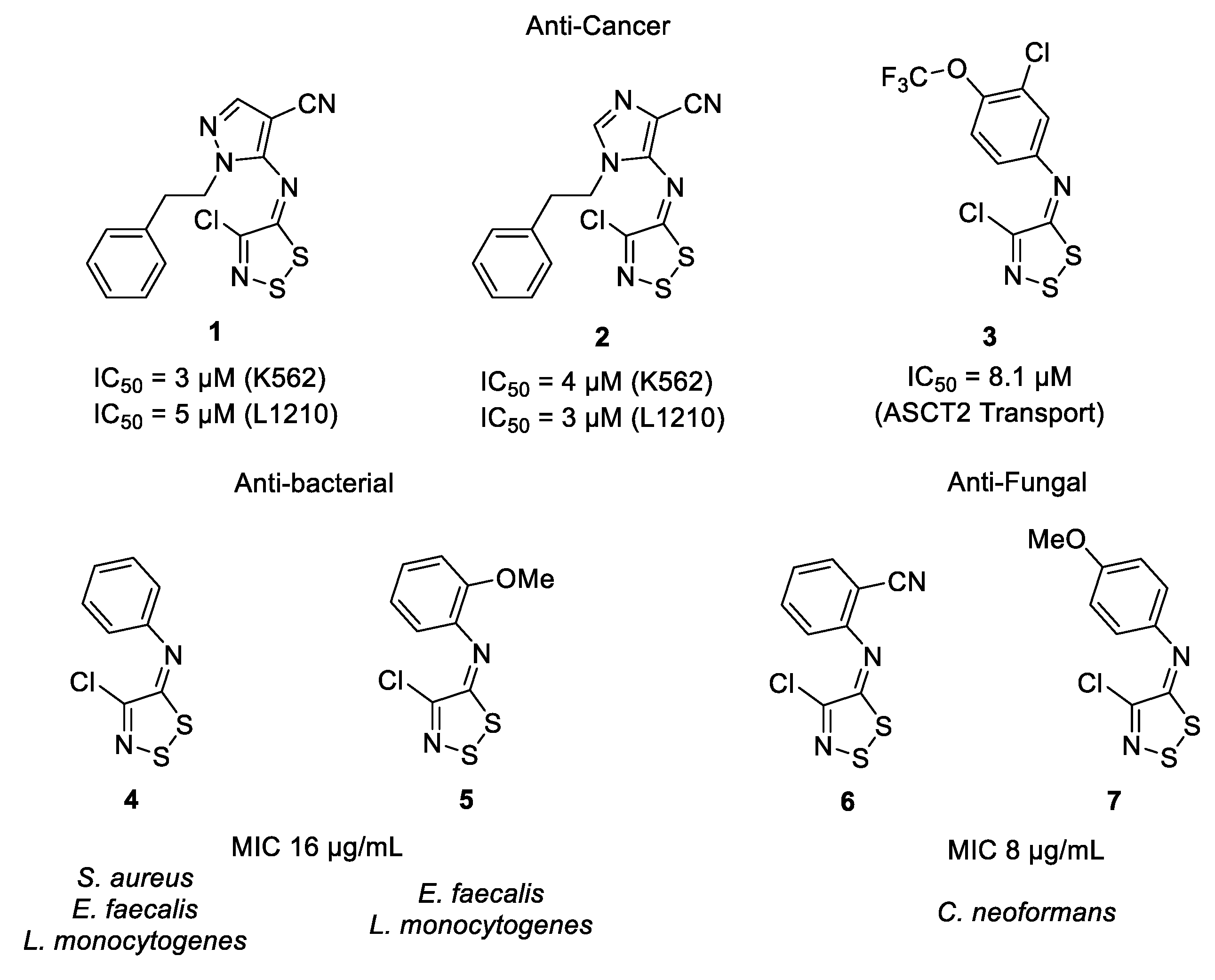
1. Introduction
2. Results
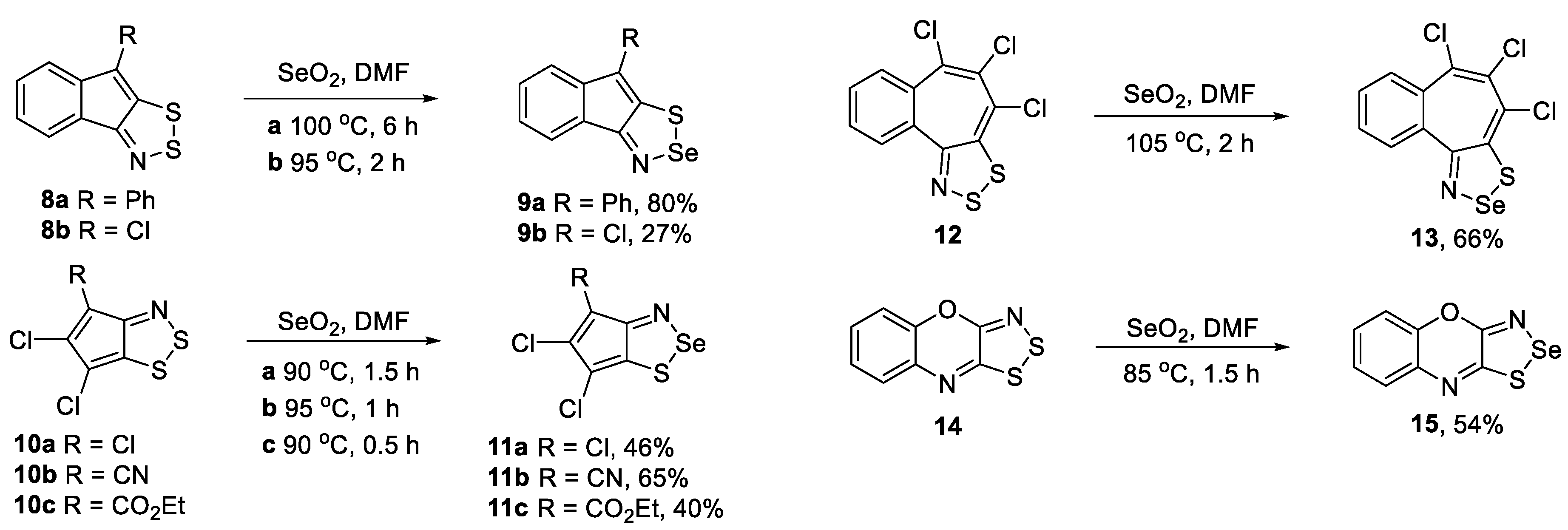
2.1. Synthesis of 1,2,3-Dithiazoles and 1,2,3-Thiaselenazoles
2.2. Evaluation of 1,2,3-Dithiazoles and 1,2,3-Thiaselenazoles
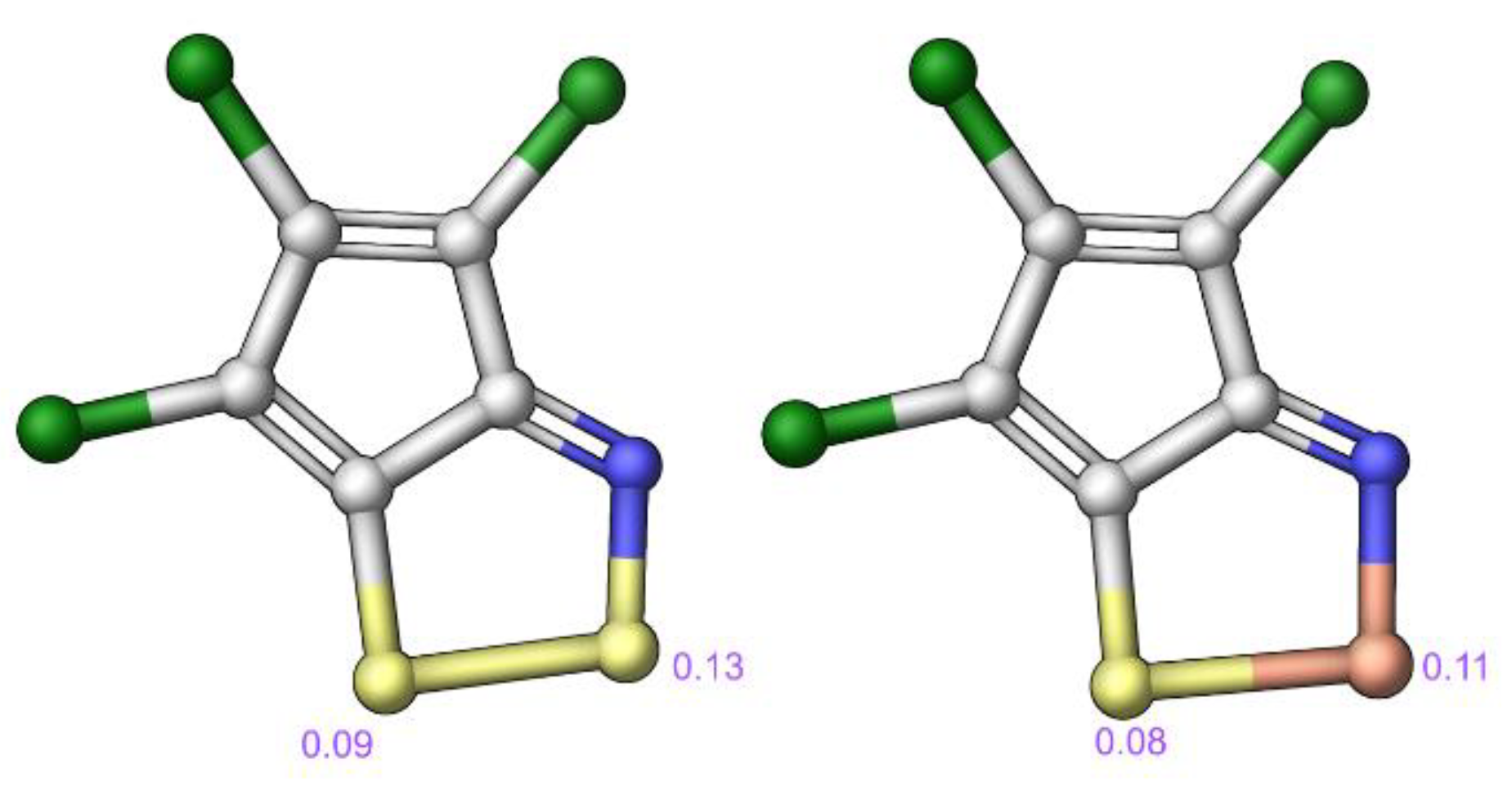
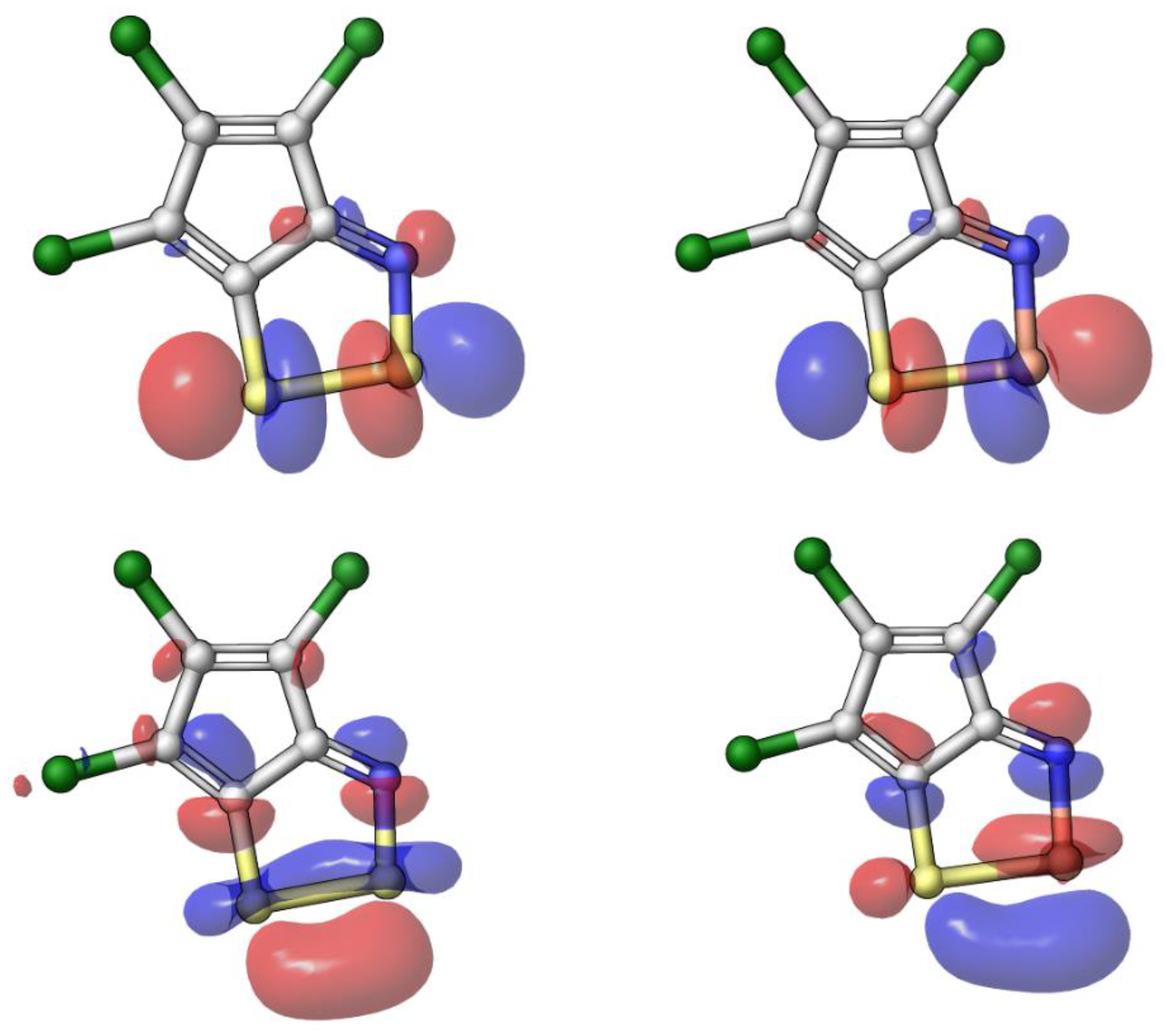
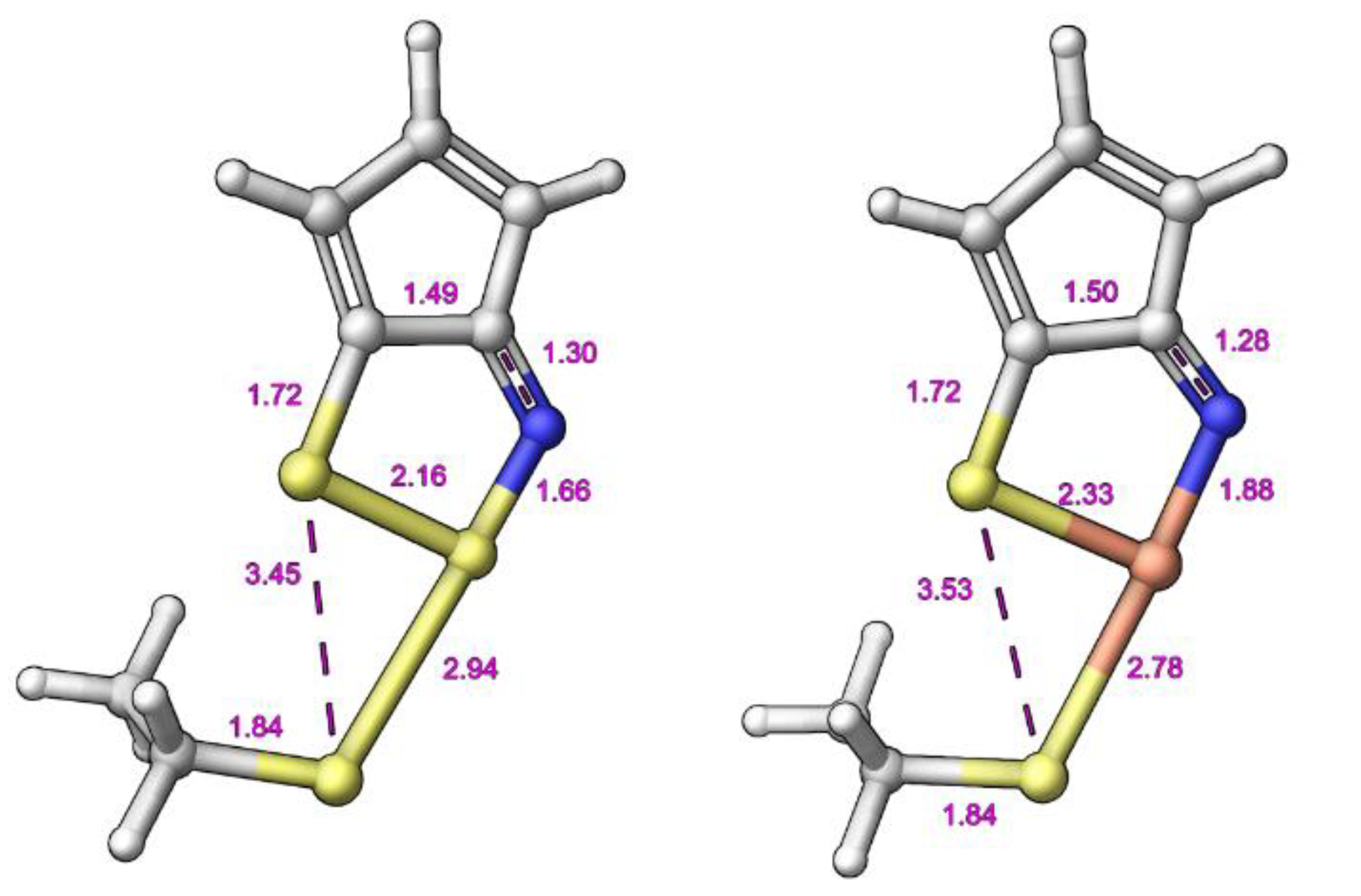
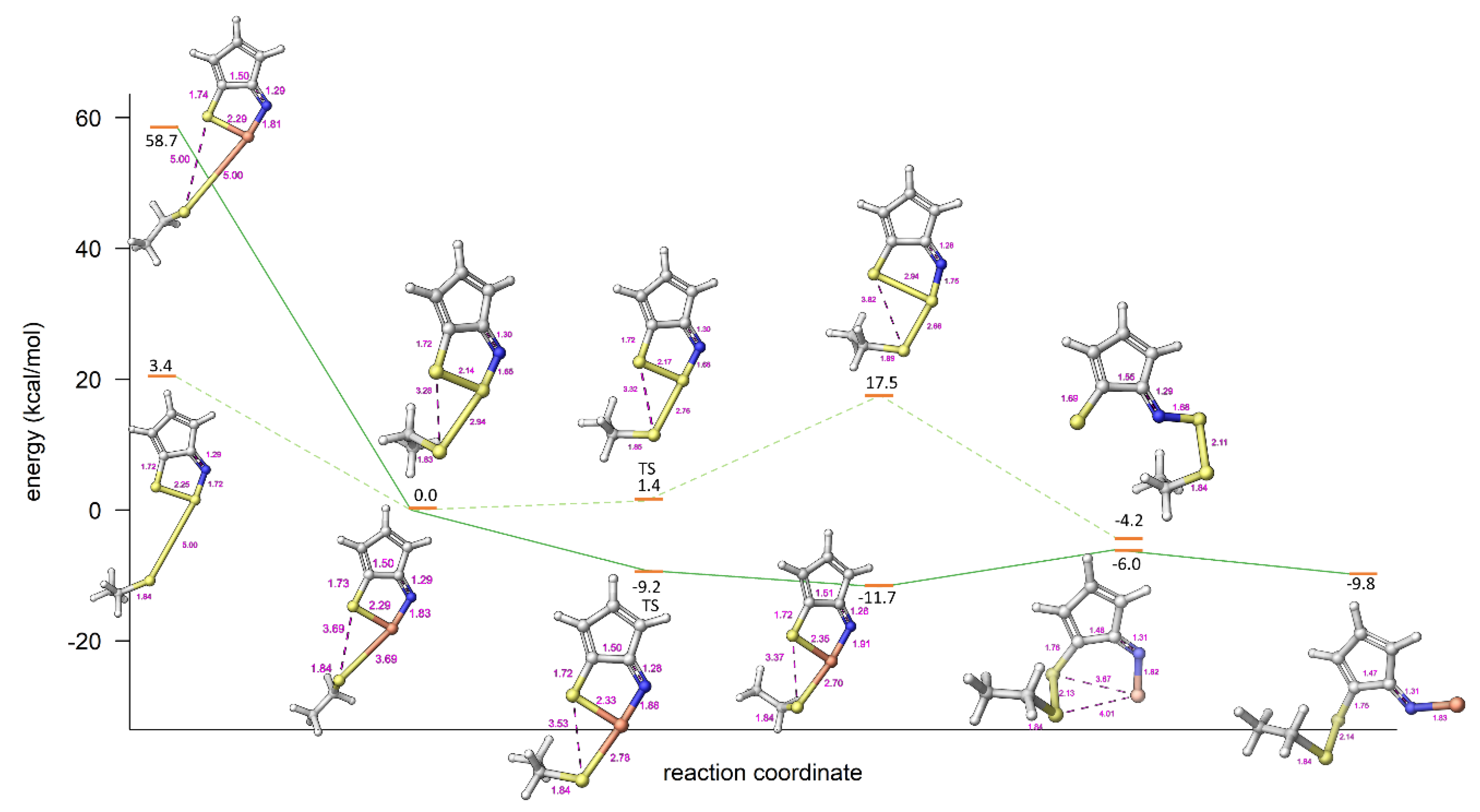
2.3. Modelling Investigation
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. General Procedure for Synthesis of 1,2,3-dithiazoles.
4.1.2. General Procedure for Synthesis of 1,2,3-Thiaselenazoles
4.2. Molecular Simulations and Modelling
4.2.1. General Modelling and Electronic Structure Calculations
4.2.2. Vibrational Analysis
4.2.3. Transition State Optimization
4.3. Biology
4.3.1. Antibacterial Assay
4.3.2. Antifungal Assay
4.3.3. Cytotoxicity Assay
4.3.4. Haemolysis Assay
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brown, E.D.; Wright, G.D. Antibacterial drug discovery in the resistance era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Aminov, R. History of antimicrobial drug discovery: Major classes and health impact. Biochem. Pharm. 2017, 133, 4–19. [Google Scholar] [CrossRef]
- Cowen, L.E. The evolution of fungal drug resistance: Modulating the trajectory from genotype to phenotype. Nat. Rev. Microbiol. 2008, 6, 187–198. [Google Scholar] [CrossRef]
- Cowen, L.E.; Steinbach, W.J. Stress, drugs, and evolution: The role of cellular signaling in fungal drug resistance. Eukaryot. Cell 2008, 7, 747–764. [Google Scholar] [CrossRef] [PubMed]
- Perfect, J.R. The antifungal pipeline: A reality check. Nat. Rev. Drug. Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How Antibiotics Kill Bacteria: From Targets to Networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.M.; Clay, M.C.; Cioffi, A.G.; Diaz, K.A.; Hisao, G.S.; Tuttle, M.D.; Nieuwkoop, A.J.; Comellas, G.; Maryum, N.; Wang, S.; et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 2014, 10, 400–406. [Google Scholar] [CrossRef]
- Maertens, J.A. History of the development of azole derivatives. Clin. Microbiol. Infect. 2004, 10, 1–10. [Google Scholar] [CrossRef]
- Perlin, D.S. Current perspectives on echinocandin class drugs. Future Microbiol. 2011, 6, 441–457. [Google Scholar] [CrossRef]
- Mroczyńska, M.; Brillowska-Dąbrowska, A. Review on Current Status of Echinocandins Use. Antibiotics 2020, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Konstantinova, L.S.; Bol’shakov, O.I.; Obruchnikova, N.V.; Laborie, H.; Tanga, A.; Sopéna, V.; Lanneluc, I.; Picot, L.; Sablé, S.; Thiéry, V.; et al. One-pot synthesis of 5-phenylimino, 5-thieno or 5-oxo-1,2,3-dithiazoles and evaluation of their antimicrobial and antitumor activity. Bioorg. Med. Chem. Lett. 2008, 19, 136–141. [Google Scholar] [CrossRef]
- Oppedisano, F.; Catto, M.; Koutentis, P.A.; Nicolotti, O.; Pochini, L.; Koyioni, M.; Introcaso, A.; Michaelidou, S.S.; Carotti, A.; Indiveri, C. Inactivation of the Glutamine/Amino Acid Transporter ASCT2 by 1,2,3-dithiazoles: Proteoliposomes as a Tool to Gain Insights in the Molecular Mechanism of Action and of Antitumor Activity. Toxicol. Appl. Pharmacol. 2012, 265, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, L.; Scalise, M.; Koyioni, M.; Koutentis, P.; Catto, M.; Eberini, I.; Parravicini, C.; Palazzolo, L.; Pisani, L.; Galluccio, M.; et al. Potent Inhibitors of Human LAT1 (SLC7A5) Transporter Based on Dithiazole and Dithiazine Compounds for Development of Anticancer Drugs. Biochem. Pharmacol. 2017, 143, 39–52. [Google Scholar] [CrossRef]
- Cottenceau, G.; Besson, T.; Gautier, V.; Rees, C.W.; Pons, A.-M. Antibacterial Evaluation of Novel N-Arylimino-1,2,3-dithiazoles and N-Arylcyanothioformamides. Bioorg. Med. Chem. Lett. 1996, 6, 529–532. [Google Scholar] [CrossRef]
- Besson, T.; Rees, C.W.; Cottenceau, G.; Pons, A.-M. Antimicrobial evaluation of 3,1-benzoxazin-4-ones, 3,1-benzothiazin-4-ones, 4-alkoxyquinazolin-2-carbonitriles and N-arylimino-1,2,3-dithiazoles. Bioorg. Med. Chem. Lett. 1996, 6, 2343–2348. [Google Scholar] [CrossRef]
- Thiéry, V.; Rees, C.W.; Besson, T.; Cottenceau, G.; Pons, A.-M. Antimicrobial activity of novel N-quinolinyl and N-naphthylimino-1,2,3-dithiazoles. Eur. J. Med. Chem. 1998, 33, 149–153. [Google Scholar] [CrossRef]
- Joseph, R.W.; Antes, D.L.; Osei-Gyimah, P. (Rohm & Haas Co.), Antimicrobial Compounds with Quick Speed of Kill. US Patent No. US5688744 A, 18 November 1997. [Google Scholar]
- Moore, J.E. (Chevron Research Co.), Certain 4-Halo-5-aryl-1,2,3-dithiazole Compounds and Their Preparation. US Patent No. US4059590, 22 November 1977. [Google Scholar]
- Moore, J.E. (Chevron Research Co.), Method for Control of Fungi Using 4-Halo-5-aryl-1,2,3,-dithiazoles. US Patent No. US4119722 A, 10 October 1978. [Google Scholar]
- Appel, R.; Janssen, H.; Haller, I.; Plempel, M. (Bayer AG), 1,2,3-Dithiazolderivate, Verfahren zu ihrer Herstellung Sowie ihre Verwendung als Arzneimittel. Germany Patent No. DE2848221 A1, 14 May 1980. [Google Scholar]
- Mayer, R.; Fçrster, E.; Matauschek, B.D. Verfahren zur Herstellung von Aromatisch oder Heteroaromatisch Substituierten Cyanthioformamiden. Germany Patent No. DD212387, 8 August 1984. [Google Scholar]
- Benting, J.; Dahmen, P.; Wachendorff-Neumann, U.; Hadano, H.; Vors, J.-P. (Bayer Cropscience AG), Int. 5-Heteroarylimino-1,2,3-dithiazoles. PCT Publication No. WO2012045726 A2, 12 April 2012. [Google Scholar]
- Asquith, C.R.M.; Konstantinova, L.S.; Meli, M.L.; Laitinen, T.; Poso, A.; Rakitin, O.A.; Hofmann-Lehmann, R.; Hilton, S.T. Evaluation of Substituted 1,2,3-Dithiazoles as Inhibitors of the Feline Immunodeficiency Virus (FIV) Nucleocapsid Protein via a Proposed Zinc Ejection Mechanism. Chem. Med. Chem. 2016, 11, 2119–2126. [Google Scholar] [CrossRef]
- Asquith, C.R.M.; Meili, T.; Laitinen, T.; Baranovsky, I.V.; Konstantinova, L.S.; Poso, A.; Rakitin, O.A.; Hofmann-Lehmann, R. Synthesis and comparison of substituted 1,2,3-dithiazole and 1,2,3-thiaselenazole as inhibitors of the feline immunodeficiency virus (FIV) nucleocapsid protein as a model for HIV infection. Bioorg. Med. Chem. Lett. 2019, 29, 1765–1768. [Google Scholar] [CrossRef]
- Charalambous, A.; Koyioni, M.; Antoniades, I.; Pegeioti, D.; Eleftheriou, I.; Michaelidou, S.S.; Amelichev, S.A.; Konstantinova, L.S.; Rakitin, O.A.; Koutentis, P.A.; et al. 1,2,3-Dithiazoles – new reversible melanin synthesis inhibitors: A chemical genomics study. MedChemComm 2015, 6, 935–946. [Google Scholar] [CrossRef]
- Mazum, T.K.; Bricker, B.A.; Flores-Rozas, H.; Ablordeppey, S.Y. The Mechanistic Targets of Antifungal Agents: An Overview. Mini. Rev. Med. Chem. 2016, 16, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, J.; Van Gerven, F.; Fransen, J.; Schrooten, P.; Janssen, P.A. The in vitro antifungal activity of ketoconazole, zinc pyrithione, and selenium sulfide against Pityrosporum and their efficacy as a shampoo in the treatment of experimental pityrosporosis in guinea pigs. J. Am. Acad. Dermatol. 1990, 22, 993–998. [Google Scholar] [CrossRef]
- Cohen, P.R.; Anderson, C.A. Topical Selenium Sulfide for the Treatment of Hyperkeratosis. Dermatol. Ther. (Heidelb) 2018, 8, 639–646. [Google Scholar] [CrossRef]
- Asquith, C.R.M.; Machado, A.C.S.; de Miranda, L.H.M.; Konstantinova, L.S.; Almeida-Paes, R.; Rakitin, O.A.; Pereira, S.A. Synthesis and Identification of Pentathiepin-Based Inhibitors of Sporothrix brasiliensis. Antibiotics 2019, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Appel, R.; Janssen, H.; Siray, M.; Knoch, F. Synthese und Reaktionen des 4,5-Dichlor-1,2,3-dithiazolium-chlorids. Chem. Ber. 1985, 118, 1632–1643. [Google Scholar] [CrossRef]
- Rees, C.W. Polysulfur-nitrogen Heterocyclic Chemistry. J. Heterocycl. Chem. 1992, 29, 639–651. [Google Scholar] [CrossRef]
- Cuadro, A.M.; Alvarez-Buila, J. 4,5-Dichloro-1,2,3-dithiazolium Chloride (Appel’s Salt): Reactions with N-Nucleophiles. Tetrahedron 1994, 50, 10037–10046. [Google Scholar] [CrossRef]
- Rakitin, O.A.; Rees, C.W.; Vlasova, O.G. Direct Synthesis of 2-Cyano-benzimidazoles and the Generation of S2. Tetrahedron Lett. 1996, 37, 4589–4592. [Google Scholar] [CrossRef]
- English, R.F.; Rakitin, O.A.; Rees, C.W.; Vlasova, O.G. Conversion of Imino-1,2,3-dithiazoles into 2-Cyanobenzothiazoles, Cyanoimidoyl Chlorides and Diatomic Sulfur. J. Chem. Soc. Perkin Trans. 1 1997, 27, 201–206. [Google Scholar] [CrossRef]
- Kim, K. Recent Advances in 1,2,3-Dithiazole Chemistry. Phosphorus Sulfur Silicon Relat. Elem. 1997, 120, 229–244. [Google Scholar] [CrossRef]
- Besson, T.; Guillaumet, G.; Lamazzi, C.; Rees, C.W. Synthesis of 3,1-Benzoxazines, 3,1-Benzothiazines and 3,1-Benzoxazepines via N-Arylimino-1,2,3-dithiazoles. Synlett 1997, 28, 704–706. [Google Scholar] [CrossRef]
- Kim, K. Synthesis and Reactions of 1,2,3-Dithiazoles. J. Sulfur Chem. 1998, 21, 147–207. [Google Scholar] [CrossRef]
- Besson, T.; Dozias, M.J.; Guillard, J.; Rees, C.W. New Route to 2-Cyano-benzothiazoles via N-Arylimino-1,2,3-dithiazoles. J. Chem. Soc. Perkin Trans. 1 1998, 30, 3925–3926. [Google Scholar] [CrossRef]
- Christoforou, I.C.; Koutentis, P.A.; Michaelidou, S.S. 1,2,3-Dithiazole Chemistry in Heterocyclic Synthesis. Arkivoc 2006, 7, 207–223. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Koutentis, P.A. The Reaction of 4,5-Dichloro-1,2,3-dithiazolium Chloride with Sulfimides: A New Synthesis of N-Aryl-1,2,3-dithiazolimines. Molecules 2009, 14, 2356–2362. [Google Scholar] [CrossRef]
- Koyioni, M.; Manoli, M.; Manos, M.J.; Koutentis, P.A. Reinvestigating the Reaction of 1H-Pyrazol-5-amines with 4,5-Dichloro-1,2,3-dithiazolium Chloride: A Route to Pyrazolo[3,4-c]isothiazoles and Pyrazolo[3,4-d]thiazoles. J. Org. Chem. 2014, 79, 4025–4037. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Bol’shakov, O.I.; Baranovsky, I.V.; Bogacheva, A.M.; Strunyasheva, V.V.; Rakitin, O.A. A short and efficient synthesis of 5,5′-bi-1,2,3-dithiazoles. Mendeleev Commun. 2015, 25, 427–428. [Google Scholar] [CrossRef]
- Koyioni, M.; Manoli, M.; Koutentis, P.A. The Reaction of DABCO with 4-Chloro-5H-1,2,3-dithiazoles: Synthesis and Chemistry of 4-[N-(2-Chloroethyl)piperazin-1-yl]-5H-1,2,3-dithiazoles. J. Org. Chem. 2016, 81, 615–631. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Baranovsky, I.V.; Strunyasheva, V.V.; Kalogirou, A.S.; Popov, V.V.; Lyssenko, K.A.; Koutentis, P.A.; Rakitin, O.A. The Conversion of 5,5′-Bi(1,2,3-dithiazolylidenes) into Isothiazolo[5,4-d]isothiazoles. Molecules 2018, 23, 1257. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Baranovsky, I.V.; Pritchina, E.A.; Mikhailov, M.S.; Bagryanskaya, I.Y.; Semenov, N.A.; Irtegova, I.G.; Salnikov, G.E.; Lyssenko, K.A.; Gritsan, N.P.; et al. Fused 1,2,3-Thiaselenazoles Synthesized from 1,2,3-Dithiazoles through Selective Chalcogen Exchange. Chem. Eur. J. 2017, 23, 17037–17047. [Google Scholar] [CrossRef]
- Rakitin, O.A.; Zibarev, A.V. Synthesis and Applications of 5-Membered Chalcogen-Nitrogen π-Heterocycles with Three Heteroatoms. Asian J. Org. Chem. 2018, 7, 2397–2416. [Google Scholar] [CrossRef]
- Beer, L.; Britten, J.F.; Cordes, A.W.; Clements, O.P.; Oakley, R.T.; Pink, M.; Reed, R.W. Benzo[2,1-c:3,4-c′]bis(1,2,3-thiaselenazole) (BSe) and its charge transfer chemistry. Crystal and electronic structure of [BSe]3[ClO4]2. Inorg. Chem. 2001, 40, 4705–4709. [Google Scholar] [CrossRef] [PubMed]
- Konstantinova, L.S.; Baranovsky, I.V.; Irtegova, I.G.; Bagryanskaya, I.Y.; Shundrin, L.A.; Zibarev, A.V.; Rakitin, O.A. Fused 1,2,3-Dithiazoles: Convenient Synthesis, Structural Characterization, and Electrochemical Properties. Molecules 2016, 21, 596. [Google Scholar] [CrossRef]
- Blaskovich, M.A.; Zuegg, J.; Elliott, A.G.; Cooper, M.A. Helping chemists discover new antibiotics. ACS Infect. Dis. 2015, 1, 285–287. [Google Scholar] [CrossRef]
- Gordon, R.J.; Lowy, F.D. Pathogenesis of Methicillin-Resistant Staphylococcus aureus Infection. Clin. Infect. Dis. 2008, 46, S350–S359. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a Successful Pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.-J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front. Cell. Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef]
- Sudbery, P.E. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 2011, 9, 737–748. [Google Scholar] [CrossRef]
- Capilla, J.; Maffei, C.M.; Clemons, K.V.; Sobel, R.A.; Stevens, D.A. Experimental systemic infection with Cryptococcus neoformans var. grubii and Cryptococcus gattii in normal and immunodeficient mice. Med. Mycol. 2006, 44, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Litvintseva, A.P.; Mitchell, T.G. Most Environmental Isolates of Cryptococcus neoformans var. grubii (Serotype A) Are Not Lethal for Mice. Infect. Immu. 2009, 77, 3188–3195. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef]
- Husain, S.; Wagener, M.M.; Singh, N. Cryptococcus neoformans infection in organ transplant recipients: Variables influencing clinical characteristics and outcome. Emerg. Infect. Dis. 2001, 7, 375–381. [Google Scholar] [CrossRef]
- Mitchell, T.G.; Perfect, J.R. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 1995, 8, 515–548. [Google Scholar] [CrossRef]
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. 2017. Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/ (accessed on 6 June 2020).
- O’Keefe, J.P.; Dustin, C.M.; Barber, D.; Snider, G.W.; Hondal, R.J. A “Seleno Effect” Differentiates the Roles of Redox Active Cysteine Residues in Plasmodium falciparum Thioredoxin Reductase. Biochemistry 2018, 57, 1767–1778. [Google Scholar] [CrossRef]
- Senior, K. Can we keep up with hospital-acquired infections? Lancet Infect. Dis. 2001, 1, 8. [Google Scholar] [CrossRef]
- Gilbert, P.; Moore, L.E. Cationic Antiseptics: Diversity of Action under a Common Epithet. J. Appl. Microbiol. 2005, 99, 703–715. [Google Scholar] [CrossRef]
- Augustine, J.K.; Kumar, R.; Bombrun, A.; Mandal, A.B. An efficient catalytic method for the Beckmann rearrangement of ketoximes to amides and aldoximes to nitriles mediated by propylphosphonic anhydride (T3P®). Tetrahedron Lett. 2011, 52, 1074–1077. [Google Scholar] [CrossRef]
- Plater, M.J.; Rees, C.W.; Roe, D.G.; Torroba, T. Cyclopenta-1,2,3-dithiazoles and related compounds. J. Chem. Soc. Perkin Trans. 1 1993, 3, 293–294. [Google Scholar]
- Gómez, T.; Macho, S.; Miguel, D.; Neo, A.G.; Rodríguez, T.; Torroba, T. Cyclopentathiadiazines, Cyclohepta- and Cyclopentadithiazoles: New Materials and a Rich Heterocyclic Chemistry of Cyclic Enaminonitriles. Eur. J. Org. Chem. 2005, 2005, 5055–5066. [Google Scholar] [CrossRef]
- Macho, S.; Miguel, D.; Gomez, T.; Rodriguez, T.; Torroba, T. From cyclopentanone oximes to bis[1,2,3]dithiazolo-s-indacenes, cyclopenta[c][1,2]thiazine, pentathiepino-, tetrathiino-, and thienocyclopenta[1,2,3]dithiazoles as a rich source of new materials. J. Org. Chem. 2005, 70, 9314–9325. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.S.; Michaelidou, S.S.; Koyioni, M.; Koutentis, P.A. Ring transformations of 2-hydroxy-(4-chloro-5H-1,2,3-dithiazol-5-ylideneamino)arenes. Tetrahedron 2015, 71, 7181–7190. [Google Scholar] [CrossRef]
| Compound | X | Initial Screen a | Antibacterial | Antifungal | CC50 b | Hc10 c | |||
|---|---|---|---|---|---|---|---|---|---|
| A. baumannii | S. aureus | C. albicans | C. neoformans | ||||||
| MIC (μg mL−1) | MIC (μg mL−1) | ||||||||
| 10a | 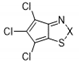 | S | Inactive | - | - | - | - | - | - |
| 11a | Se | Active | ≤0.25 | ≤0.25 | ≤0.25 | ≤0.25 | 0.52 | >32 | |
| 10b |  | S | Active | >32 | >32 | ≤0.25 | ≤0.25 | >32 | >32 |
| 11b | Se | Active | >32 | ≤0.25 | ≤0.25 | ≤0.25 | >32 | >32 | |
| 10c | 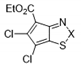 | S | Active | >32 | >32 | ≤0.25 | >32 | >32 | >32 |
| 11c | Se | Active | >32 | ≤0.25 | ≤0.25 | ≤0.25 | 7 | >32 | |
| 12 | 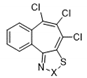 | S | Inactive | - | - | - | - | - | - |
| 13 | Se | Active | >32 | ≤0.25 | ≤0.25 | ≤0.25 | 0.48 | >32 | |
| 8a | 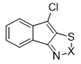 | S | Inactive | - | - | - | - | - | - |
| 9a | Se | Active | >32 | >32 | ≤0.25 | ≤0.25 | >32 | >32 | |
| 8b |  | S | Inactive | - | - | - | - | - | - |
| 9b | Se | Inactive | - | - | - | - | - | - | |
| 14 | 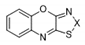 | S | Inactive | - | - | - | - | - | - |
| 15 | Se | Active | >32 | >32 | ≤0.25 | 2 | >32 | >32 | |
| Vibration | 10a | Vibration | 11a | ||
|---|---|---|---|---|---|
| Frequencies | Intensities | Frequencies | Intensities | ||
| 1 | 81.19 | 0.090 | 1 | −1106.90 | 1.59 |
| 2 | 97.19 | 0.050 | 2 | −428.76 | 4.23 |
| 3 | 126.77 | 0.53 | 3 | 85.37 | 0.070 |
| 4 | 159.42 | 0.33 | 4 | 121.91 | 0.49 |
| 5 | 168.71 | 0.050 | 5 | 174.60 | 0.22 |
| 6 | 189.77 | 1.02 | 6 | 197.10 | 0.27 |
| 7 | 238.27 | 0.27 | 7 | 209.31 | 0.56 |
| 8 | 326.79 | 0.55 | 8 | 297.64 | 0.40 |
| 9 | 351.68 | 1.23 | 9 | 306.99 | 2.03 |
| 10 | 372.54 | 2.73 | 10 | 354.64 | 2.95 |
| 11 | 397.68 | 7.85 | 11 | 382.75 | 0.40 |
| 12 | 426.85 | 1.18 | 12 | 421.39 | 0.91 |
| 13 | 440.19 | 7.82 | 13 | 531.84 | 0.56 |
| 14 | 560.53 | 3.48 | 14 | 587.19 | 69.88 |
| 15 | 644.34 | 0.13 | 15 | 642.56 | 0.12 |
| 16 | 656.69 | 11.16 | 16 | 670.91 | 37.5 |
| 17 | 708.68 | 3.56 | 17 | 712.86 | 3.37 |
| 18 | 718.86 | 86.30 | 18 | 772.86 | 28.48 |
| 19 | 796.88 | 81.17 | 19 | 816.30 | 1.46 |
| 20 | 881.86 | 24.82 | 20 | 857.16 | 27.67 |
| 21 | 1007.65 | 0.60 | 21 | 997.94 | 1.43 |
| 22 | 1100.15 | 71.57 | 22 | 1092.41 | 70.45 |
| 23 | 1205.19 | 147.80 | 23 | 1201.72 | 177.49 |
| 24 | 1283.36 | 123.21 | 24 | 1271.67 | 108.05 |
| 25 | 1493.57 | 5.90 | 25 | 1513.89 | 7.19 |
| 26 | 1581.56 | 102.24 | 26 | 1584.26 | 94.69 |
| 27 | 1596.95 | 28.40 | 27 | 1609.23 | 33.50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laitinen, T.; Baranovsky, I.V.; Konstantinova, L.S.; Poso, A.; Rakitin, O.A.; Asquith, C.R.M. Antimicrobial and Antifungal Activity of Rare Substituted 1,2,3-Thiaselenazoles and Corresponding Matched Pair 1,2,3-Dithiazoles. Antibiotics 2020, 9, 369. https://doi.org/10.3390/antibiotics9070369
Laitinen T, Baranovsky IV, Konstantinova LS, Poso A, Rakitin OA, Asquith CRM. Antimicrobial and Antifungal Activity of Rare Substituted 1,2,3-Thiaselenazoles and Corresponding Matched Pair 1,2,3-Dithiazoles. Antibiotics. 2020; 9(7):369. https://doi.org/10.3390/antibiotics9070369
Chicago/Turabian StyleLaitinen, Tuomo, Ilia V. Baranovsky, Lidia S. Konstantinova, Antti Poso, Oleg A. Rakitin, and Christopher R. M. Asquith. 2020. "Antimicrobial and Antifungal Activity of Rare Substituted 1,2,3-Thiaselenazoles and Corresponding Matched Pair 1,2,3-Dithiazoles" Antibiotics 9, no. 7: 369. https://doi.org/10.3390/antibiotics9070369
APA StyleLaitinen, T., Baranovsky, I. V., Konstantinova, L. S., Poso, A., Rakitin, O. A., & Asquith, C. R. M. (2020). Antimicrobial and Antifungal Activity of Rare Substituted 1,2,3-Thiaselenazoles and Corresponding Matched Pair 1,2,3-Dithiazoles. Antibiotics, 9(7), 369. https://doi.org/10.3390/antibiotics9070369







