Antifungal In Vitro Activity of Pilosulin- and Ponericin-Like Peptides from the Giant Ant Dinoponera quadriceps and Synergistic Effects with Antimycotic Drugs
Abstract
1. Introduction
2. Results
2.1. Peptides
2.2. Evaluation of Antifungal Activity of Synthetic Pilosulin- and Ponericin-Like Peptides from D. quadriceps Venom
2.2.1. Minimum Inhibitory Concentration (MIC) and Minimum Lethal Concentration (MLC)
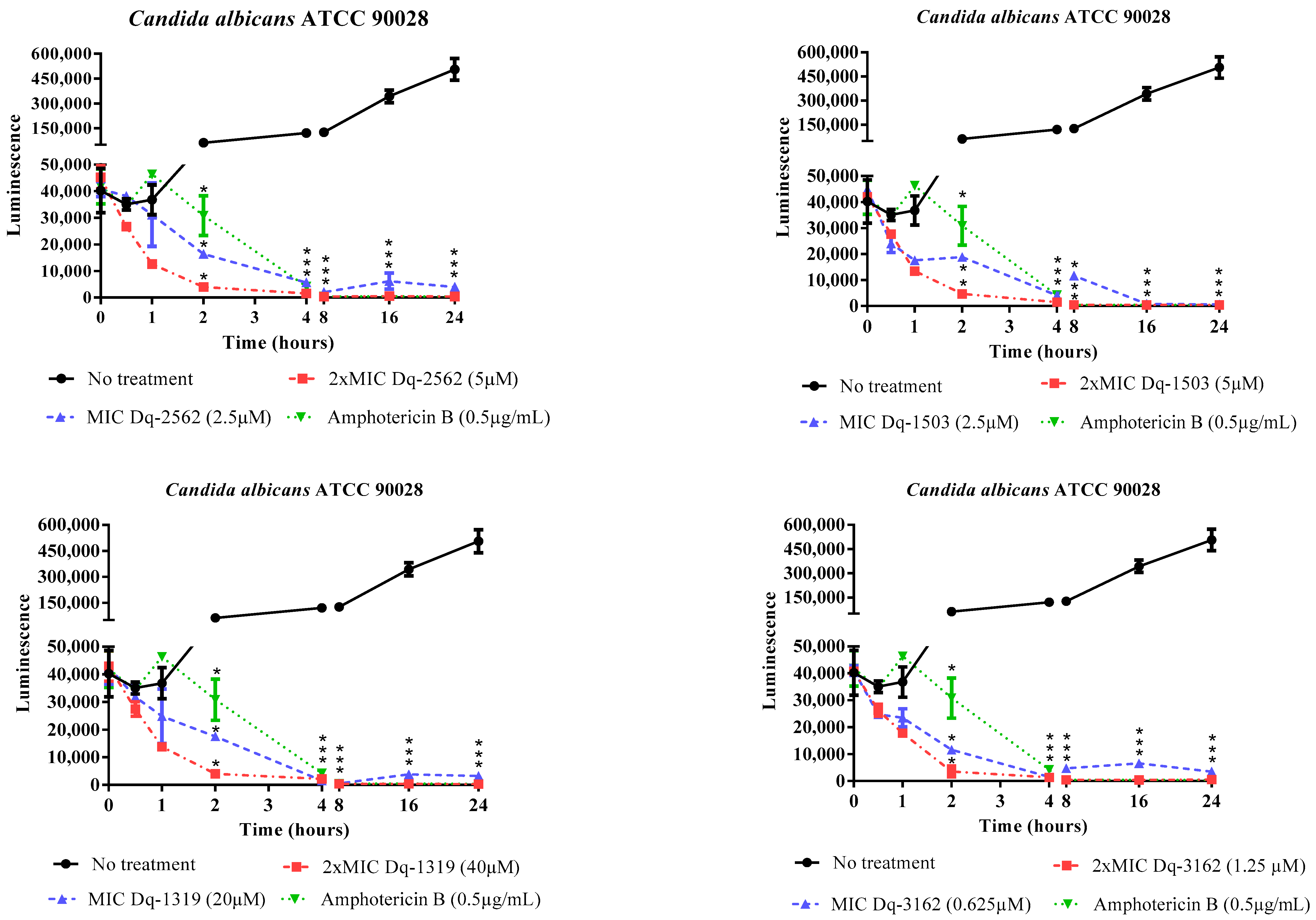
2.2.2. Time-Dependent Antifungal Activity of D. quadriceps Peptides
2.3. Antifungal Activity of D. quadriceps Peptides in Combination with Antimycotyc Drugs
2.3.1. Checkerboard Test
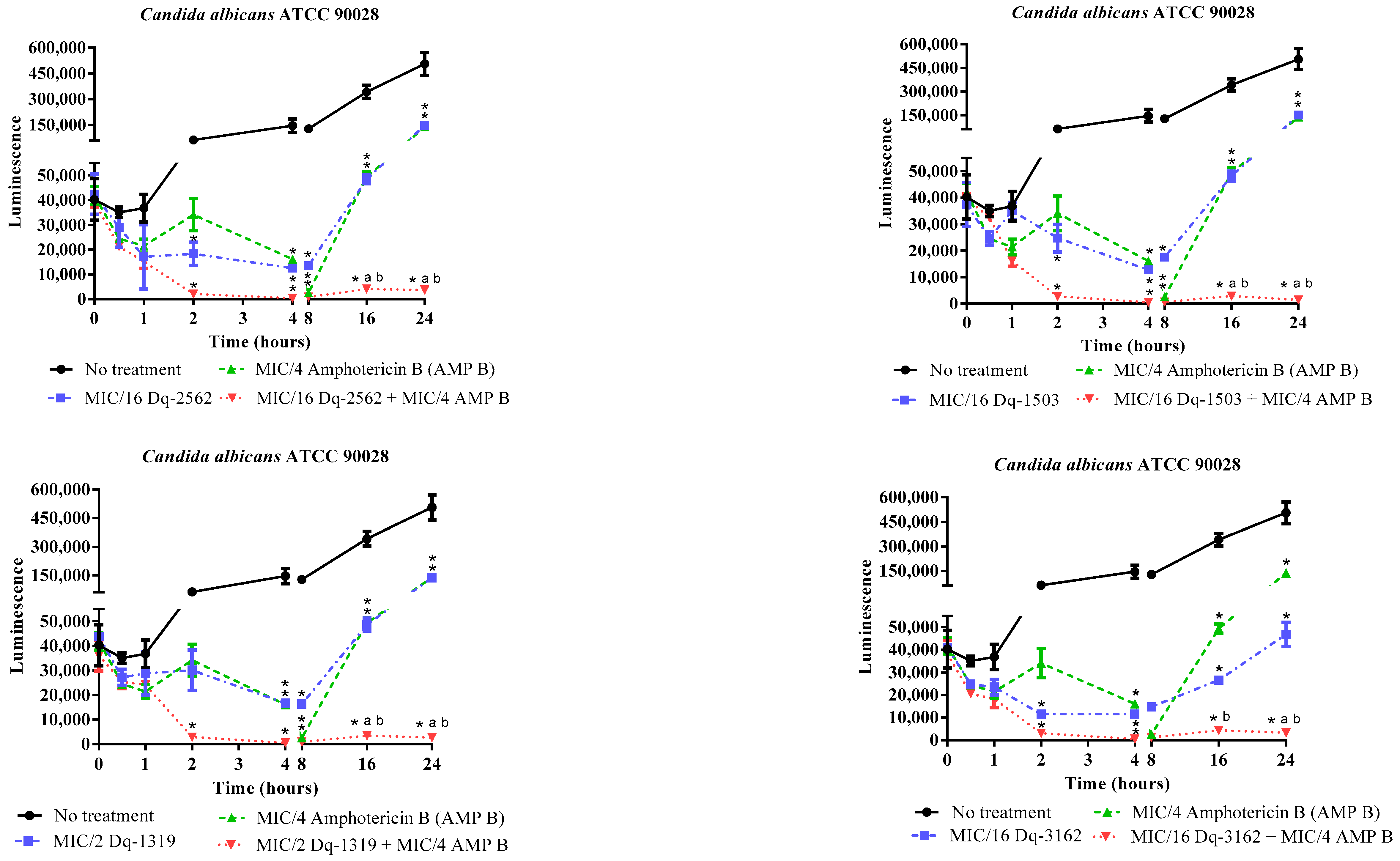
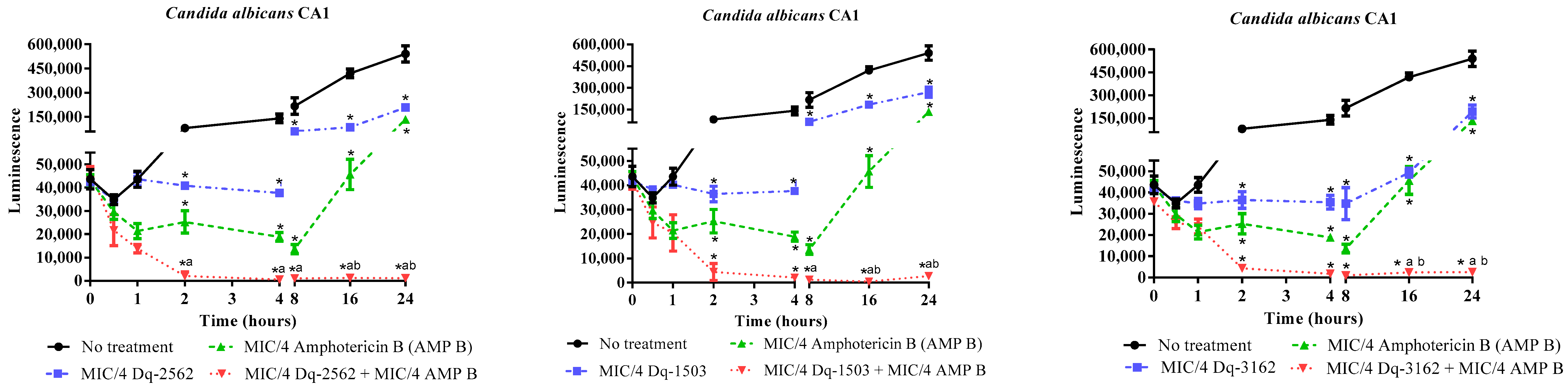
2.3.2. Time-Kill Effect of the Combinations of D. quadriceps Antimicrobial Peptides and Amphotericin B on the Candida Cell Viability
2.3.3. Membrane Permeabilization Induced by Pilosulin- and Ponericin-like Peptides
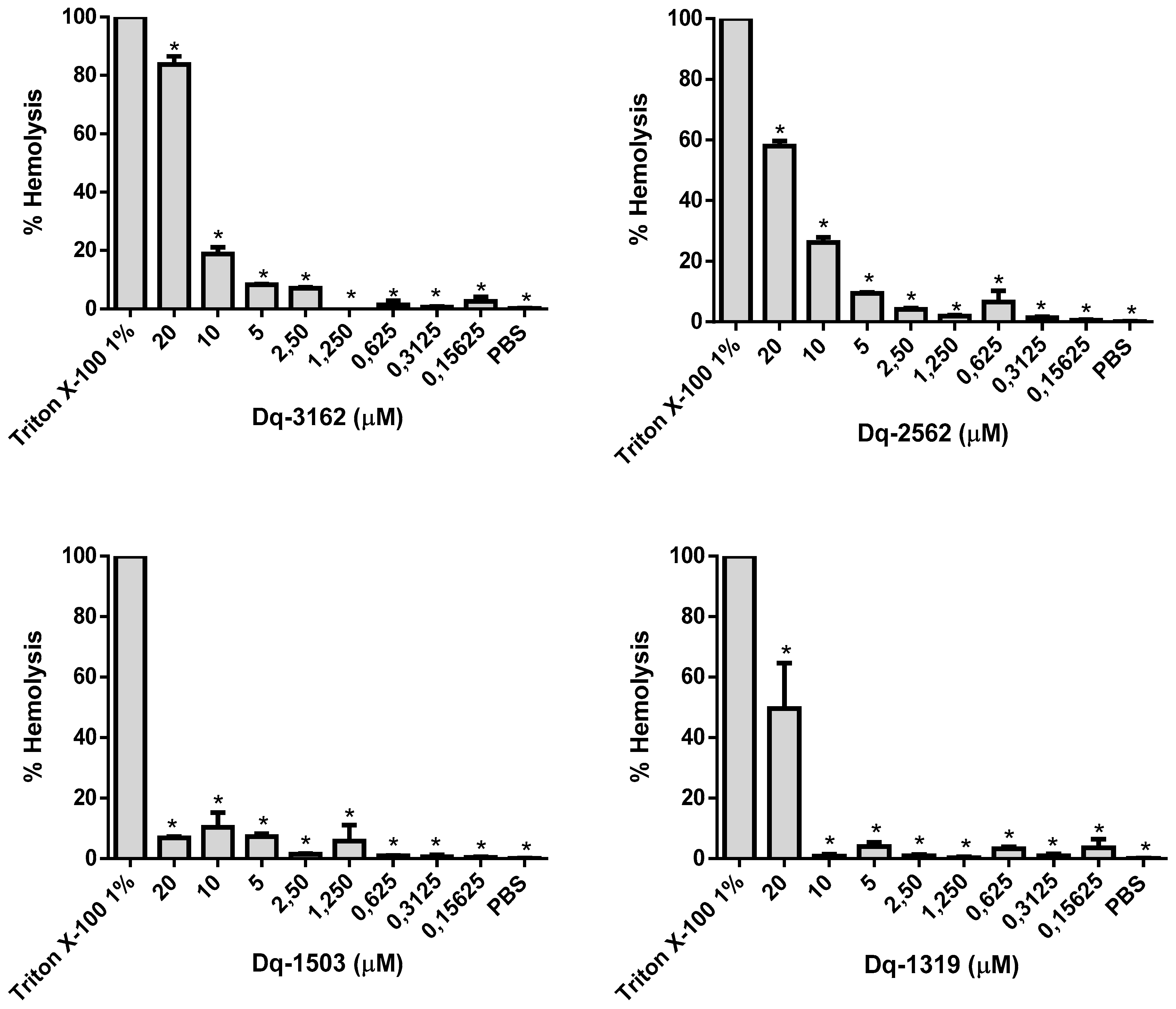
2.4. Hemolytic Activity of D. quadriceps Antimicrobial Peptides Alone and in Combinations with Amphotericin B
2.5. Effect of Soluble Ergosterol on the Antifungal Activity of Peptides
3. Discussion
4. Materials and Methods
4.1. Pilosulin- and Ponericin-Like Peptides from the Giant Ant D. quadriceps
4.2. Evaluation of Antifungal Activity of Peptides
4.2.1. Microorganisms
4.2.2. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Lethal Concentration (MLC)
4.2.3. Determination of Time-Kill Kinetics of Peptide Activity Against C. albicans
4.3. Antifungal Activity of the Combinations of D. quadriceps Antimicrobial Peptides and Antimycotycs
4.3.1. Checkerboard Test
4.3.2. Determination of the Time-Kill Kinetics of the Combination of D. quadriceps Antimicrobial Peptides and Amphotericin B on Candida Cell Viability
4.3.3. Membrane Permeabilization Induced by pilosulin- (Dq-2562) and ponericin-like (Dq-3162) Peptides and Combinations with Amphotericin B
4.4. Hemolytic Assay
4.5. Influence of Ergosterol on the MIC of Peptides and Amphotericin B
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Knabl, L.; Lass-Flörl, C. Antifungal susceptibility testing in Candida species: Current methods and promising new tools for shortening the turnaround time. Expert Rev. Anti-Infect. Ther. 2020, 1–9. [Google Scholar] [CrossRef]
- Poulain, D. Candida albicans, plasticity and pathogenesis. Crit. Rev. Microbiol. 2015, 41, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Z.; Chen, Z.; Li, Y.; Su, S.; Sun, S. Potential Antifungal Targets Based on Glucose Metabolism Pathways of Candida albicans. Front. Microbiol. 2020, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Mba, I.E.; Nweze, E.I. Mechanism of Candida pathogenesis: Revisiting the vital drivers. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2020, 1–23. [Google Scholar] [CrossRef]
- Köhler, J.R.; Acosta-Zaldívar, M.; Qi, W. Phosphate in Virulence of Candida albicans and Candida glabrata. J. Fungi 2020, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Tóth, R.; Gácser, A. Mechanisms of Pathogenic Candida Species to Evade the Host Complement Attack. Front. Cell Infect. Microbiol. 2020, 10, 94. [Google Scholar] [CrossRef]
- Antibiotic Resistance Threats in the United States 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 23 June 2020). [CrossRef]
- Chen, H.; Zhou, X.; Ren, B.; Cheng, L. The regulation of hyphae growth in Candida albicans. Virulence 2020, 11, 337–348. [Google Scholar] [CrossRef]
- Wiederhold, N.P. The antifungal arsenal: Alternative drugs and future targets. Int. J. Antimicrob. Agents 2018, 51, 333–339. [Google Scholar] [CrossRef]
- Jamiu, A.T.; Albertyn, J.; Sebolai, O.M.; Pohl, C.H. Update on Candida krusei, a potential multidrug-resistant pathogen. Med. Mycol. 2020, myaa031. [Google Scholar] [CrossRef]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. Lancet. Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2019, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Pei, X.; Ren, S.; Chen, X.; Wang, L.; Ma, C.; Xi, X.; Chen, T.; Shaw, C.; Zhou, M. Identification and Rational Design of a Novel Antibacterial Peptide Dermaseptin-AC from the Skin Secretion of the Red-Eyed Tree Frog Agalychnis callidryas. Antibiotics 2020, 9, 243. [Google Scholar] [CrossRef] [PubMed]
- Greber, K.E.; Dawgul, M. Antimicrobial Peptides Under Clinical Trials. Curr. Top. Med. Chem. 2017, 17, 620–628. [Google Scholar] [CrossRef]
- Nicolas, P.; El Amri, C. The dermaseptin superfamily: A gene-based combinatorial library of antimicrobial peptides. Biochim. Et Biophys. Acta 2009, 1788, 1537–1550. [Google Scholar] [CrossRef]
- Koehbach, J.; Craik, D.J. The Vast Structural Diversity of Antimicrobial Peptides. Trends Pharmacol. Sci. 2019, 40, 517–528. [Google Scholar] [CrossRef]
- Fernández de Ullivarri, M.; Arbulu, S.; Garcia-Gutierrez, E.; Cotter, P.D. Antifungal Peptides as Therapeutic Agents. Front. Cell. Infect. Microbiol. 2020, 10, 105. [Google Scholar] [CrossRef]
- Di Somma, A.; Moretta, A.; Canè, C.; Cirillo, A.; Duilio, A. Antimicrobial and Antibiofilm Peptides. Biomolecules 2020, 10, 652. [Google Scholar] [CrossRef]
- Radis-Baptista, G. Snake Venoms; Springer: Dordrecht, The Netherlands, 2015; pp. 1–25. [Google Scholar]
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Insects, arachnids and centipedes venom: A powerful weapon against bacteria. A literature review. Toxicon Off. J. Int. Soc. Toxinol. 2017, 130, 91–103. [Google Scholar] [CrossRef]
- Touchard, A.; Aili, S.R.; Fox, E.G.P.; Escoubas, P.; Orivel, J.; Nicholson, G.M.; Dejean, A. The Biochemical Toxin Arsenal from Ant Venoms. Toxins 2016, 8, 30. [Google Scholar] [CrossRef]
- Aili, S.R.; Touchard, A.; Escoubas, P.; Padula, M.P.; Orivel, J.; Dejean, A.; Nicholson, G.M. Diversity of peptide toxins from stinging ant venoms. Toxicon Off. J. Int. Soc. Toxinol. 2014, 92, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Aili, S.R.; Touchard, A.; Hayward, R.; Robinson, S.D.; Pineda, S.S.; Lalagüe, H.; Mrinalini; Vetter, I.; Undheim, E.A.B.; Kini, R.M.; et al. An Integrated Proteomic and Transcriptomic Analysis Reveals the Venom Complexity of the Bullet Ant Paraponera clavata. Toxins 2020, 12, 324. [Google Scholar] [CrossRef] [PubMed]
- Ceolin Mariano, D.O.; de Oliveira, U.C.; Zaharenko, A.J.; Pimenta, D.C.; Radis-Baptista, G.; Prieto-da-Silva, A.R.B. Bottom-Up Proteomic Analysis of Polypeptide Venom Components of the Giant Ant Dinoponera Quadriceps. Toxins 2019, 11, 448. [Google Scholar] [CrossRef]
- Kazuma, K.; Masuko, K.; Konno, K.; Inagaki, H. Combined Venom Gland Transcriptomic and Venom Peptidomic Analysis of the Predatory Ant Odontomachus monticola. Toxins 2017, 9, 323. [Google Scholar] [CrossRef] [PubMed]
- Tani, N.; Kazuma, K.; Ohtsuka, Y.; Shigeri, Y.; Masuko, K.; Konno, K.; Inagaki, H. Mass Spectrometry Analysis and Biological Characterization of the Predatory Ant Odontomachus monticola Venom and Venom Sac Components. Toxins 2019, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Barassé, V.; Touchard, A.; Téné, N.; Tindo, M.; Kenne, M.; Klopp, C.; Dejean, A.; Bonnafé, E.; Treilhou, M. The Peptide Venom Composition of the Fierce Stinging Ant Tetraponera aethiops (Formicidae: Pseudomyrmecinae). Toxins 2019, 11, 732. [Google Scholar] [CrossRef]
- Cologna, C.T.; Rodrigues, R.S.; Santos, J.; de Pauw, E.; Arantes, E.C.; Quinton, L. Peptidomic investigation of Neoponera villosa venom by high-resolution mass spectrometry: Seasonal and nesting habitat variations. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 6. [Google Scholar] [CrossRef]
- Heep, J.; Klaus, A.; Kessel, T.; Seip, M.; Vilcinskas, A.; Skaljac, M. Proteomic Analysis of the Venom from the Ruby Ant Myrmica rubra and the Isolation of a Novel Insecticidal Decapeptide. Insects 2019, 10, 42. [Google Scholar] [CrossRef]
- Torres, A.F.; Huang, C.; Chong, C.M.; Leung, S.W.; Prieto-da-Silva, A.R.; Havt, A.; Quinet, Y.P.; Martins, A.M.; Lee, S.M.; Radis-Baptista, G. Transcriptome analysis in venom gland of the predatory giant ant Dinoponera quadriceps: Insights into the polypeptide toxin arsenal of hymenopterans. PLoS ONE 2014, 9, e87556. [Google Scholar] [CrossRef]
- Cologna, C.T.; Cardoso Jdos, S.; Jourdan, E.; Degueldre, M.; Upert, G.; Gilles, N.; Uetanabaro, A.P.; Costa Neto, E.M.; Thonart, P.; de Pauw, E.; et al. Peptidomic comparison and characterization of the major components of the venom of the giant ant Dinoponera quadriceps collected in four different areas of Brazil. J. Proteom. 2013, 94, 413–422. [Google Scholar] [CrossRef]
- Radis-Baptista, G.; Dodou, H.V.; Prieto-da-Silva, A.R.B.; Zaharenko, A.J.; Kazuma, K.; Nihei, K.I.; Inagaki, H.; Mori-Yasumoto, K.; Konno, K. Comprehensive analysis of peptides and low molecular weight components of the giant ant Dinoponera quadriceps venom. Biol. Chem. 2020. [Google Scholar] [CrossRef]
- Lima, D.B.; Torres, A.F.; Mello, C.P.; de Menezes, R.R.; Sampaio, T.L.; Canuto, J.A.; da Silva, J.J.; Freire, V.N.; Quinet, Y.P.; Havt, A.; et al. Antimicrobial effect of Dinoponera quadriceps (Hymenoptera: Formicidae) venom against Staphylococcus aureus strains. J. Appl. Microbiol. 2014, 117, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.B.; Sousa, P.L.; Torres, A.F.; Rodrigues, K.A.; Mello, C.P.; Menezes, R.R.; Tessarolo, L.D.; Quinet, Y.P.; de Oliveira, M.R.; Martins, A.M. Antiparasitic effect of Dinoponera quadriceps giant ant venom. Toxicon Off. J. Int. Soc. Toxinol. 2016, 120, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.B.; Mello, C.P.; Bandeira, I.C.J.; Pessoa Bezerra de Menezes, R.R.P.; Sampaio, T.L.; Falcao, C.B.; Morlighem, J.R.L.; Radis-Baptista, G.; Martins, A.M.C. The dinoponeratoxin peptides from the giant ant Dinoponera quadriceps display in vitro antitrypanosomal activity. Biol. Chem. 2018, 399, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.P.; Pereira, G.R.; Barros, E.; Ramos, H.J.O.; Oliveira, L.L.; Serrão, J.E. Antibacterial activity of the venom of the Ponerine ant Pachycondyla striata (Formicidae: Ponerinae). Int. J. Trop. Insect Sci. 2020, 40, 393–402. [Google Scholar] [CrossRef]
- Pluzhnikov, K.A.; Kozlov, S.A.; Vassilevski, A.A.; Vorontsova, O.V.; Feofanov, A.V.; Grishin, E.V. Linear antimicrobial peptides from Ectatomma quadridens ant venom. Biochimie 2014, 107 Pt B, 211–215. [Google Scholar] [CrossRef]
- Johnson, S.R.; Copello, J.A.; Evans, M.S.; Suarez, A.V. A biochemical characterization of the major peptides from the Venom of the giant Neotropical hunting ant Dinoponera australis. Toxicon Off. J. Int. Soc. Toxinol. 2010, 55, 702–710. [Google Scholar] [CrossRef]
- Wu, Q.; Patočka, J.; Kuča, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef]
- Radis-Baptista, G.; Konno, K. Arthropod Venom Components and Their Potential Usage. Toxins 2020, 12, 82. [Google Scholar] [CrossRef]
- Kang, S.J.; Park, S.J.; Mishig-Ochir, T.; Lee, B.J. Antimicrobial peptides: Therapeutic potentials. Expert Rev. Anti-Infect. Ther. 2014, 12, 1477–1486. [Google Scholar] [CrossRef]
- Yin, L.M.; Edwards, M.A.; Li, J.; Yip, C.M.; Deber, C.M. Roles of hydrophobicity and charge distribution of cationic antimicrobial peptides in peptide-membrane interactions. J. Biol. Chem. 2012, 287, 7738–7745. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Vasil, A.I.; Hale, J.D.; Hancock, R.E.W.; Vasil, M.L.; Hodges, R.S. Effects of net charge and the number of positively charged residues on the biological activity of amphipathic alpha-helical cationic antimicrobial peptides. Biopolymers 2008, 90, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Avci, F.G.; Akbulut, B.S.; Ozkirimli, E. Membrane Active Peptides and Their Biophysical Characterization. Biomolecules 2018, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Dathe, M.; Wieprecht, T. Structural features of helical antimicrobial peptides: Their potential to modulate activity on model membranes and biological cells. Biochim. Et Biophys. Acta 1999, 1462, 71–87. [Google Scholar] [CrossRef]
- Lee, W.; Lee, D.G. Fungicidal mechanisms of the antimicrobial peptide Bac8c. Biochim. Et Biophys. Acta (Bba)-Biomembr. 2015, 1848, 673–679. [Google Scholar] [CrossRef]
- Silva, J.R.d.; Souza, A.Z.d.; Pirovani, C.P.; Costa, H.; Silva, A.; Dias, J.C.T.; Delabie, J.H.C.; Fontana, R. Assessing the Proteomic Activity of the Venom of the Ant Ectatomma tuberculatum (Hymenoptera: Formicidae: Ectatomminae). Psyche 2018, 2018, 7915464. [Google Scholar] [CrossRef]
- Keskin, M.; Duymaz, A.; Tosun, Z.; Savaci, N. Tissue necrosis following a honey bee sting. Ann. Plast. Surg. 2005, 55, 114–115. [Google Scholar] [CrossRef]
- Ma, L.; Ye, X.; Sun, P.; Xu, P.; Wang, L.; Liu, Z.; Huang, X.; Bai, Z.; Zhou, C. Antimicrobial and antibiofilm activity of the EeCentrocin 1 derived peptide EC1-17KV via membrane disruption. EBioMedicine 2020, 55, 102775. [Google Scholar] [CrossRef]
- Li, Y.; Xiang, Q.; Zhang, Q.; Huang, Y.; Su, Z. Overview on the recent study of antimicrobial peptides: Origins, functions, relative mechanisms and application. Peptides 2012, 37, 207–215. [Google Scholar] [CrossRef]
- Ruiz, J.; Calderon, J.; Rondón-Villarreal, P.; Torres Sáez, R. Analysis of Structure and Hemolytic Activity Relationships of Antimicrobial Peptides (AMPs); Springer: Cham, Switzerland, 2014; Volume 232, pp. 253–258. [Google Scholar]
- Slootweg, J.C.; Prochnow, P.; Bobersky, S.; Bandow, J.E.; Metzler-Nolte, N. Exploring Structure–Activity Relationships in Synthetic Antimicrobial Peptides (synAMPs) by a Ferrocene Scan. Eur. J. Inorg. Chem. 2017, 2017, 360–367. [Google Scholar] [CrossRef]
- Butts, A.; Palmer, G.E.; Rogers, P.D. Antifungal adjuvants: Preserving and extending the antifungal arsenal. Virulence 2017, 8, 198–210. [Google Scholar] [CrossRef]
- Enoch, D.A.; Ludlam, H.A.; Brown, N.M. Invasive fungal infections: A review of epidemiology and management options. J. Med. Microbiol. 2006, 55, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Taveira, G.B.; Carvalho, A.O.; Rodrigues, R.; Trindade, F.G.; Da Cunha, M.; Gomes, V.M. Thionin-like peptide from Capsicum annuum fruits: Mechanism of action and synergism with fluconazole against Candida species. BMC Microbiol. 2016, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Mora-Navarro, C.; Caraballo-León, J.; Torres-Lugo, M.; Ortiz-Bermúdez, P. Synthetic antimicrobial β-peptide in dual-treatment with fluconazole or ketoconazole enhances the in vitro inhibition of planktonic and biofilm Candida albicans. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2015, 21, 853–861. [Google Scholar] [CrossRef]
- Cavalcante, C.S.P.; de Aguiar, F.L.L.; Fontenelle, R.O.S.; de Menezes, R.; Martins, A.M.C.; Falcão, C.B.; Andreu, D.; Rádis-Baptista, G. Insights into the candidacidal mechanism of Ctn[15–34]-a carboxyl-terminal, crotalicidin-derived peptide related to cathelicidins. J. Med. Microbiol. 2018, 67, 129–138. [Google Scholar] [CrossRef]
- de Aguiar, F.L.L.; Cavalcante, C.; Dos Santos Fontenelle, R.O.; Falcão, C.B.; Andreu, D.; Rádis-Baptista, G. The antiproliferative peptide Ctn[15–34] is active against multidrug-resistant yeasts Candida albicans and Cryptococcus neoformans. J. Appl. Microbiol. 2020, 128, 414–425. [Google Scholar] [CrossRef]
- Casciaro, B.; Cappiello, F.; Verrusio, W.; Cacciafesta, M.; Mangoni, M.L. Antimicrobial Peptides and Their Multiple Effects at Sub-Inhibitory Concentrations. Curr. Top. Med. Chem. 2020. [Google Scholar] [CrossRef]
- Pérez-Peinado, C.; Dias, S.A.; Domingues, M.M.; Benfield, A.H.; Freire, J.M.; Rádis-Baptista, G.; Gaspar, D.; Castanho, M.; Craik, D.J.; Henriques, S.T.; et al. Mechanisms of bacterial membrane permeabilization by crotalicidin (Ctn) and its fragment Ctn(15–34), antimicrobial peptides from rattlesnake venom. J. Biol. Chem. 2018, 293, 1536–1549. [Google Scholar] [CrossRef]
- Pérez-Peinado, C.; Defaus, S.; Andreu, D. Hitchhiking with Nature: Snake Venom Peptides to Fight Cancer and Superbugs. Toxins 2020, 12, 255. [Google Scholar] [CrossRef]
- Miron, D.; Battisti, F.; Silva, F.K.; Lana, A.D.; Pippi, B.; Casanova, B.; Gnoatto, S.; Fuentefria, A.; Mayorga, P.; Schapoval, E.E.S. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Rev. Bras. De Farmacogn. 2014, 24, 660–667. [Google Scholar] [CrossRef]
- Silva Junior, I.F.; Raimondi, M.; Zacchino, S.; Cechinel Filho, V.; Noldin, V.F.; Rao, V.S.; Lima, J.C.S.; Martins, D.T.O. Evaluation of the antifungal activity and mode of action of Lafoensia pacari A. St.-Hil., Lythraceae, stem-bark extracts, fractions and ellagic acid. Rev. Bras. De Farmacogn. 2010, 20, 422–428. [Google Scholar] [CrossRef]
- Rodrigues, M.L. The Multifunctional Fungal Ergosterol. mBio 2018, 9, e01755-01718. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; Approved Standard CLSI document M27-A3; The Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Shanholtzer, C.J.; Peterson, L.R.; Mohn, M.L.; Moody, J.A.; Gerding, D.N. MBCs for Staphylococcus aureus as determined by macrodilution and microdilution techniques. Antimicrob. Agents Chemother. 1984, 26, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C.; Schoenknecht, F.D.; Sherris, J.C.; Linner, E.C. Determination of minimum bactericidal concentrations of oxacillin for Staphylococcus aureus: Influence and significance of technical factors. Antimicrob. Agents Chemother. 1983, 23, 142–150. [Google Scholar] [CrossRef]
- Pillai, S.K.; Moellering, R.C., Jr.; Eliopoulos, G.M. Antimicrobial Combinations. In: Antibiotics in Laboratory Medicine, 5th ed.; Lorian, V., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Shin, S.; Lim, S. Antifungal effects of herbal essential oils alone and in combination with ketoconazole against Trichophyton spp. J. Appl. Microbiol. 2004, 97, 1289–1296. [Google Scholar] [CrossRef]
- EUCAST. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, ix–xv. [Google Scholar] [CrossRef]
- Falcao, C.B.; de La Torre, B.G.; Pérez-Peinado, C.; Barron, A.E.; Andreu, D.; Rádis-Baptista, G. Vipericidins: A novel family of cathelicidin-related peptides from the venom gland of South American pit vipers. Amino Acids 2014, 46, 2561–2571. [Google Scholar] [CrossRef]

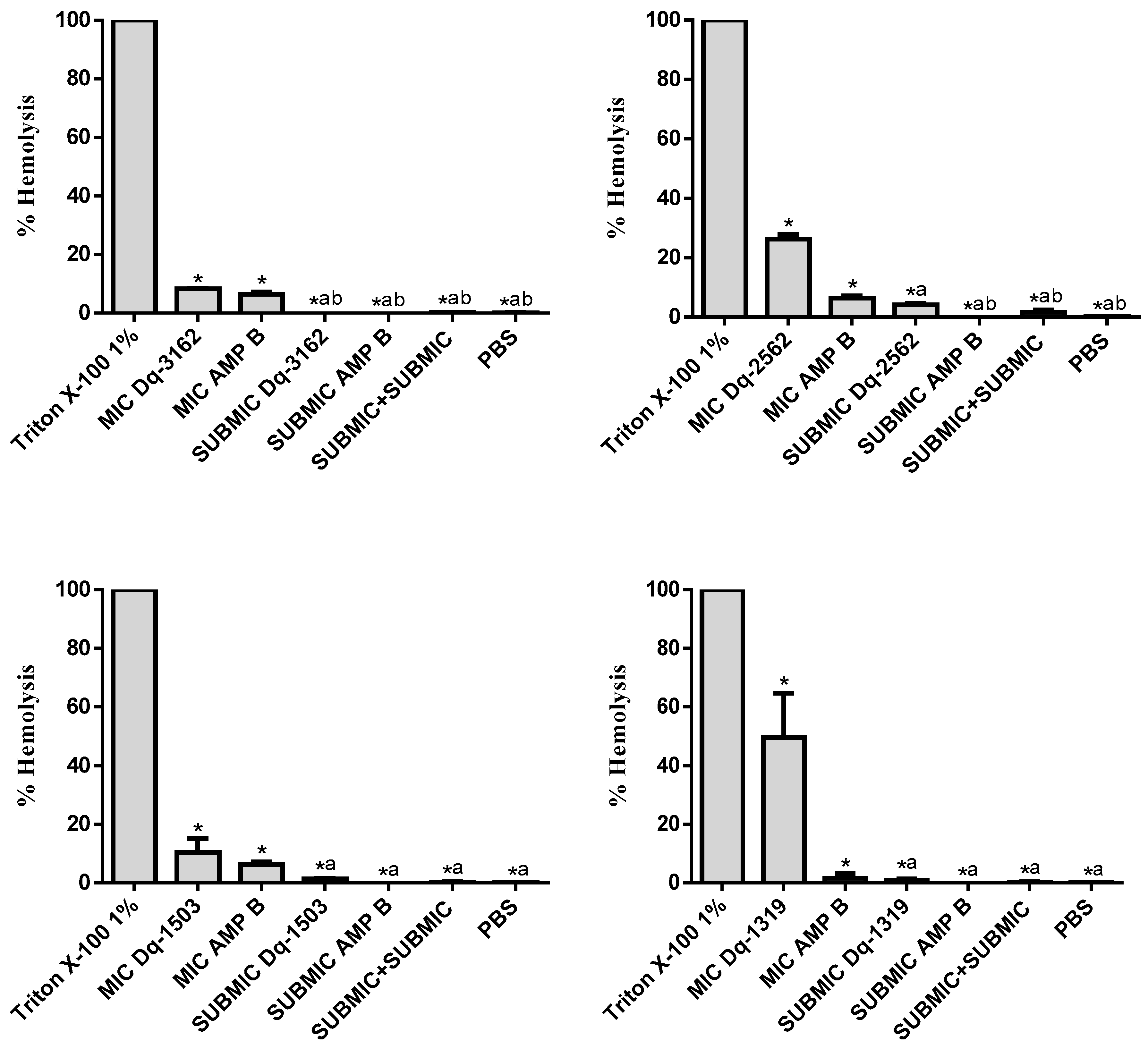
| Peptide | Primary and Secondary Structures (a) | Helical Wheel Plot (b) | Physicochemical Properties (c,d) |
|---|---|---|---|
| Dq-1319 | 1FWGTLAKWALK11 | 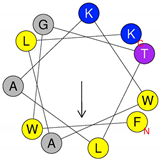 | Exp. MW = 1320.75 Net charge = +2 H = 0.781 µH = 0.523 Hydrophobic face: none |
| Dq-1503 | 1FWGTLAKWALKAI13 | 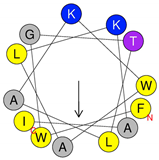 | Exp. MW = 1504.86 Net charge = +2 H = 0.823 µH = 0.531 Hydrophobic face: none |
| Dq-2562 | 1FWGTLAKWALKAIPAAMGMKQNK23 | 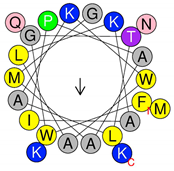 | Exp. MW = 2561.38 Net charge = +4 H = 0.509 µH = 0.248 Hydrophobic face: IAML |
| Dq-3162 | 1GLKDWWNKHKDKIVKVVKEMGKAGINAA28-NH2 |  | Exp. MW = 3162.75 Net charge = +5 H = 0.485 µH = 0.194 Hydrophobic face: LMIWGV |
| Strain | Conc.* | Dq-3162 | Dq-2562 | Dq-1503 | Dq-1319 |
|---|---|---|---|---|---|
| Candida albicans ATCC 90029 | MIC MLC | 10 (32) 20 (64) | 10 (25) 10 (25) | - - | - - |
| Candida albicans ATCC 90028 | MIC MLC | 0.625 (2.0) 1.25 (4.0) | 2.5 (6.25) 5.0 (12.5) | 2.5 (3.75) 2.5 (3.75) | 20 (26) - |
| Candida tropicalis ATCC 13803 | MIC MLC | 0.625 (2.0) 0.625 (2.0) | 2.5 (6.25) 5.0 (12.5) | 2.5 (3.75) 5.0 (7.5) | 20 (26) - |
| Candida tropicalis ATCC 750 | MIC MLC | 10 (32) 20 (64) | 10 (25) 20 (50) | - - | - - |
| Candida parapsilosis ATCC 90018 | MIC MLC | 10 (32) 10 (32) | 10 (25) 10 (25) | 20 (30) 20 (30) | - - |
| Candida parapsilosis ATCC 40038 | MIC MLC | 5.0 (16) 5.0 (16) | 5.0 (12.5) 10 (12.5) | 10 (15) 10 (15) | - - |
| Candida krusei ATCC 40095 | MIC MLC | 2.5 (8) 5.0 (16) | 2.5 (6.25) 5.0 (12.5) | 10 (15) 10 (15) | - - |
| Candida krusei ATCC 40147 | MIC MLC | 2.5 (8) 5.0 (16) | 5.0 (12.5) 5.0 (12.5) | 10 (15) 10 (15) | - - |
| Candida albicans CA1 | MIC MLC | 5.0 (16) 10 (32) | 10 (25) 20 (50) | 10 (15) 20 (30) | - - |
| Peptide/ Antifungal | C. albicans ATCC 90028 | C. tropicalis ATCC 13803 | C. krusei ATCC 40095 | C. parapsilosis ATCC 40038 | C. albicans CA1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| FICI | Reduction [ATF] | FICI | Reduction [ATF] | FICI | Reduction [ATF] | FICI | Reduction [ATF] | FICI | Reduction [ATF] | |
| Dq-3162/ | ||||||||||
| AMP B | 0.3125 (S) | 4x | 0.3125 (S) | 4x | 0.3125 (S) | 4x | 0.375 (S) | 8x | 0.5 (S) | 4x |
| MICO | - | - | - | - | 0.5 (S) | 4x | 0.3125 (S) | 16x | 0.5625 (A) | 16x |
| CICL | 0.3125 (S) | 4x | 0.5625 (A) | 2x | 0.3125 (S) | 4x | 0.3125 (S) | 16x | 0.5625 (A) | 16x |
| FLUC | - | - | - | - | 0.5625 (A) | 16x | 0.375 (S) | 8x | 0.5625 (A) | 16x |
| NYST | 0.1875 (S) | 8x | 0.1875 (S) | 8x | 0.1875 (S) | 8x | 0.1875 (S) | 8x | 0.5625 (A) | 16x |
| Dq-2562/ | ||||||||||
| AMP B | 0.3125 (S) | 4x | 0.3125 (S) | 4x | 0.3125 (S) | 4x | 0.3125 (S) | 4x | 0.5 (S) | 4x |
| MICO | 0.5625 (A) | 16x | 0.5625 (A) | 16x | 0.5625 (A) | 16x | 0.3125 (S) | 16x | 0.5625 (A) | 16x |
| CICL | 0.375 (S) | 8x | 0.5625 (A) | 16x | 0.3125 (S) | 4x | 0.3125 (S) | 16x | 0.5625 (A) | 16x |
| FLUC | - | - | - | - | 0.5625 (A) | 2x | 0.5625 (A) | 16x | 0.5625 (A) | 16x |
| NYST | 0.1875 (S) | 8x | 0.1875 (S) | 8x | 0.1875 (S) | 8x | 0.1875 (S) | 8x | 0.5625 (A) | 16x |
| Dq-1503/ | ||||||||||
| AMP B | 0.3125 (S) | 4x | 0.3125 (S) | 4x | 0.3125 (S) | 4x | 0.3125 (S) | 4x | 0.5 (S) | 4x |
| MICO | - | - | - | - | 0.3125 (S) | 16x | 0.375 (S) | 8x | 0.5625 (A) | 16x |
| CICL | 0.3125 (S) | 4x | 0.625 (A) | 8x | 0.3125 (S) | 16x | 0.3125 (S) | 16x | 0.5625 (A) | 16x |
| FLUC | 0.625 (A) | 8x | 0.625 (A) | 8x | 0.3125 (S) | 16x | 0.3125 (S) | 16x | 0.5625 (A) | 16x |
| NYST | 0.1875 (S) | 8x | 0.1875 (S) | 8x | 0.3125 (S) | 16x | 0.1875 (S) | 8x | 0.5625 (A) | 16x |
| Dq-1319 | ||||||||||
| AMP B | 0.75 (A) | 4x | 0.375 (S) | 4x | ND | ND | ND | |||
| MICO | 0.5625 (A) | 16x | 0.5625 (A) | 16x | ND | ND | ND | |||
| CICL | 0.5 (S) | 4x | 0.5625 (A) | 16x | ND | ND | ND | |||
| FLUC | 0.5625 (A) | 16x | 0.5625 (A) | 16x | ND | ND | ND | |||
| NYST | 0.1875 (S) | 8x | 0.1875 (S) | 8x | ND | ND | ND | |||
| Peptide/Drug a | Concentration b | SYTOX® Green Stained-Cells c (%) | |
|---|---|---|---|
| C. albicans ATCC 90028 | C. albicans CA1 (d) | ||
| Dq-2562 | MIC | 75 | 56 |
| Dq-3162 | MIC | 76 | 54 |
| Amphotericin B | MIC | 48 | 58 |
| Dq-2562/AMP B | SUBMICs | 73 | 69 |
| Dq-3162/AMP B | SUBMICs | 50 | 59 |
| - | - | 5 | 4 |
| Peptide | C. albicans ATCC 90028 | C. tropicalis ATCC 13803 | C. krusei ATCC 40095 | C. parapsilosis ATCC 40038 |
|---|---|---|---|---|
| Dq-3162 | ||||
| Without ergosterol | 0.0625 (2) | 0.0625 (2) | 2.5 (8) | 5.0 (16) |
| With ergosterol (µg/mL) | ||||
| 100 | 1.25 (4) | 1.25 (4) | 5.0 (16) | 5.0 (16) |
| 200 | 1.25 (4) | 2.5 (8) | 5.0 (16) | 10 (32) |
| 400 | 5.0 (16) | 5.0 (16) | 10 (32) | 20 (64) |
| 800 | 10 (32) | 10 (32) | 20 (64) | 20 (64) |
| Dq-2562 | ||||
| Without ergosterol | 2.5 (6.25) | 2.5 (6.25) | 2.5 (6.25) | 5.0 (12.5) |
| With ergosterol (µg/mL) | ||||
| 100 | 5.0 (12.5) | 5.0 (12.5) | 5.0 (12.5) | 5.0 (12.5) |
| 200 | 5.0 (12.5) | 5.0 (12.5) | 5.0 (12.5) | 5.0 (12.5) |
| 400 | 20 (50) | 20 (50) | 10 (25) | 20 (25) |
| 800 | 20 (50) | 20 (50) | 20 (50) | 20 (25) |
| Dq-1503 | ||||
| Without ergosterol | 2.5 (3.75) | 2.5 (3.75) | 10 (15) | 10 (15) |
| With ergosterol (µg/mL) | ||||
| 100 | 2.5 (3.75) | 5.0 (7.5) | 10 (15) | 10 (15) |
| 200 | 5.0 (7.5) | 5.0 (7.5) | 10 (15) | 20 (30) |
| 400 | 10 (15) | 10 (15) | 20 (30) | 20 (30) |
| 800 | 20 (30) | 10 (15) | 20 (30) | 40 (60) |
| Dq-1319 | ||||
| Without ergosterol | 20 (26) | 20 (26) | - | - |
| With ergosterol (µg/mL) | ||||
| 100 | 20 (26) | 20 (26) | - | - |
| 200 | 40 (52) | 40 (52) | - | - |
| 400 | 80 (104) | 40 (52) | - | - |
| 800 | 80 (104) | 80 (104) | - | - |
| Amphotericin B | ||||
| Without ergosterol | 0.5 (0.5) | 0.5 (0.5) | 1.0 (0.9) | 0.5 (0.5) |
| With ergosterol (µg/mL) | ||||
| 100 | 4.0 (3.7) | 2.0 (1.8) | 4.0 (3.7) | 4.0 (3.7) |
| 200 | 4.0 (7.4) | 4.0 (3.7) | 4.0 (3.7) | 16 (15) |
| 400 | 16 (15) | 16 (15) | 16 (15) | 32 (30) |
| 800 | 32 (30) | 32 (30) | 32 (30) | 32 (30) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dodou Lima, H.V.; de Paula Cavalcante, C.S.; Rádis-Baptista, G. Antifungal In Vitro Activity of Pilosulin- and Ponericin-Like Peptides from the Giant Ant Dinoponera quadriceps and Synergistic Effects with Antimycotic Drugs. Antibiotics 2020, 9, 354. https://doi.org/10.3390/antibiotics9060354
Dodou Lima HV, de Paula Cavalcante CS, Rádis-Baptista G. Antifungal In Vitro Activity of Pilosulin- and Ponericin-Like Peptides from the Giant Ant Dinoponera quadriceps and Synergistic Effects with Antimycotic Drugs. Antibiotics. 2020; 9(6):354. https://doi.org/10.3390/antibiotics9060354
Chicago/Turabian StyleDodou Lima, Hilania Valéria, Carolina Sidrim de Paula Cavalcante, and Gandhi Rádis-Baptista. 2020. "Antifungal In Vitro Activity of Pilosulin- and Ponericin-Like Peptides from the Giant Ant Dinoponera quadriceps and Synergistic Effects with Antimycotic Drugs" Antibiotics 9, no. 6: 354. https://doi.org/10.3390/antibiotics9060354
APA StyleDodou Lima, H. V., de Paula Cavalcante, C. S., & Rádis-Baptista, G. (2020). Antifungal In Vitro Activity of Pilosulin- and Ponericin-Like Peptides from the Giant Ant Dinoponera quadriceps and Synergistic Effects with Antimycotic Drugs. Antibiotics, 9(6), 354. https://doi.org/10.3390/antibiotics9060354






