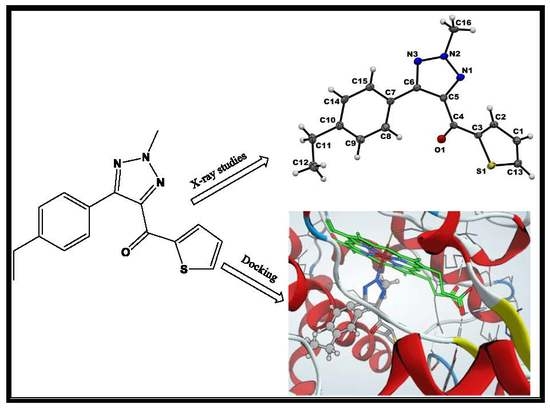
Crystallography, in Silico Studies, and In Vitro Antifungal Studies of 2,4,5 Trisubstituted 1,2,3-Triazole Analogues
Abstract
1. Introduction
2. Results and Discussion
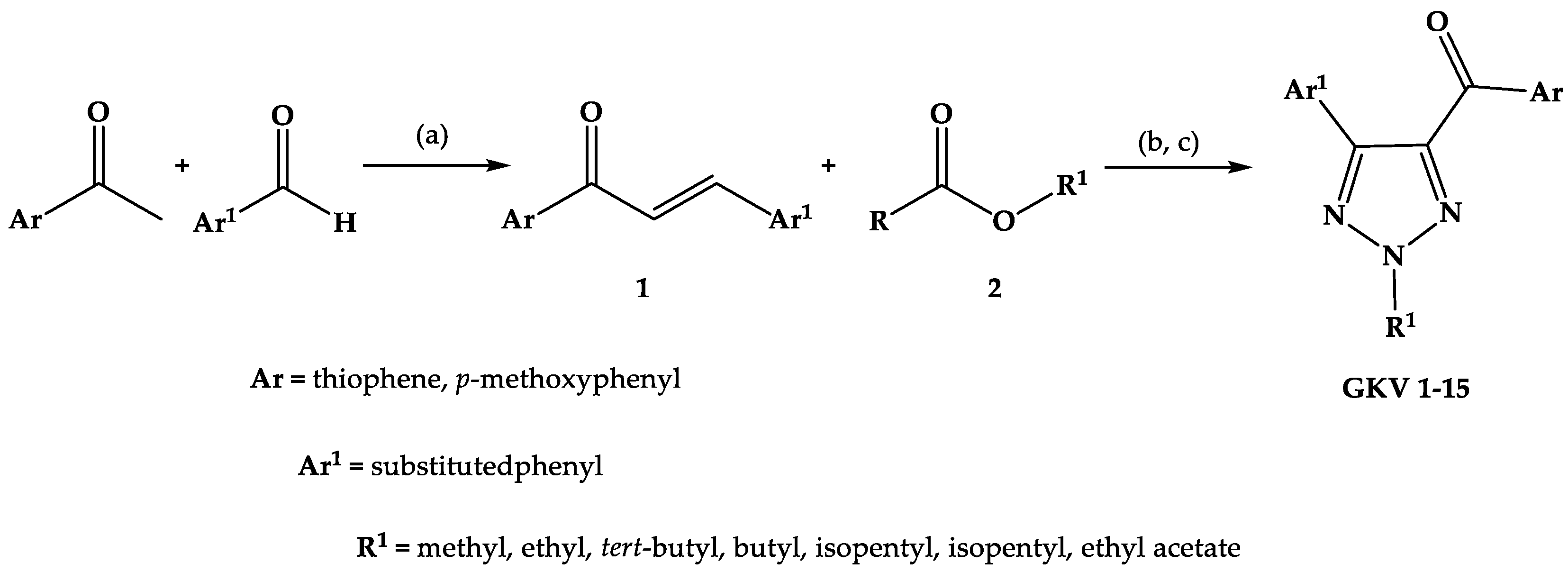
2.1. Chemistry
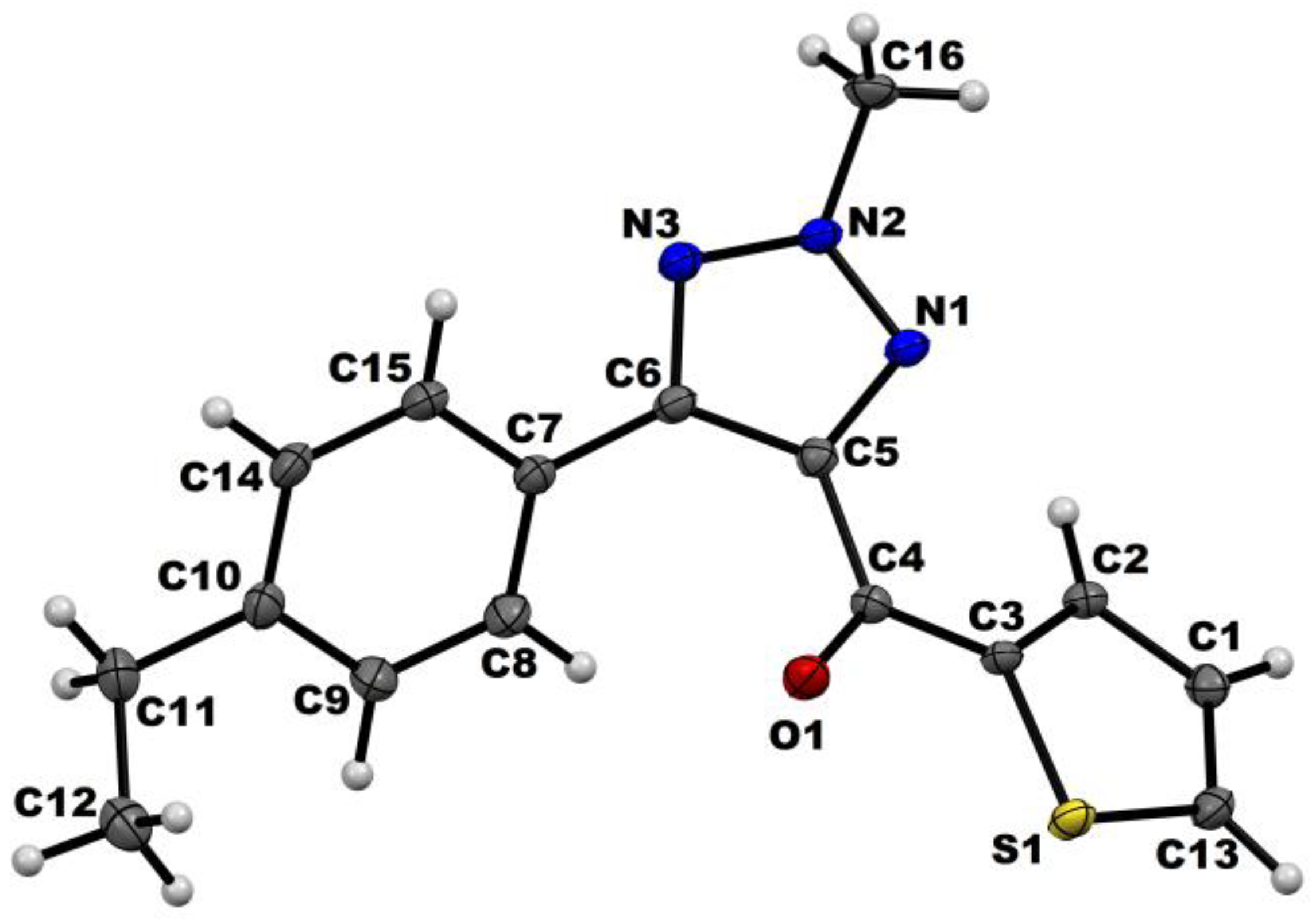
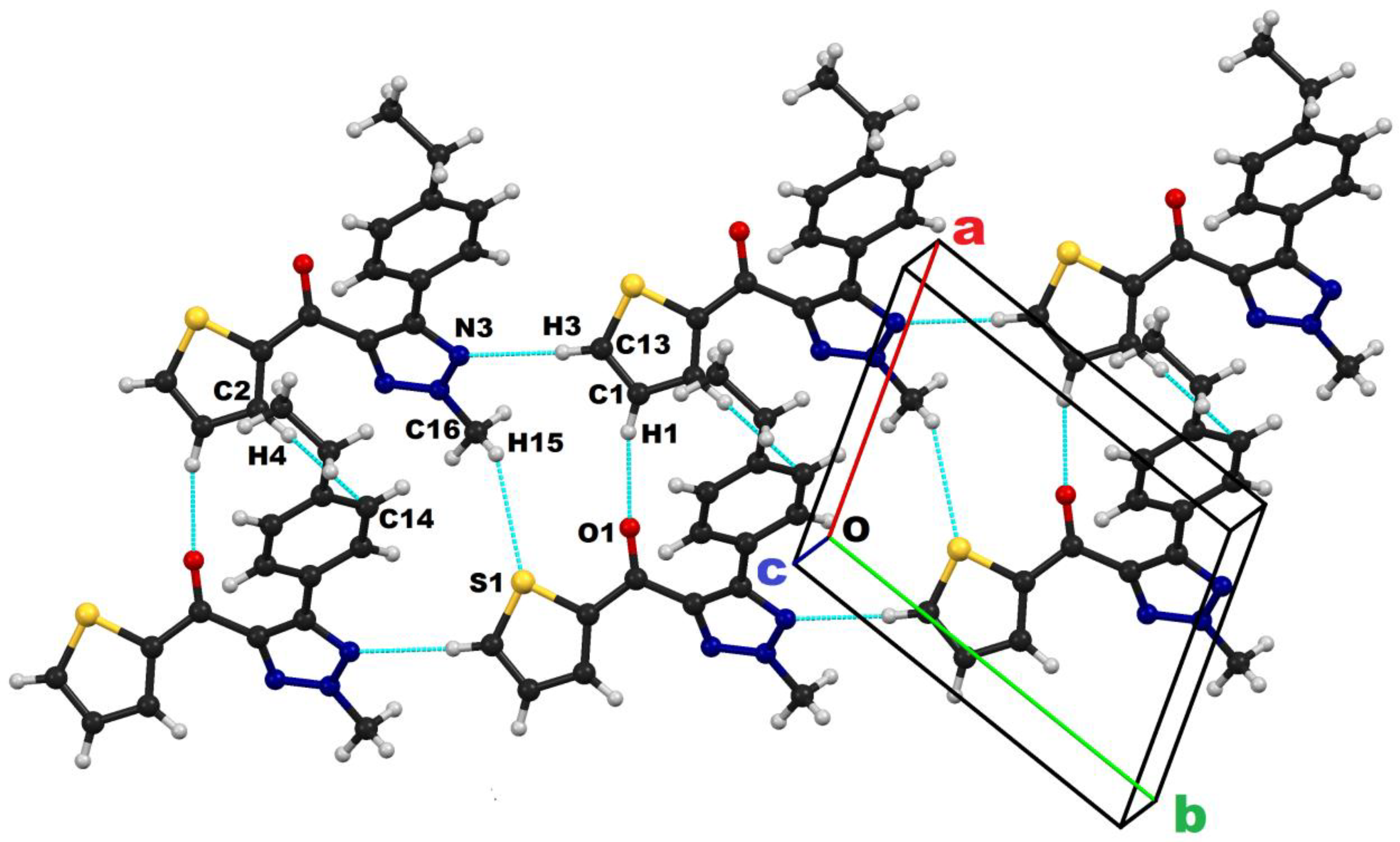
2.2. Crystallography
2.3. Pharmacology
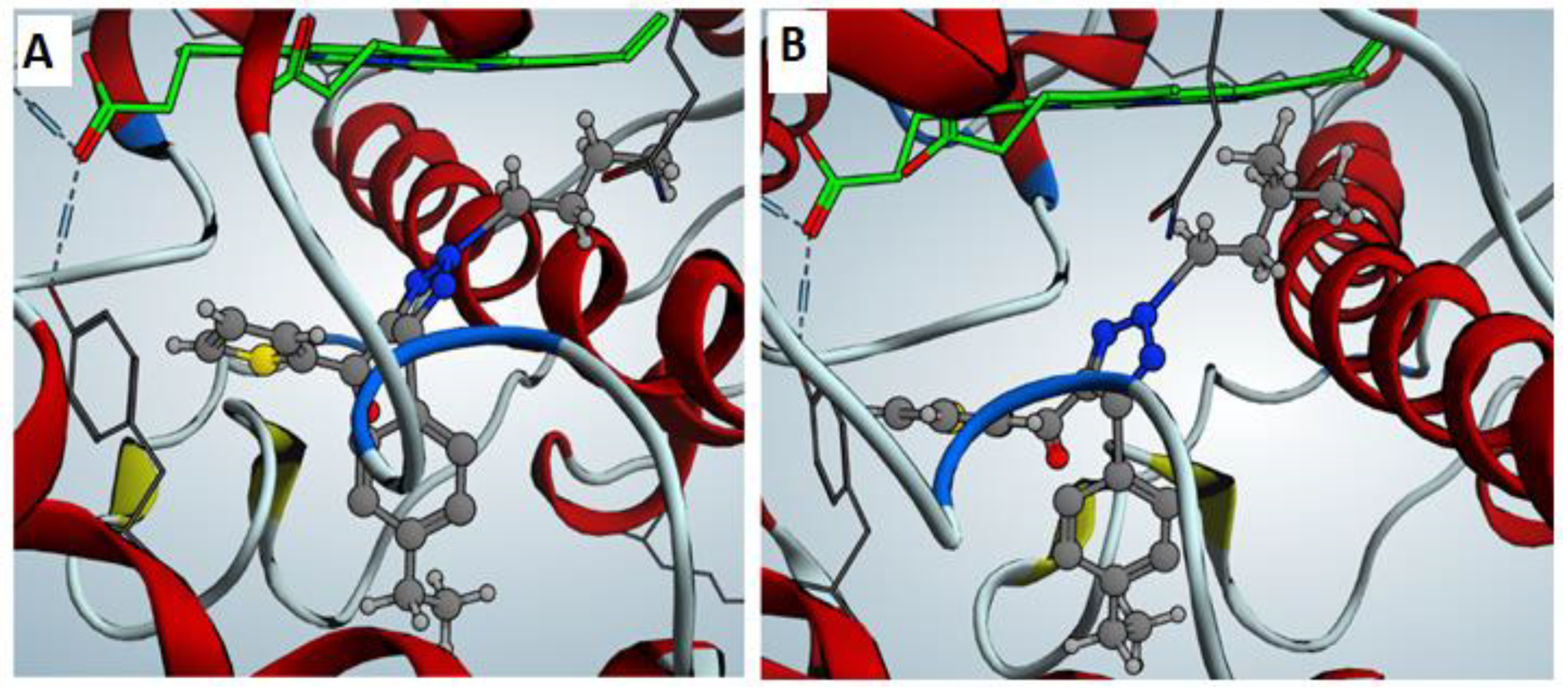
2.4. Molecular Docking
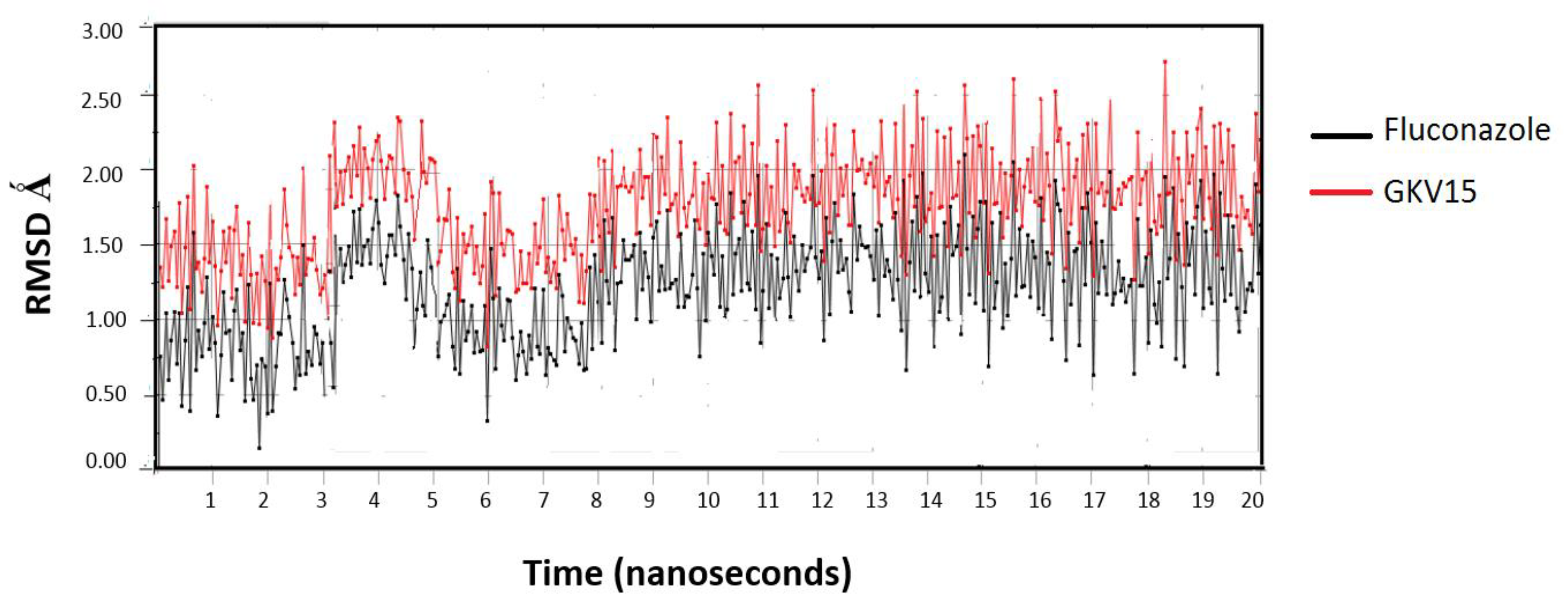
2.5. Molecular Dynamics
3. Materials and Methods
3.1. General
3.2. Single-Crystal X-Ray Data Collection and Refinement Details
3.3. Antifungal Screening
3.4. Molecular Docking
3.5. Molecular Dynamics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Panackal, A.A.; Williamson, P.R. Fungal Infections of the Central Nervous System. Contin. Lifelong Learni. Neurol. 2015, 21, 1662–1678. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Chatterjee, S.S.; Shivaprakash, M.R. Overview of opportunistic fungal infections in India. Nihon Ishinkin Gakkai Zasshi 2008, 49, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.K.; Mahadevan, A.; Sundaram, C.; Sarkar, C.; Chacko, G.; Lanjewar, D.N.; Santosh, V.; Yasha, T.C.; Radhakrishnan, V.V. Pathobiology of fungal infections of the central nervous system with special reference to the Indian scenario. Neurology 2007, 55, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Chatterjee, S.S.; Das, A.; Shivaprakash, M.R. Invasive aspergillosis in developing countries. Med. Mycol. 2011, 49, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Khoza, S.; Moyo, I.; Ncube, D. Comparative Hepatotoxicity of Fluconazole, Ketoconazole, Itraconazole, Terbinafine, and Griseofulvin in Rats. J. Toxicol. 2017, 2017, 6746989. [Google Scholar] [CrossRef] [PubMed]
- Siafaka, P.I.; Üstündağ Okur, N.; Mone, M.; Giannakopoulou, S.; Er, S.; Pavlidou, E.; Karavas, E.; Bikiaris, D.N. Two Different Approaches for Oral Administration of Voriconazole Loaded Formulations: Electrospun Fibers versus β-Cyclodextrin Complexes. Int. J. Mol. Sci. 2016, 17, 282. [Google Scholar] [CrossRef]
- Khedr, M.A. Stepwise design, synthesis, and in vitro antifungal screening of (Z)-substituted-propenoic acid derivatives with potent broad-spectrum antifungal activity. Drug Des. Dev. Ther. 2015, 9, 4501–4513. [Google Scholar] [CrossRef]
- Paeshuyse, J.; Dallmeier, K.; Neyts, J. Ribavirin for the treatment of chronic hepatitis C virus infection: A review of the proposed mechanisms of action. Curr. Opin. Virol. 2011, 1, 590–598. [Google Scholar] [CrossRef]
- Mhasalkar, M.Y.; Shah, M.H.; Nikam, S.T.; Anantanarayanan, K.G.; Deliwala, C.V. 4-alkyl-5-aryl-4H-1,2,4-triazole-3-thiols as hypoglycemic agents. J. Med. Chem. 1970, 13, 672–674. [Google Scholar] [CrossRef]
- Özdemir, A.; Turan-Zitouni, G.; Asim Kaplancikli, Z.; Chevallet, P. Synthesis of some 4-arylidenamino-4H-1,2,4-triazole-3-thiols and their antituberculosis activity. J. Enzym. Inhib. Med. Chem. 2007, 22, 511–516. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Dharma Rao, G.B.; Bhandary, S.; Pillay, M.; Chopra, D.; Aldhubiab, B.E.; Attimarad, M.; Alwassil, O.I.; Harsha, S.; Mlisana, K. Design, synthesis, and characterization of (1-(4-aryl)- 1H-1,2,3-triazol-4-yl)methyl, substituted phenyl-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylates against Mycobacterium tuberculosis. Drug Des. Devel. Ther. 2016, 10, 2681–2690. [Google Scholar] [CrossRef] [PubMed]
- Raghu Prasad, M.; Prashanth, J.; Shilpa, K.; Pran Kishore, D. Synthesis and antibacterial activity of some novel triazolothienopyrimidines. Chem. Pharm. Bull. 2007, 55, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Jayashree, B.S.; Sahu, A.R.; Srinivasa, M.M.; Venugopala, K.N. Synthesis, characterization and determination of partition coefficient of some triazole derivatives of coumarins for their anti-microbial activity. Asian J. Chem. 2007, 19, 73–78. [Google Scholar]
- Czarnocka-Janowicz, A.; Foks, H.; Nasal, A.; Petrusewicz, J.; Damasiewicz, B.; Radwanska, A.; Kaliszan, R. Synthesis and pharmacological activity of 5-substituted-s-triazole-3-thiols. Pharmazie 1991, 46, 109–112. [Google Scholar] [PubMed]
- Jayashree, B.S.; Arora, S.; Venugopala, K.N. Microwave assisted synthesis of substituted coumarinyl chalcones as reaction intermediates for biologically important coumarinyl heterocycles. Asian J. Chem. 2008, 20, 1–7. [Google Scholar]
- Chandrashekharappa, S.; Venugopala, K.N.; Nayak, S.K.M.; Gleiser, R.; García, D.A.; Kumalo, H.M.; Kulkarni, R.S.; Mahomoodally, F.M.; Venugopala, R.; Mohan, M.K.; et al. One-pot microwave assisted synthesis and structural elucidation of novel ethyl 3-substituted-7-methylindolizine-1-carboxylates with larvicidal activity against Anopheles arabiensis. J. Mol. Struct. 2018, 1156, 377–384. [Google Scholar] [CrossRef]
- Rao, G.K.; Venugopala, K.N.; Pai, P.N.S. Microwave-assisted synthesis of some 6-chloro-3-[2-(substituted anilino)-1,3-thiazol-4-yl]-2H-1-benzopyran-2-ones as antibacterial agents. Indian J. Heterocycl. Chem. 2008, 17, 397–400. [Google Scholar]
- Venugopala, K.N. Design, microwave assisted synthesis and characterization of substituted 1, 2, 4-oxadiazole analogues as promising pharmacological agents. Asian J. Chem. 2017, 29, 1767–1770. [Google Scholar] [CrossRef]
- Raghu, P.M.; Deb, P.K. Multistep, microwave assisted, solvent free synthesis and antibacterial activity of 6-substituted-2,3,4-trihydropyrimido[1,2-c]9,10,11,12-tetrahydrobenzo[b]thieno[3,2-e]pyrimidines. Chem. Pharm Bull. 2007, 55, 776–779. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Uppar, V.; Sandeep, C.; Abdallah, H.H.; Pillay, M.; Deb, P.K.; Morsy, M.A.; Aldhubiab, B.E.; Attimarad, M.; Nair, A.B.; et al. Cytotoxicity and antimycobacterial properties of pyrrolo[1,2-a]quinoline derivatives: Molecular target identification and molecular docking studies. Antibiotics 2020, 9, 233. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Tratrat, C.; Pillay, M.; Chandrashekharappa, S.; Al-Attraqchi, O.H.A.; Aldhubiab, B.E.; Attimarad, M.; Alwassil, O.I.; Nair, A.B.; Sreeharsha, N.; et al. In silico design and synthesis of tetrahydropyrimidinones and tetrahydropyrimidinethiones as potential thymidylate kinase inhibitors exerting anti-TB activity against Mycobacterium tuberculosis. Drug Des. Dev. Ther. 2020, 14, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Venugopala, K.N.; Tratrat, C.; Pillay, M.; Mahomoodally, F.M.; Bhandary, S.; Chopra, D.; Morsy, M.A.; Haroun, M.; Aldhubiab, B.E.; Attimarad, M.; et al. Anti-tubercular activity of substituted 7-methyl and 7-formylindolizines and in silico study for prospective molecular target identification. Antibiotics 2019, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Venugopala, K.N.; Tratrat, C.; Chandrashekharappa, S.; Attimarad, M.; Sreeharsha, N.; Nair, A.B.; Pottathil, S.; Venugopala, R.; Al-Attraqchi, O.H.A.; Morsy, M.A.; et al. Anti-tubercular potency and computationally-assessed drug-likeness and toxicology of diversely substituted indolizines. Indian J. Pharm. Edu. Res. 2019, 53, 545–552. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Khedr, M.A.; Pillay, M.; Nayak, S.K.; Chandrashekharappa, S.; Aldhubiab, B.E.; Harsha, S.; Attimard, M.; Odhav, B. Benzothiazole analogs as potential anti-TB agents: Computational input and molecular dynamics. J. Biomol. Struct. Dyn. 2019, 37, 1830–1842. [Google Scholar] [CrossRef] [PubMed]
- Venugopala, K.N.; Chandrashekharappa, S.; Pillay, M.; Bhandary, S.; Kandeel, M.; Mahomoodally, F.M.; Morsy, M.A.; Chopra, D.; Aldhubiab, B.E.; Attimarad, M.; et al. Synthesis and structural elucidation of novel benzothiazole derivatives as anti-tubercular agents: In-silico screening for possible target identification. Med. Chem. 2019, 15, 311–326. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Sandeep, C.; Pillay, M.; Hassan, H.A.; Fawzi, M.M.; Bhandary, S.; Chopra, D.; Attimarad, M.; Bandar, E.A.; Nair, A.B.; et al. Computational, crystallographic studies, cytotoxicity and anti-tubercular activity of substituted 7-methoxy-indolizine analogues. PLoS ONE 2019, 14, e0217270. [Google Scholar] [CrossRef]
- Chandrashekharappa, S.; Venugopala, K.N.; Venugopala, R.; Padmashali, B. Qualitative anti-tubercular activity of synthetic ethyl 7-acetyl-2-substituted-3-(4-substituted benzoyl) indolizine-1-carboxylate analogues. J. Appl. Pharm. Sci. 2019, 9, 124–128. [Google Scholar] [CrossRef]
- Khedr, M.A.; Pillay, M.; Chandrashekharappa, S.; Chopra, D.; Aldhubiab, B.E.; Attimarad, M.; Alwassil, O.I.; Mlisana, K.; Odhav, B.; Venugopala, K.N. Molecular modeling studies and anti-TB activity of trisubstituted indolizine analogues; molecular docking and dynamic inputs. J. Biomol. Struct. Dyn. 2018, 36, 2163–2178. [Google Scholar] [CrossRef]
- Alveera, S.; Venugopala, K.N.; Khedr, M.A.; Pillay, M.; Nwaeze, K.U.; Coovadia, Y.; Shode, F.; Odhav, B. Antimycobacterial, docking and molecular dynamic studies of pentacyclic triterpenes from Buddleja saligna leaves. J. Biomol. Struct. Dyn. 2017, 35, 2654–2664. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Nayak, S.K.; Pillay, M.; Prasanna, R.; Coovadia, Y.M.; Odhav, B. Synthesis and antitubercular activity of 2-(substituted phenyl/benzyl-amino)-6-(4-chlorophenyl)-5-(methoxycarbonyl)-4-methyl-3,6-dihydropyrimidin-1-ium chlorides. Chem. Biol. Drug Des 2013, 81, 219–227. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Albericio, F.; Coovadia, Y.M.; Kruger, H.G.; Maguire, G.E.M.; Pillay, M.; Govender, T. Total synthesis of a depsidomycin analogue by convergent solid-phase peptide synthesis and macrolactonization strategy for antitubercular activity. J. Pept. Sci 2011, 17, 683–689. [Google Scholar] [CrossRef]
- Morsy, M.A.; Ali, E.M.; Kandeel, M.; Venugopala, K.N.; Nair, A.B.; Greish, K.; El-Daly, M. Screening and molecular docking of novel benzothiazole derivatives as potential antimicrobial agents. Antibiotics 2020, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Rai, N.P.; Venugopala, K.N.; Sheena, S.; Pirama, N.A. Synthesis, characterization and anti-bacterial activity of 2-[1-(5-chloro-2-methoxy-phenyl)-5-methyl-1H-pyrazol-4-yl]-5-(substituted-phenyl)-[1,3,4] oxadiazoles. Eur. J. Med. Chem. 2009, 44, 4522–4527. [Google Scholar] [CrossRef] [PubMed]
- Venugopala, K.N.; Jayashree, B.S. Microwave-induced synthesis of Schiff bases of aminothiazolyl bromocoumarins as antibacterials. Indian J. Pharm. Sci. 2008, 70, 88–91. [Google Scholar] [CrossRef]
- Rai, N.P.; Narayanaswamy, V.K.; Govender, T.; Manuprasad, B.K.; Shashikanth, S.; Arunachalam, P.N. Design, synthesis, characterization, and antibacterial activity of [35]-(phenyl)-methanones. Eur. J. Med. Chem. 2010, 45, 2677–2682. [Google Scholar] [CrossRef]
- Girish, Y.R.; Sharath Kumar, K.S.; Muddegowda, U.; Lokanath, N.K.; Rangappa, K.S.; Shashikanth, S. ZrO2-supported Cu(ii)-[small beta]-cyclodextrin complex: Construction of 2,4,5-trisubstituted-1,2,3-triazoles via azide-chalcone oxidative cycloaddition and post-triazole alkylation. RSC Adv. 2014, 4, 55800–55806. [Google Scholar] [CrossRef]
- Bhandary, S.; Girish, Y.R.; Venugopala, K.N.; Deepak, C. Crystal structure analysis of [5-(4-methoxyphenyl)-2-methyl-2H-1,2,3-triazol-4-yl](thiophen-2-yl)-methanone. Acta Cryst. E 2018, 74, 1178–1181. [Google Scholar] [CrossRef]
- Apex2, Version 2 User Manual, M86-E01078; Bruker Analytical X-ray Systems: Madison, WI, USA, 2006.
- Siemens. SMART System; Siemens Analytical X-ray Instruments Inc.: Madison, MI, USA, 1995. [Google Scholar]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M.C.; Polidori, G.; Camalli, M. SIRPOW.92-a program for automatic solution of crystal structures by direct methods optimized for powder data. J. Appl. Crystallogr. 1994, 27, 435–436. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Farrugia, L. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS; Bruker AXS, Inc.: Madison, WI, USA, 2007. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Van de Streek, J.; Wood, P.A. Mercury CSD 2.0-new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Nardelli, M. PARST95-an update to PARST: A system of Fortran routines for calculating molecular structure parameters from the results of crystal structure analyses. J. Appl. Crystallogr. 1995, 28, 659. [Google Scholar] [CrossRef]
- Spek, A. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Cambridge Crystallographic Data Centre. Available online: https://www.Ccdc.Cam.Ac.Uk (accessed on 28 December 2019).
- Anaissie, E.J.; Paetznick, V.L.; Ensign, L.G.; Espinel-Ingroff, A.; Galgiani, J.N.; Hitchcock, C.A.; LaRocco, M.; Patterson, T.; Pfaller, M.A.; Rex, J.H.; et al. Microdilution antifungal susceptibility testing of Candida albicans and Cryptococcus neoformans with and without agitation: An eight-center collaborative study. Antimicrob. Agents Chemother. 1996, 40, 2387–2391. [Google Scholar] [CrossRef] [PubMed]
- Deb, P.K.; Al-Attraqchi, O.; Mohammed, N.A.Q.; Raghuprasad, M.; Tekade, R.K. Chapter-19: Applications of Computers in Pharmaceutical Product Formulation; Dosage form design considerations: Advances in pharmaceutical product development and research series, Vol. II. Tekade, R.K., Ed.; Academic Press: London, UK, 2018; pp. 665–703. [Google Scholar]




| Compound Code | Minimum Inhibitory Concentration (μg/mL) | ||||
|---|---|---|---|---|---|
| C. parapsilosis | C. albicans | C. tropicalis | A. niger | T. rubrum | |
| GKV1 | 125 | 62.5 | 62.5 | 125 | 125 |
| GKV2 | 31.25 | 15.63 | 15.63 | 62.5 | 62.5 |
| GKV3 | 125 | 62.5 | 62.5 | 125 | 125 |
| GKV4 | 15.63 | 15.63 | 15.63 | 31.25 | 31.25 |
| GKV5 | 125 | 62.5 | 62.5 | 125 | 125 |
| GKV6 | 31.5 | 15.63 | 15.63 | 31.25 | 31.25 |
| GKV7 | 7.81 | 7.81 | 3.9 | 15.63 | 15.63 |
| GKV8 | 7.81 | 7.81 | 3.9 | 15.63 | 15.63 |
| GKV9 | 3.9 | 3.9 | 1.98 | 7.81 | 15.63 |
| GKV10 | 0.98 | 0.98 | 0.49 | 1.95 | 1.95 |
| GKV11 | 0.98 | 0.98 | 0.49 | 0.98 | 1.95 |
| GKV12 | 15.63 | 15.63 | 15.63 | 31.25 | 31.25 |
| GKV13 | 15.63 | 15.63 | 7.81 | 15.63 | 15.63 |
| GKV14 | 7.81 | 7.81 | 3.9 | 7.81 | 15.63 |
| GKV15 | 0.98 | 0.98 | 0.49 | 0.98 | 0.98 |
| Fluconazole | 0.49 | 0.49 | 0.98 | 0.49 | 1.95 |
| Compound | Computational Parameters | |||
|---|---|---|---|---|
| ΔG(kcal/mol) | Affinity | Clash Score | RMSD (Ǻ) | |
| GKV1 | −22.64 | 25.88 | 3.50 | 1.11 |
| GKV2 | −22.41 | 25.72 | 3.01 | 1.25 |
| GKV3 | −22.94 | 26.32 | 2.95 | 1.03 |
| GKV4 | −22.33 | 26.10 | 3.00 | 1.30 |
| GKV5 | −22.04 | 26.34 | 2.84 | 1.25 |
| GKV6 | −22.83 | 24.97 | 2.65 | 1.25 |
| GKV7 | −23.98 | 29.45 | 2.33 | 1.01 |
| GKV8 | −23.88 | 29.33 | 2.38 | 0.97 |
| GKV9 | −23.71 | 28.95 | 2.35 | 0.94 |
| GKV10 | −24.33 | 29.80 | 2.40 | 0.87 |
| GKV11 | −24.54 | 29.87 | 2.51 | 0.85 |
| GKV12 | −21.95 | 25.12 | 3.25 | 1.50 |
| GKV13 | −21.67 | 26.74 | 3.13 | 1.61 |
| GKV14 | −22.89 | 25.74 | 2.81 | 1.44 |
| GKV15 | −24.75 | 30.01 | 2.25 | 0.81 |
| Data | Compound GKV10 |
|---|---|
| CCDC | 1443666 |
| Formula | C16 H15 N3 O S |
| Formula weight | 297.37 |
| Temperature/K | 110 (2) |
| Wavelength (Å) | 0.71073 |
| Solvent system, Temperature | Toluene, 25 °C |
| Crystal system | Triclinic |
| Space group | P-1 |
| a (Å) | 7.6676 (4) |
| b (Å) | 9.8450 (5) |
| c (Å) | 10.8781 (6) |
| α (°) | 94.319 (3) |
| β (°) | 109.220 (3) |
| γ (°) | 107.332 (3) |
| V (Å3) | 726.36 (7) |
| Zʹ, Z | 1, 2 |
| Density (g cm−3) | 1.360 |
| μ (mm−1) | 0.225 |
| F (000) | 312 |
| θ (min, max) | 2.21, 30.02 |
| hmin, max, kmin, max, lmin, max | −10 10, −13 12, −14 14 |
| No. of ref. | 10812 |
| No. of unique ref./obs. Ref. | 3573/3098 |
| No. parameters | 172 |
| Rall, Robs | 0.0503, 0.0442 |
| wR2all, wR2obs | 0.1301, 0.1249 |
| ∆ρmin, max (eÅ−3) | −0.249, 0.446 |
| Goodness of Fit (G. O. F.) | 1.066 |
| Motifs | D–H···A | Symmetry | Geometry | ||
|---|---|---|---|---|---|
| D···A/Å | H···A/Å | ˂D–H···A/° | |||
| I | C1-H1···O1 | x + 1, +y, +z | 3.235 (2) | 2.18 | 164 |
| II | C11-H5···N3 | x + 1, +y, +z | 3.750 (3) | 2.71 | 161 |
| III | C13 -H3···N3 | x + 1, +y + 1, +z | 3.430 (2) | 2.35 | 175 |
| IV | C16 -H14···O1 | −x, −y, −z + 1 | 3.546 (2) | 2.51 | 159 |
| V | C16 -H13···N1 | −x + 1, −y, −z + 1 | 3.824 (3) | 2.76 | 167 |
| VI | C2-H4···C14(π) | x + 1, y, z | 3.875 (2) | 2.99 | 154 |
| VII | Cg1···Cg1 | −x + 1, −y+1, −z + 1 | 3.901 (2) | − | − |
| VIII | C9-H11···Cg1 | −x + 1, −y + 1,−z + 2 | 3.911 (2) | 3.07 | 149 |
| IX | C16-H15···S1 | x, y − 1, z | 3.790 (1) | 3.08 | 131 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venugopala, K.N.; Khedr, M.A.; Girish, Y.R.; Bhandary, S.; Chopra, D.; Morsy, M.A.; Aldhubiab, B.E.; Deb, P.K.; Attimarad, M.; Nair, A.B.; et al. Crystallography, in Silico Studies, and In Vitro Antifungal Studies of 2,4,5 Trisubstituted 1,2,3-Triazole Analogues. Antibiotics 2020, 9, 350. https://doi.org/10.3390/antibiotics9060350
Venugopala KN, Khedr MA, Girish YR, Bhandary S, Chopra D, Morsy MA, Aldhubiab BE, Deb PK, Attimarad M, Nair AB, et al. Crystallography, in Silico Studies, and In Vitro Antifungal Studies of 2,4,5 Trisubstituted 1,2,3-Triazole Analogues. Antibiotics. 2020; 9(6):350. https://doi.org/10.3390/antibiotics9060350
Chicago/Turabian StyleVenugopala, Katharigatta N., Mohammed A. Khedr, Yarabahally R. Girish, Subhrajyoti Bhandary, Deepak Chopra, Mohamed A. Morsy, Bandar E. Aldhubiab, Pran Kishore Deb, Mahesh Attimarad, Anroop B. Nair, and et al. 2020. "Crystallography, in Silico Studies, and In Vitro Antifungal Studies of 2,4,5 Trisubstituted 1,2,3-Triazole Analogues" Antibiotics 9, no. 6: 350. https://doi.org/10.3390/antibiotics9060350
APA StyleVenugopala, K. N., Khedr, M. A., Girish, Y. R., Bhandary, S., Chopra, D., Morsy, M. A., Aldhubiab, B. E., Deb, P. K., Attimarad, M., Nair, A. B., Sreeharsha, N., V, R., Kandeel, M., Akrawi, S. H., Reddy M B, M., Shashikanth, S., Alwassil, O. I., & Mohanlall, V. (2020). Crystallography, in Silico Studies, and In Vitro Antifungal Studies of 2,4,5 Trisubstituted 1,2,3-Triazole Analogues. Antibiotics, 9(6), 350. https://doi.org/10.3390/antibiotics9060350