A Novel Derivative of Thioridazine Shows Low Toxicity and Efficient Activity against Gram-Positive Pathogens
Abstract
1. Introduction
2. Results
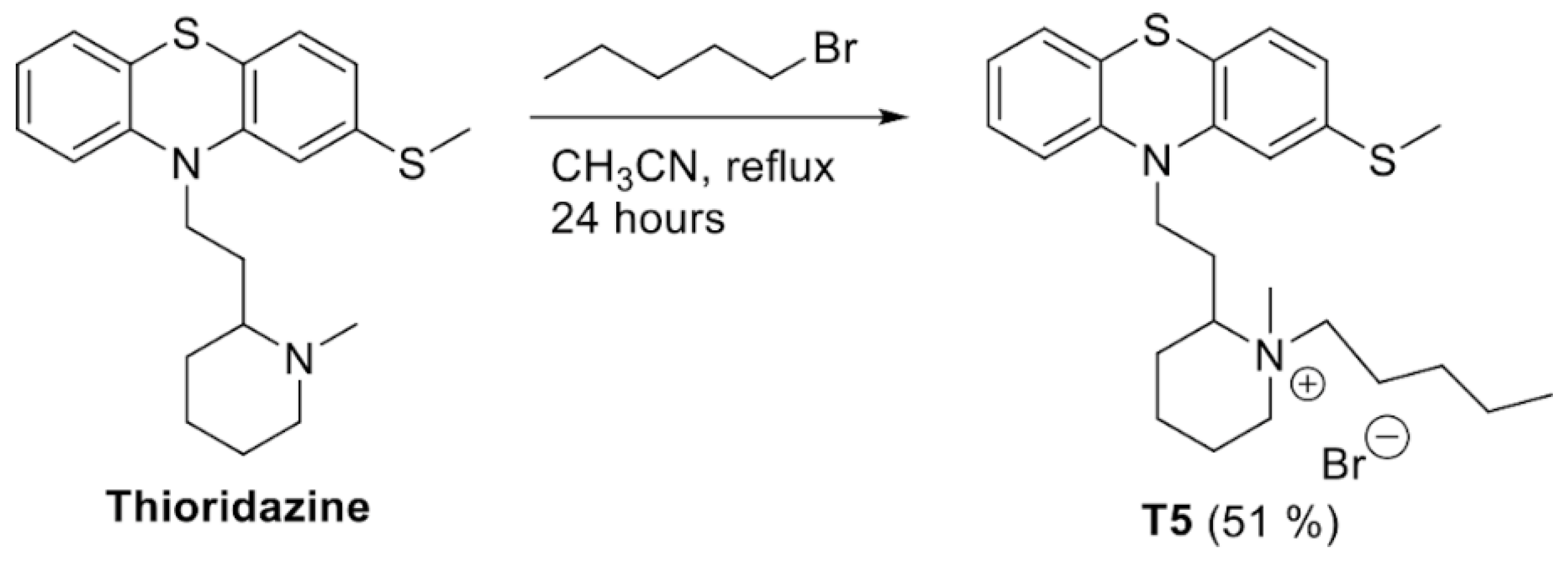
2.1. Structure, Yield, and Chemical Characteristics of T5
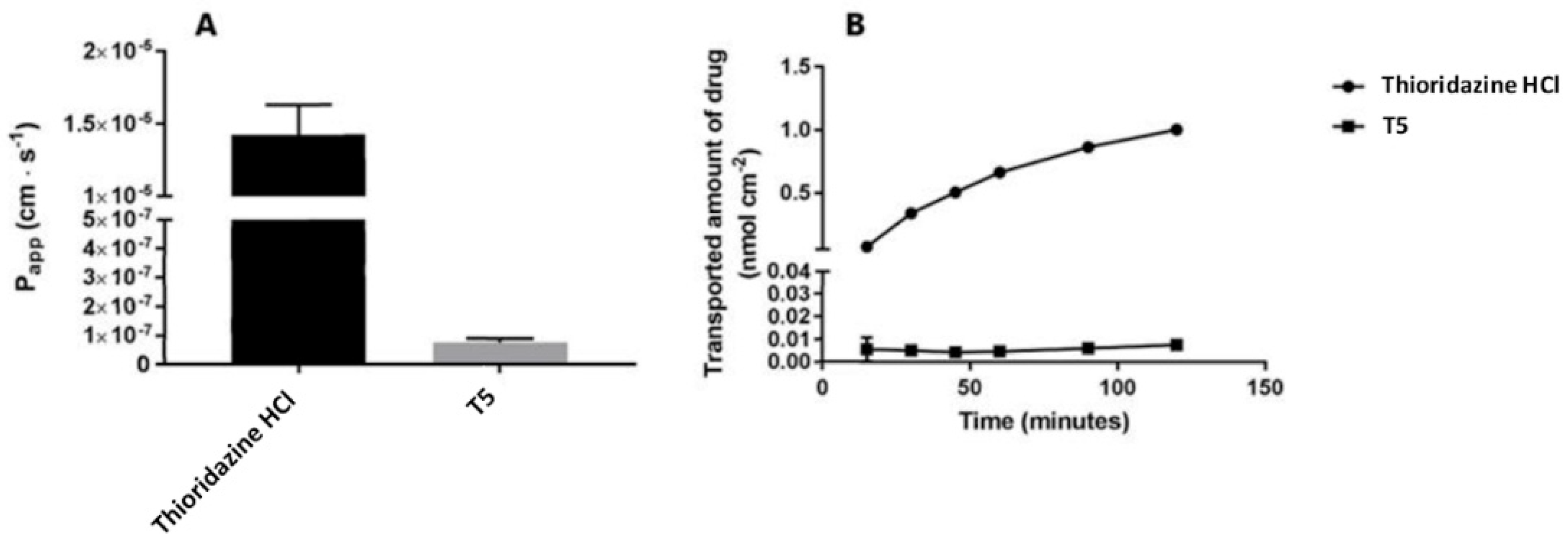
2.2. Cellular Permeability and Cytotoxicity of T5
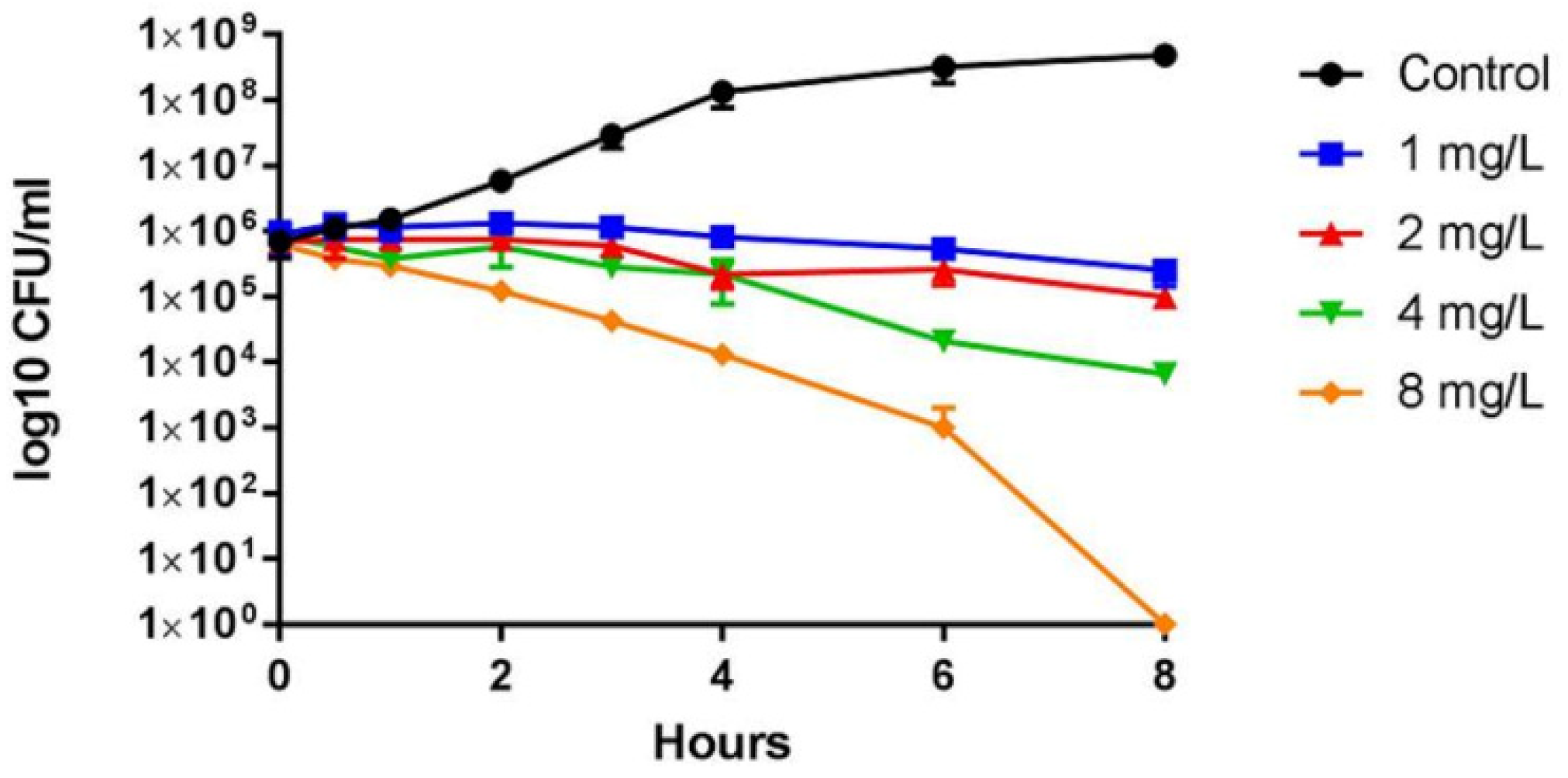
2.3. Antimicrobial Activity of T5
2.4. In Silico Analysis of T5
3. Discussion
4. Materials and Methods
4.1. Synthesis of T5 (1-methyl-2-(2-(2-(methylthio)-10H-phenothiazin-10-yl)ethyl)-1-pentylpiperidin-1-umbromide)
4.2. Transport Assay
4.3. Lactate Dehydrogenase Colorimetric Assay
4.4. Antimicrobial Activity
4.5. Growth and Viability Assays
4.6. Oxacillin Synergy Assessment
4.7. In Silico Analysis of Pharmacokinetics and Toxicity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choo, E.J.; Chambers, H.G. Treatment of Methicillin-Resistant Staphylococcus aureus Bacteremia. Infect. Chemother. 2016, 48, 267–273. [Google Scholar] [CrossRef]
- Coates, A.R.; Halls, G.; Hu, Y. Novel classes of antibiotics or more of the same? Br. J. Pharmacol. 2011, 163, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.C.; Kirchhelle, C.; Roberts, A.P. Reinventing the antimicrobial pipeline in response to the global crisis of antimicrobial-resistant infections. F1000Research 2019, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis: Causes and threats. P. T. J. 2015, 40, 277–283. [Google Scholar]
- Kristiansen, J.E.; Dastidar, S.G.; Palchoudhuri, S.; Roy, D.S.; Das, S.; Hendricks, O.; Christensen, J.B. Phenothiazines as a solution for multidrug resistant tuberculosis: From the origin to present. Int. Microbiol. 2015, 18, 1–12. [Google Scholar]
- Kristiansen, J.E.; Hendricks, O.; Delvin, T.; Butterworth, T.S.; Aagaard, L.; Christensen, J.B.; Flores, V.C.; Keyzer, H. Reversal of resistance in microorganisms by help of non-antibiotics. J. Antimicrob. Chemother. 2007, 59, 1271–1279. [Google Scholar] [CrossRef]
- Amaral, L.; Viveiros, M.; Kristiansen, J. “Non-Antibiotics”: Alternative Therapy for the Management of MDRTB and MRSA in Economically Disadvantaged Countries. Curr. Drug Targets 2006, 7, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, M.M. Phenothiazines alter resistance of methicillin-resistant strains of Staphylococcus aureus (MRSA) to oxacillin in vitro. Int. J. Antimicrob. Agents 2003, 22, 250–253. [Google Scholar] [CrossRef]
- Kristiansen, M.M.; Leandro, C.; Ordway, D.; Martins, M.; Viveiros, M.; Pacheco, T.; Molnár, J.; Kristiansen, J.E.; Amaral, L. Thioridazine reduces resistance of methicillin-resistant staphylococcus aureus by inhibiting a reserpine-sensitive efflux pump. In Vivo 2006, 20, 361–366. [Google Scholar]
- Stenger, M.; Behr-Rasmussen, C.; Klein, K.; Grønnemose, R.B.; Andersen, T.E.; Klitgaard, J.K.; Kolmos, H.J.; Lindholt, J.S. Systemic thioridazine in combination with dicloxacillin against early aortic graft infections caused by Staphylococcus aureus in a porcine model: In vivo results do not reproduce the in vitro synergistic activity. PLoS ONE 2017, 12, e0173362. [Google Scholar] [CrossRef]
- Stenger, M.; Hendel, K.K.; Bollen, P.; Licht, P.B.; Kolmos, H.J.; Klitgaard, J.K. Assessments of Thioridazine as a Helper Compound to Dicloxacillin against Methicillin-Resistant Staphylococcus aureus: In Vivo Trials in a Mouse Peritonitis Model. PLoS ONE 2015, 10, e0135571. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, T.; Cohen, B.M. Blood to brain distribution of neuroleptics. Psychiatry Res. 1987, 20, 299–305. [Google Scholar] [CrossRef]
- Bourquin, J.-P.; Schwarb, G.; Gamboni, G.; Fischer, R.; Ruesch, L.; Guldimann, S. Synthesen auf dem Phenothiazin-Gebiet. Helv. Chim. Acta 1958, 59, 1072–1108. [Google Scholar] [CrossRef]
- Kucerova, G.; Kalíková, K.; Tesařová, E. Enantioselective potential of polysaccharide-based chiral stationary phases in supercritical fluid chromatography. Chirality 2017, 29, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Haggart, D.; Toll, L.; Cuny, G.D. Synthesis, receptor binding and functional studies of mesoridazine stereoisomers. Bioorg. Med. Chem. Lett. 2004, 14, 4379–4382. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.B.; Hendricks, O.; Chaki, S.; Mukherjee, S.; Das, A.; Pal, T.K.; Dastidar, S.G.; Kristiansen, J.E. A comparative Analysis of In Vitro and In Vivo Efficacies of the Enantiomers of Thioridazine and Its Racemate. PLoS ONE 2013, 8, e57493. [Google Scholar] [CrossRef]
- Nehme, H.; Saulnier, P.; Ramadan, A.A.; Cassisa, V.; Guillet, C.; Eveillard, M.; Umerska, A. Antibacterial activity of antipsychotic agents, their association with lipid nanocapsules and its impact on the properties of the nanocarriers and on antibacterial activity. PLoS ONE 2018, 13, e0189950. [Google Scholar] [CrossRef]
- Blaskovich, M.A.; Elliott, A.G.; Kavanagh, A.M.; Ramu, S.; Cooper, M.A. In vitro Antimicrobial Activity of Acne Drugs Against Skin-Associated Bacteria. Sci. Rep. 2019, 9, 14658. [Google Scholar] [CrossRef]
- Ng, V.; Kuehne, S.A.; Chan, W.C. Rational Design and Synthesis of Modified Teixobactin Analogues: In Vitro Antibacterial Activity against Staphylococcus aureus, Propionibacterium acnes and Pseudomonas aeruginosa. Chem.-A Eur. J. 2018, 24, 9136–9147. [Google Scholar] [CrossRef]
- Appelbaum, P.C. The emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 2006, 12, 16–23. [Google Scholar] [CrossRef]
- Pohl, A.; Lübke-Becker, A.; Heuwieser, W. Minimum inhibitory concentrations of frequently used antibiotics against Escherichia coli and Trueperella pyogenes isolated from uteri of postpartum dairy cows. J. Dairy Sci. 2018, 101, 1355–1364. [Google Scholar] [CrossRef]
- Breakpoint Tables for Interpretation of MICs and Zone Diameters. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_10.0_Breakpoint_Tables.pdf (accessed on 1 January 2020).
- Bonde, M.; Højland, D.H.; Kallipolitis, B.H.; Kolmos, H.J.; Klitgaard, J.K. Thioridazine affects transcription of genes involved in cell wall biosynthesis in methicillin-resistant Staphylococcus aureus. FEMS Microbiol. Lett. 2011, 318, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Thorsing, M.; Klitgaard, J.K.; Atilano, M.; Skov, M.N.; Kolmos, H.J.; Filipe, S.R.; Kallipolitis, B.H. Thioridazine Induces Major Changes in Global Gene Expression and Cell Wall Composition in Methicillin-Resistant Staphylococcus aureus USA300. PLoS ONE 2013, 8, e64518. [Google Scholar] [CrossRef]
- French, G.L. Bactericidal agents in the treatment of MRSA infections--the potential role of daptomycin. J. Antimicrob. Chemother. 2006, 58, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef]
- Kvist, M.; Hancock, V.; Klemm, P. Inactivation of Efflux Pumps Abolishes Bacterial Biofilm Formation. Appl. Environ. Microbiol. 2008, 74, 7376–7382. [Google Scholar] [CrossRef]
- Zeitlinger, M.A.; Derendorf, H.; Mouton, J.W.; Cars, O.; Craig, W.A.; Andes, D.; Theuretzbacher, U. Protein binding: Do we ever learn? Antimicrob. Agents. Chemother. 2011, 55, 3067–3074. [Google Scholar] [CrossRef]
- Dutta, P.; Mitra, U.; Dutta, S.; De, A.; Chatterjee, M.K.; Bhattacharya, S.K. Ceftriaxone therapy in ciprofloxacin treatment failure typhoid fever in children. Indian J. Med Res. 2001, 113, 210–213. [Google Scholar]
- Marrella, A.; Buratti, P.; Markus, J.; Firpo, G.; Presenti, M.; Landry, T. In vitro demonstration of intestinal absorption mechanisms of different sugars using 3D organotypic tissues in a fluidic device. ALTEX 2019, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Barakat, K. Development of Safe Drugs: The hERG Challenge. Med. Res. Rev. 2017, 38, 525–555. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.S.; Pennisi, C.P.; Sevcencu, C.; Christensen, J.B.; Kristiansen, J.E.; Struijk, J.J. Differential effects of thioridazine enantiomers on action potential duration in rabbit papillary muscle. Eur. J. Pharmacol. 2015, 747, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Rong, H.; Feng, B. Demystifying Brain Penetration in Central Nervous System Drug Discovery. J. Med. Chem. 2012, 56, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Saaby, L.; Helms, H.C.C.; Brodin, B. IPEC-J2 MDR1, a Novel High-Resistance Cell Line with Functional Expression of Human P-glycoprotein (ABCB1) for Drug Screening Studies. Mol. Pharm. 2016, 13, 640–652. [Google Scholar] [CrossRef] [PubMed]
- PreADMET. Available online: Preadmet.bmdrc.kr (accessed on 28 May 2020).

| Species | Strain | Origin | MIC (µg/mL) | MBC (µg/mL) | |
|---|---|---|---|---|---|
| MH Broth | MH Broth + 20% Human Serum | ||||
| Staphylococcus aureus | JE2 (USA300) | Human clinical isolate | 2 | 16 | 2 |
| Staphylococcus aureus | ATCC BAA-1556 | Human clinical isolate | 2 | 32 | 2 |
| Staphylococcus aureus | CC398 | Veterinary clinical isolate | 1 | 16 | 1 |
| Enterococcus faecalis | 72B6 | Veterinary clinical isolate | 4 | ND | 4 |
| Enterococcus faecium | ATCC 700221 | Human clinical isolate | 8 | ND | 8 |
| Proteus vulgaris | 4663 | Veterinary clinical isolate | 64 | ND | 64 |
| Escherichia coli | E2 | Human clinical isolate | 64 | ND | 64 |
| Escherichia coli | APEC O2 | Veterinary clinical isolate | 32 | ND | 32 |
| Feature | T5 | Thioridazine HCl | Range |
|---|---|---|---|
| Plasma protein binding (%) | 90.9 | 62.5 | >90%: chemicals strongly bound |
| Blood brain barrier penetration (Cbrain/Cblood) | 0.1 | 0.5 | 2.0–0.1: middle absorption to CNS |
| Skin permeability (logKp, cm/hour) | −1.92 | −3.7 | Not defined |
| Caco-2 cell permeability | 21.78 | 31.9 | 4–70: middle permeability |
| Human intestinal absorption (%) | 98.26 | 94.4 | 70–100%: well-absorbed compounds |
| Ames test (TA100) | Negative | Positive | - |
| Carcinogenicity | Negative | Positive | - |
| hERG inhibition | Medium risk | High risk | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jørgensen, N.S.; Saaby, L.; Andersson, A.M.; Kromann, S.; Sheikhsamani, E.; Permin, A.; Ronco, T.; Svenningsen, S.W.; Christensen, J.B.; Olsen, R.H. A Novel Derivative of Thioridazine Shows Low Toxicity and Efficient Activity against Gram-Positive Pathogens. Antibiotics 2020, 9, 327. https://doi.org/10.3390/antibiotics9060327
Jørgensen NS, Saaby L, Andersson AM, Kromann S, Sheikhsamani E, Permin A, Ronco T, Svenningsen SW, Christensen JB, Olsen RH. A Novel Derivative of Thioridazine Shows Low Toxicity and Efficient Activity against Gram-Positive Pathogens. Antibiotics. 2020; 9(6):327. https://doi.org/10.3390/antibiotics9060327
Chicago/Turabian StyleJørgensen, Nadia S., Lasse Saaby, Anne M. Andersson, Sofie Kromann, Ehsan Sheikhsamani, Anders Permin, Troels Ronco, Søren W. Svenningsen, Jørn B. Christensen, and Rikke H. Olsen. 2020. "A Novel Derivative of Thioridazine Shows Low Toxicity and Efficient Activity against Gram-Positive Pathogens" Antibiotics 9, no. 6: 327. https://doi.org/10.3390/antibiotics9060327
APA StyleJørgensen, N. S., Saaby, L., Andersson, A. M., Kromann, S., Sheikhsamani, E., Permin, A., Ronco, T., Svenningsen, S. W., Christensen, J. B., & Olsen, R. H. (2020). A Novel Derivative of Thioridazine Shows Low Toxicity and Efficient Activity against Gram-Positive Pathogens. Antibiotics, 9(6), 327. https://doi.org/10.3390/antibiotics9060327





