A Silkworm Infection Model for In Vivo Study of Glycopeptide Antibiotics
Abstract
1. Introduction
2. Results
2.1. Rearing of B. mori Larvae at 37 °C
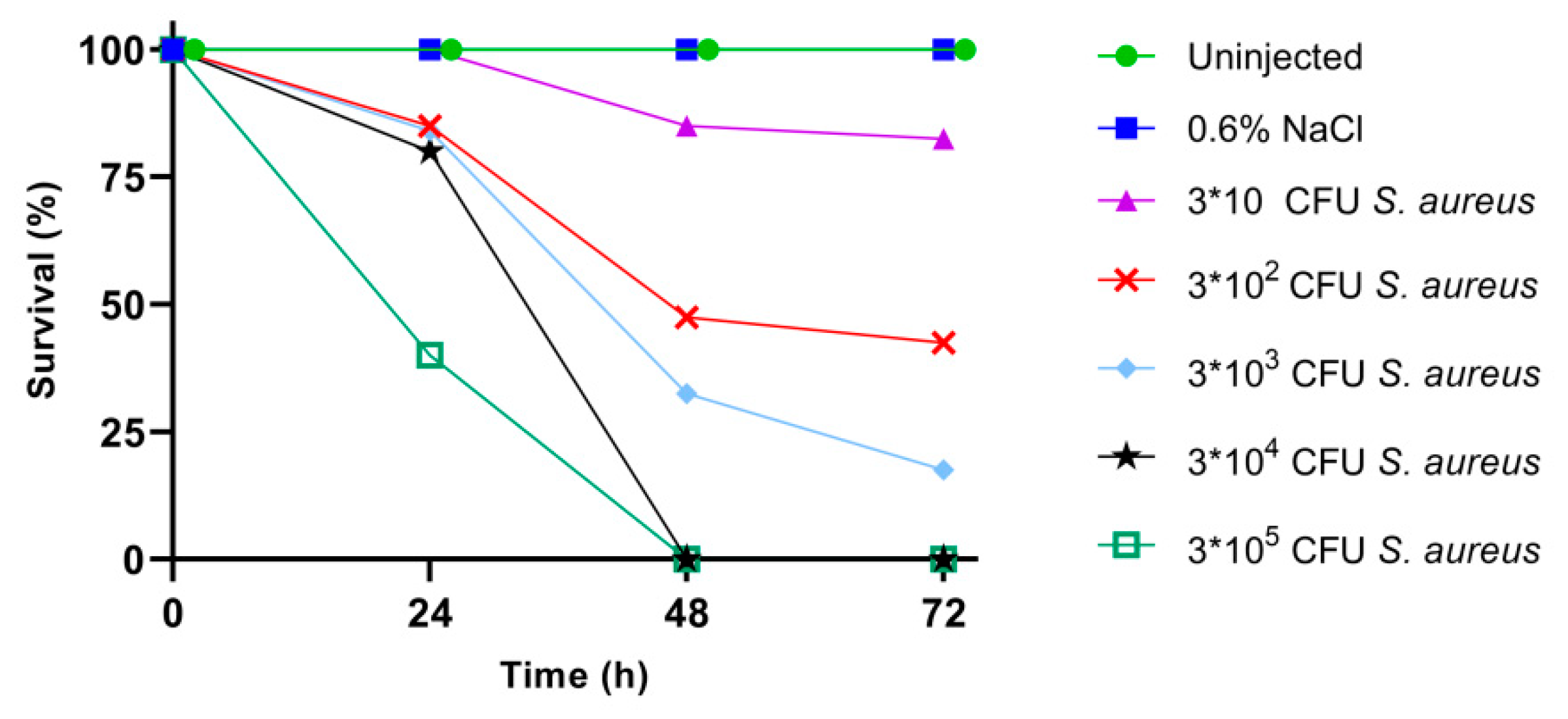
2.2. Larval Survival after S. aureus Infection
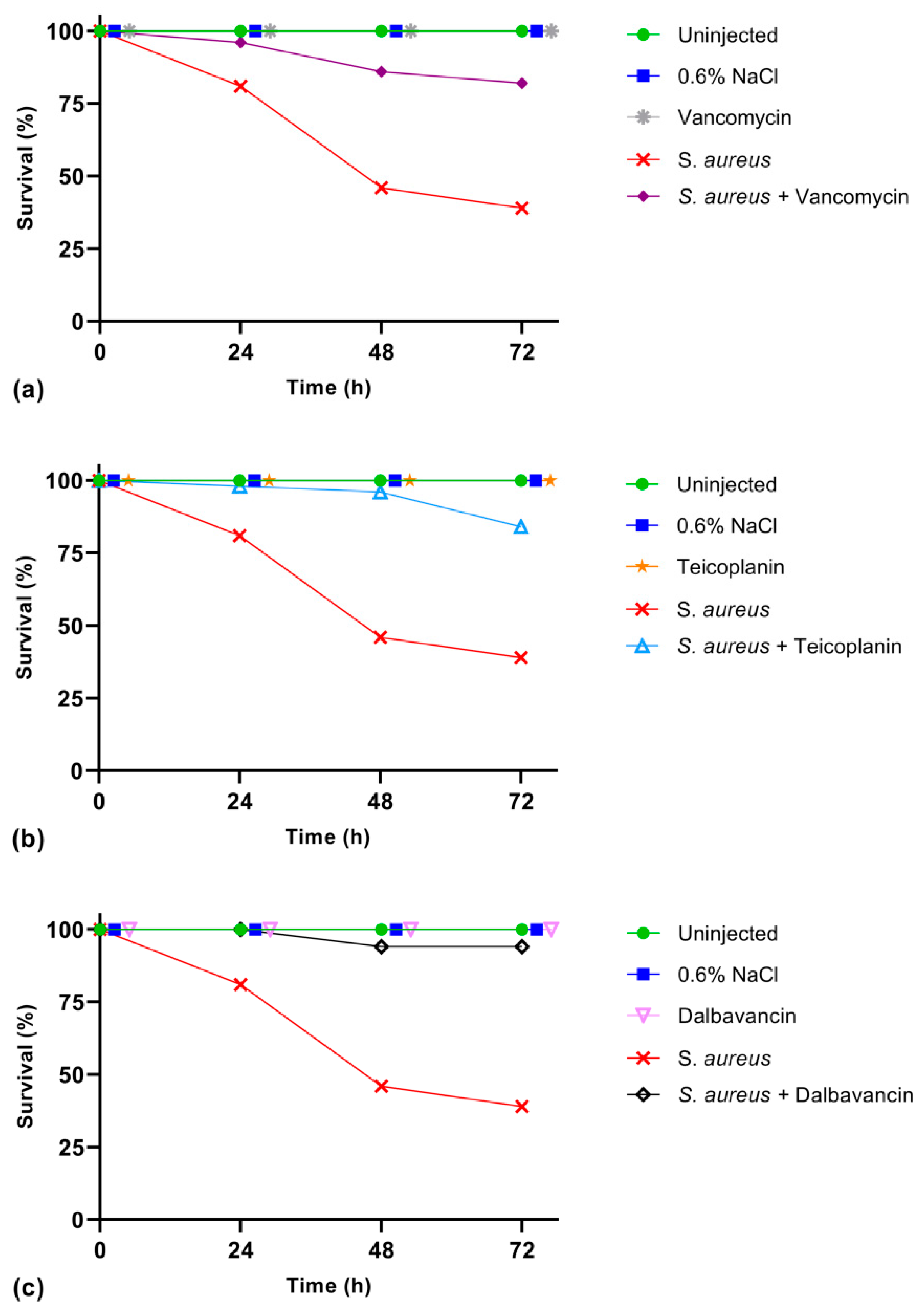
2.3. Cure of Infected Larvae by GPA Administration
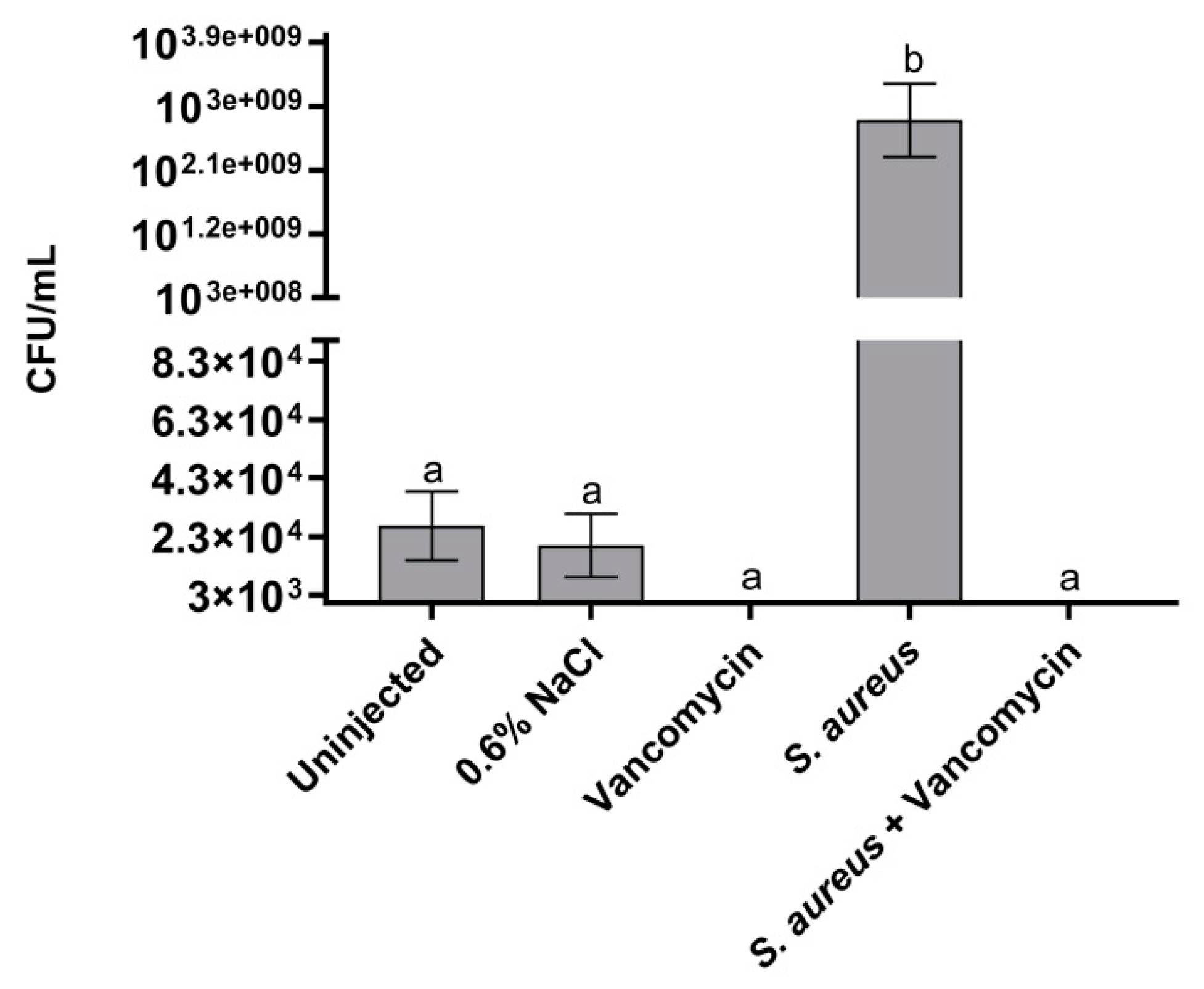
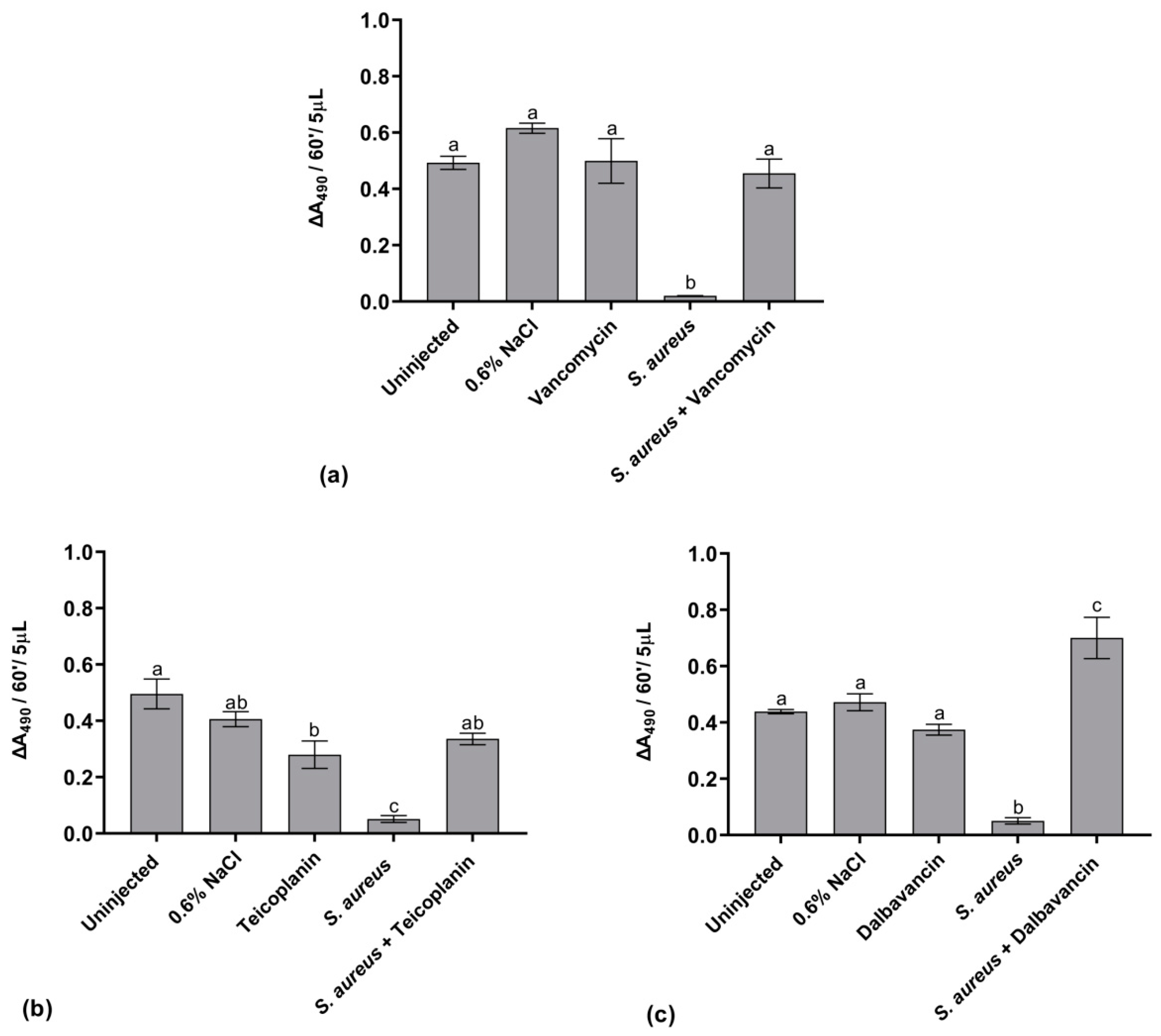
2.4. GPA Effect on Bacterial Load in the Larvae
2.5. Immunological Markers of Infection
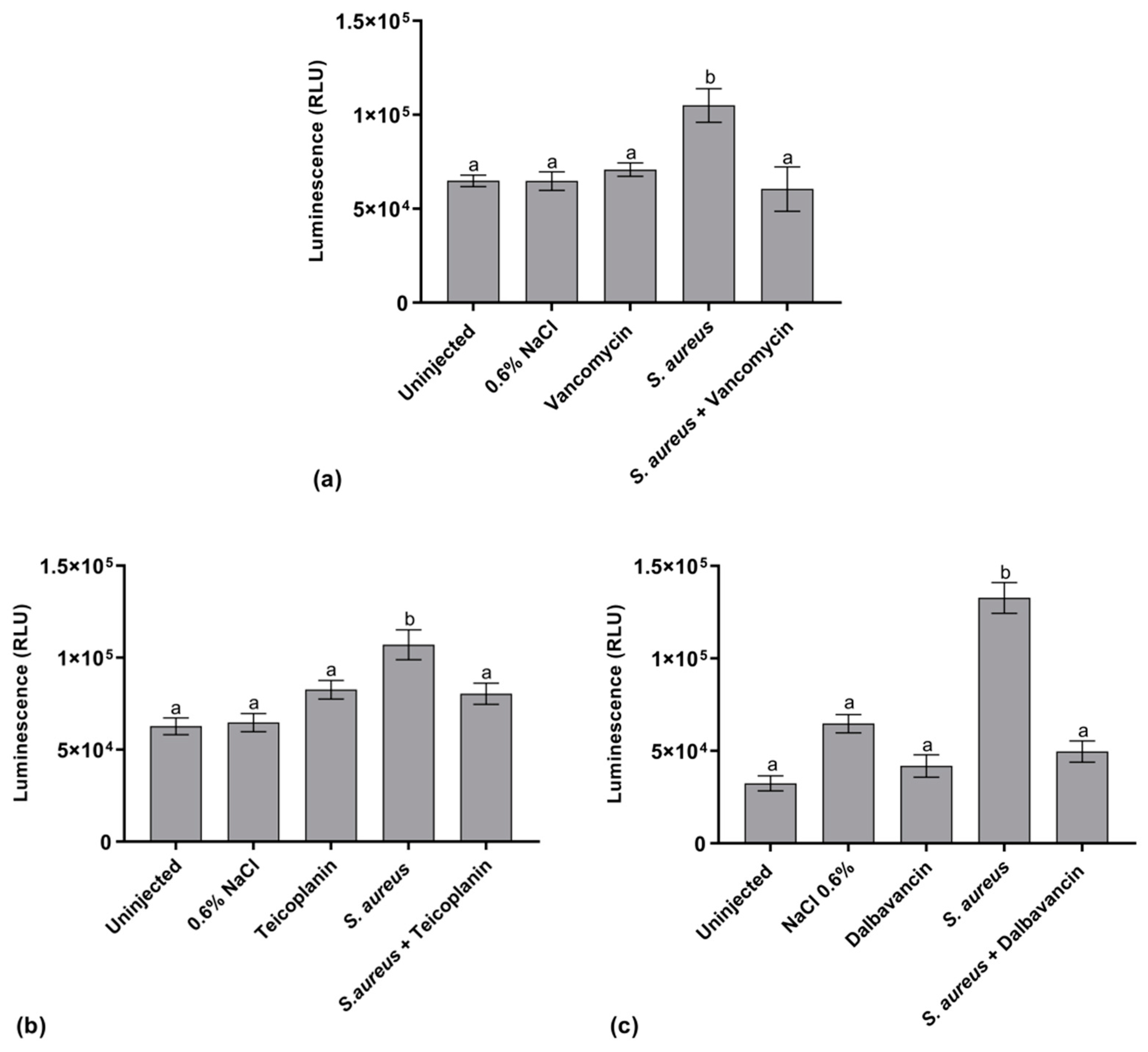
2.5.1. Hemocyte Activity
2.5.2. AMP Expression
2.5.3. Activation of the proPO System
3. Discussion
4. Materials and Methods
4.1. Experimental Model
4.2. B. mori Rearing at 37 °C
4.3. Bacterial Strains and Culture Conditions
4.4. Minimum Inhibitory Concentrations (MICs) and Minimum Bactericidal Concentrations (MBCs)
4.5. Injection of Larvae and Collection of Hemolymph
4.6. Determination of Lethal Dose 50 (LD50) for S. aureus
4.7. GPA Administration
4.7.1. Hemocyte Activity
4.7.2. AMP Expression
4.7.3. Activation of Prophenoloxidase System
4.7.4. Bacterial Load
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kållberg, C.; Årdal, C.; Salvesen Blix, H.; Klein, E.; Martinez, E.; Lindbæk, M.; Outterson, K.; Røttingen, J.A.; Laxminarayan, R. Introduction and geographic availability of new antibiotics approved between 1999 and 2014. PLoS ONE 2018, 13, e0205166. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Renwick, M.; Mossialos, E. What are the economic barriers of antibiotics R&D and how we can overcome them? Expert Opin. Drug Discov. 2018, 13, 889–892. [Google Scholar] [CrossRef]
- Paudel, A.; Panthee, S.; Urai, M.; Hamamoto, H.; Ohwada, T.; Sekimizu, K. Pharmacokinetic parameters explain the therapeutic activity of antimicrobial agents in a silkworm infection model. Sci. Rep. 2018, 8, 1578. [Google Scholar] [CrossRef] [PubMed]
- Freires, I.A.; Sardi, O.; de Castro, R.D.; Rosalen, P.L. Alternative animal and non-animal models for drug discovery and development: Bonus or burden? Pharm. Res. 2017, 34, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Ségalat, L. Invertebrate animal models of diseases as screening tools in drug discovery. ACS Chem. Biol. 2007, 2, 231–236. [Google Scholar] [CrossRef]
- Buchmann, K. Evolution of innate immunity: Clues from invertebrates via fish to mammals. Front. Immunol. 2014, 5, 459. [Google Scholar] [CrossRef]
- Tsai, C.J.; Loh, J.M.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef]
- Mistry, R.; Kounatidis, I.; Ligoxygakis, P. Exploring interactions between pathogens and the Drosophila gut. Dev. Comp. Immunol. 2016, 64, 3–10. [Google Scholar] [CrossRef]
- Kausar, S.; Abbas, M.N.; Zhao, Z.; Cui, H. Immune strategies of silkworm, Bombyx mori against microbial infections. Invert. Surviv. J. 2019, 16, 130–140. [Google Scholar]
- D’Angelo, F.; Baldelli, V.; Halliday, N.; Pantalone, P.; Polticelli, F.; Fiscarelli, E.; Williams, P.; Visca, P.; Leoni, L.; Rampioni, G. Identification of FDA-approved drugs as antivirulence agents targeting the pqs quorum-sensing system of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2018, 62, e01296-18. [Google Scholar] [CrossRef]
- Panthee, S.; Paudel, A.; Hamamoto, H.; Sekimizu, K. Advantages of the silkworm as an animal model for developing novel antimicrobial agents. Front. Microbiol. 2017, 8, 373. [Google Scholar] [CrossRef] [PubMed]
- Sekimizu, K.; Hamamoto, H. Using silkworms as a laboratory animal to evaluate medicines and foods. Drug Discov. Ther. 2016, 10, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Binda, E.; Marinelli, F.; Marcone, G.L. Old and new glycopeptide antibiotics: Action and resistance. Antibiotics 2014, 3, 572–594. [Google Scholar] [CrossRef] [PubMed]
- Marcone, G.L.; Binda, E.; Berini, F.; Marinelli, F. Old and new glycopeptide antibiotics: From product to gene and back in the post-genomic era. Biotechnol. Adv. 2018, 36, 534–554. [Google Scholar] [CrossRef]
- Rossolini, G.M.; Arena, F.; Pecile, P.; Pollini, S. Update on the antibiotic resistance crisis. Curr. Opin. Pharmacol. 2014, 18, 56–60. [Google Scholar] [CrossRef]
- Stogios, P.J.; Savchenko, A. Molecular mechanisms of vancomycin resistance. Protein Sci. 2020, 29, 654–669. [Google Scholar] [CrossRef]
- Dalmastri, C.; Gastaldo, L.; Marcone, G.L.; Binda, E.; Congiu, T.; Marinelli, F. Classification of Nonomuraea sp. ATCC 39727, an actinomycete that produces the glycopeptide antibiotic A40926, as Nonomuraea gerenzanensis sp. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 912–921. [Google Scholar] [CrossRef]
- Soriano, A.; Rossolini, G.M.; Pea, F. The role of dalbavacin in the treatment of acute bacterial skin and skin structure infections (ABSSSIs). Expert Rev. Anti. Infect. Ther. 2020, 29, 1–8. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T.; Hansford, K.A.; Butler, M.S.; Jia, Z.G.; Mark, A.E.; Cooper, M.A. Developments in glycopeptide antibiotics. ACS Infect. Dis. 2018, 4, 715–735. [Google Scholar] [CrossRef]
- Marshall, E.; Cryle, M.J.; Tailhades, J. Biological, chemical, and biochemical strategies for modifying glycopeptide antibiotics. J. Biol. Chem. 2019, 294, 18769–18783. [Google Scholar] [CrossRef] [PubMed]
- Treviño, J.; Bayón, C.; Ardá, A.; Marinelli, F.; Gandolfi, R.; Molinari, F.; Jimenez-Barbero, J.; Hernáiz, M.J. New insights into glycopeptide antibiotic binding to cell wall precursors using SPR and NMR spectroscopy. Chem. Eur. J. 2014, 20, 7363–7372. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Yarlagadda, V.; Ghosh, C.; Haldar, J. A review on cell wall synthesis inhibitors with an emphasis on glycopeptide antibiotics. Med. Chem. Commun. 2017, 8, 516–533. [Google Scholar] [CrossRef] [PubMed]
- Yarlagadda, V.; Akkapeddi, P.; Manjunath, G.B.; Haldar, J. Membrane active vancomycin analogues: A strategy to combat bacterial resistance. J. Med. Chem. 2014, 57, 4558–4568. [Google Scholar] [CrossRef]
- Szűcs, Z.; Ostorházi, E.; Kicsák, M.; Nagy, L.; Borbás, A.; Herczegh, P. New semisynthetic teicoplanin derivatives have comparable in vitro activity to that of oritavancin against clinical isolates of VRE. J. Antibiot. (Tokyo) 2019, 72, 524–534. [Google Scholar] [CrossRef]
- Zeng, P.; Xu, C.; Liu, C.; Liu, J.; Cheng, Q.; Gao, W.; Yang, X.; Chen, S.; Chan, K.F.; Wong, K.Y. De novo designed hexadecapeptides synergize glycopeptide antibiotics vancomycin and teicoplanin against pathogenic Klebsiella pneumoniae via disruption of cell permeability and potential. ACS Appl. Bio. Mater. 2020, 3, 1738–1752. [Google Scholar] [CrossRef]
- Vimberg, V.; Gazak, R.; Szűcs, Z.; Borbás, A.; Herczegh, P.; Cavanagh, J.P.; Zieglerova, L.; Závora, J.; Adámková, V.; Balikova Novotna, G. Fluorescence assay to predict activity of the glycopeptide antibiotics. J. Antibiot. 2019, 72, 114–117. [Google Scholar] [CrossRef]
- Cappellozza, L.; Cappellozza, S.; Saviane, A.; Sbrenna, G. Artificial diet rearing system for the silkworm Bombyx mori (Lepidoptera: Bombycidae): Effect of vitamin C deprivation on larval growth and cocoon production. Appl. Entomol. Zool. 2005, 40, 405–412. [Google Scholar] [CrossRef]
- Lodise, T.P.; Lomaestro, B.; Graves, J.; Drusano, G.L. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob. Agents Chemother. 2008, 52, 1330–1336. [Google Scholar] [CrossRef]
- Candiani, G.; Abbondi, M.; Borgonovi, M.; Romanò, G.; Parenti, F. In-vitro and in-vivo antibacterial activity of BI 397, a new semi-synthetic glycopeptide antibiotic. J. Antimicrob. Chemother. 1999, 44, 179–192. [Google Scholar] [CrossRef][Green Version]
- Billeter, M.; Zervos, M.J.; Chen, A.Y.; Dalovisio, J.R.; Kurukularatne, C. Dalbavacin: A novel once-weekly lipoglycopeptide antibiotic. Clin. Infect. Dis. 2008, 46, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Blow, F.; Douglas, A.E. The hemolymph microbiome of insects. J. Insect. Physiol. 2019, 115, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Tsakas, S.; Marmaras, V.J. Insect immunity and its signalling: An overview. Invert. Surviv. J. 2010, 7, 228–238. [Google Scholar]
- Tan, J.; Xu, M.; Zhang, K.; Wang, X.; Chen, S.; Li, T.; Xiang, Z.; Cui, H. Characterization of hemocytes proliferation in larval silkworm, Bombyx mori. J. Insect Physiol. 2013, 59, 595–603. [Google Scholar] [CrossRef]
- Brady, D.; Grapputo, A.; Romoli, O.; Sandrelli, F. Insect cecropins, antimicrobial peptides with potential therapeutic applications. Int. J. Mol. Sci. 2019, 20, 5862. [Google Scholar] [CrossRef]
- Romoli, O.; Saviane, A.; Bozzato, A.; D’Antona, P.; Tettamanti, G.; Squartini, A.; Cappellozza, S.; Sandrelli, F. Differential sensitivity to infections and antimicrobial peptide-mediated immune response in four silkworm strains with different geographical origin. Sci. Rep. 2017, 7, 1048. [Google Scholar] [CrossRef]
- Sugumaran, M.; Kanost, M. Regulation of insect hemolymph phenoloxidases. In Parasites and Pathogens; Beckage, N.E., Thompson, S.N., Frederick, B.A., Eds.; Academic Press: San Diego, CA, USA, 1993; Volume I, pp. 317–342. [Google Scholar] [CrossRef]
- Huang, W.; Ling, S.; Li, C.; Omenetto, F.G.; Kaplan, D.L. Silkworm silk-based materials and devices generated using bio-nanotechnology. Chem. Soc. Rev. 2018, 47, 6486–6504. [Google Scholar] [CrossRef]
- Tanaka, H.; Yamakawa, M. Regulation of the innate immune responses in the silkworm, Bombyx mori. Invert. Surviv. J. 2011, 8, 59–69. [Google Scholar]
- Yang, Y.H.; Tang, H.H.; Zhang, Y.Y.; Zhu, F.F.; Lu, P.; Yao, Q.; Chen, K.P. Research progress on the immune mechanism of the silkworm Bombyx mori. Physiologic. Entomol. 2018, 43, 159–168. [Google Scholar] [CrossRef]
- Kaito, C.; Akimitsu, N.; Watanabe, H.; Sekimizu, K. Silkworm larvae as an animal model of bacterial infection pathogenic to humans. Microb. Pathog. 2002, 32, 183–190. [Google Scholar] [CrossRef]
- Hamamoto, H.; Kurokawa, K.; Kaito, C.; Kamura, K.; Manitra Razanajatovo, I.; Kasuhara, H.; Santa, T.; Sekimizu, K. Quantitative evaluation of the therapeutic effects of antibiotics using silkworms infected with human pathogenic microorganisms. Antimicrob. Agents Chemother. 2004, 48, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Nishida, S.; Ono, Y.; Sekimizu, K. Lactic acid bacteria activating innate immunity improve survival in bacterial infection model of silkworm. Drug Discov. Ther. 2016, 10, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, F.; Matsumoto, Y.; Ishii, M.; Tatsuno, K.; Okazaki, M.; Sato, T.; Moriya, K.; Sekimizu, K. Synergistic effects of vancomycin and β-lactams against vancomycin highly resistant Staphylococcus aureus. J. Antibiot. 2017, 70, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Miyazaki, S.; Fukunaga, D.H.; Shimizu, K.; Kawamoto, S.; Sekimizu, K. Quantitative evaluation of cryptococcal pathogenesis and antifungal drugs using a silkworm infection model with Cryptococcus neoformans. J. Appl. Microbiol. 2012, 112, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, K.; Hamamoto, H.; Matsuo, M.; Nishida, S.; Yamane, N.; Lee, B.L.; Murakami, K.; Maki, H.; Sekimizu, K. Evaluation of target specificity of antibacterial agents using Staphylococcus aureus ddlA mutants and D-cycloserine in a silkworm infection model. Antimicrob. Agents Chemother. 2009, 53, 4025–4027. [Google Scholar] [CrossRef] [PubMed]
- Cutuli, M.A.; Petronio Petronio, G.; Vergalito, F.; Magnifico, I.; Pietrangelo, L.; Venditti, N.; Di Marco, R. Galleria mellonella as a consolidated in vivo model hosts: New developments in antibacterial strategies and novel drug testing. Virulence 2019, 10, 527–541. [Google Scholar] [CrossRef]
- Kato, M.; Nagayasu, K.; Hara, W.; Osamu, N. Effect of exposure of the silkworm, Bombyx mori, to high temperature on survival rate and cocoon characters. Jpn. Agric. Res. Q. 1998, 32, 61–64. [Google Scholar]
- Dong, H.L.; Zhang, S.X.; Chen, Z.H.; Tao, H.; Li, X.; Qiu, J.F.; Cui, W.Z.; Sima, Y.H.; Cui, W.Z.; Xu, S.Q. Differences in gut microbiota between silkworms (Bombyx mori) reared on fresh mulberry (Morus alba var. multicaulis) leaves or an artificial diet. RSC Adv. 2018, 8, 26188. [Google Scholar] [CrossRef]
- Sun, Z.; Kumar, D.; Cao, G.; Zhu, L.; Liu, B.; Zhu, M.; Liang, Z.; Kuang, S.; Chen, F.; Feng, Y.; et al. Effects of transient high temperature treatment on the intestinal flora of the silkworm Bombyx mori. Sci. Rep. 2017, 7, 3349. [Google Scholar] [CrossRef]
- Raad, I.; Darouiche, R.; Vazquez, J.; Lentnek, A.; Hachem, R.; Hanna, H.; Goldstein, B.; Henkel, T.; Seltzer, E. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by Gram-positive pathogens. Clin. Infec. Dis. 2005, 40, 374–380. [Google Scholar] [CrossRef]
- Shu, M.; Mang, D.; Sun Fu, G.; Tanaka, S.; Endo, H.; Kikuta, S.; Sato, R. Mechanisms of nodule-specific melanization in the hemocoel of the silkworm, Bombyx mori. Insect Biochem. Molec. Biol. 2016, 70, 10–23. [Google Scholar] [CrossRef] [PubMed]
- de Lerma Barbaro, A.; Gariboldi, M.B.; Mastore, M.; Brivio, M.F.; Giovannardi, S. In vivo effects of a pro-po system inhibitor on the phagocytosis of Xenorhabdus nematophila in Galleria mellonella larvae. Insects 2019, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.R.; Xiao, Y.; Wu, F.Q.; Ye, M.Q.; Luo, G.Q.; Xing, D.X.; Li, L.; Yang, Q. Analysis of midgut gene expression profiles from different silkworm varieties after exposure to high temperature. Gene 2014, 549, 85–96. [Google Scholar] [CrossRef]
- Franzetti, E.; Romanelli, D.; Caccia, S.; Cappellozza, S.; Congiu, T.; Rajagopalan, M.; Grimaldi, A.; de Eguileor, M.; Casartelli, M.; Tettamanti, G. The midgut of the silkmoth Bombyx mori is able to recycle molecules derived from degeneration of the larval midgut epithelium. Cell Tissue Res. 2015, 361, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Casati, B.; Terova, G.; Cattaneo, A.G.; Rimoldi, S.; Franzetti, E.; de Eguileor, M.; Tettamanti, G. Molecular cloning, characterization and expression analysis of ATG1 in the silkworm, Bombyx mori. Gene 2012, 511, 326–337. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Romanelli, D.; Casartelli, M.; Cappellozza, S.; de Eguileor, M.; Tettamanti, G. Roles and regulation of autophagy and apoptosis in the remodelling of the lepidopteran midgut epithelium during metamorphosis. Sci. Rep. 2016, 6, 32939. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Uda, A.; Watanabe, K.; Shimizu, T.; Watarai, M. Symbiosis with Francisella tularensis provides resistance to pathogens in the silkworm. Sci. Rep. 2016, 6, 31476. [Google Scholar] [CrossRef] [PubMed]


| Antibiotic | MIC (μg/mL) | MBC (μg/mL) |
|---|---|---|
| Vancomycin | 1 | >128 |
| Teicoplanin | 1 | 128 |
| Dalbavancin | 0.5 | 16 |
| Gene | Accession Number | Primer Sequences |
|---|---|---|
| BmRP49 | NM_001098282.1 | F: AGGCATCAATCGGATCGCTATG R: TTGTGAACTAGGACCTTACGGAATC |
| BmCECE | DQ233467.1 | F: GTGTGTGCGAGCGTTATGGC R: CCCATGAGCGATGGTCGCC |
| BmCECB1 | BGIBMGA000024-RA | F: TTCGCTCTGGTGCTGGCTTTG R: GGCCCGCTTTGACGATGCC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montali, A.; Berini, F.; Brivio, M.F.; Mastore, M.; Saviane, A.; Cappellozza, S.; Marinelli, F.; Tettamanti, G. A Silkworm Infection Model for In Vivo Study of Glycopeptide Antibiotics. Antibiotics 2020, 9, 300. https://doi.org/10.3390/antibiotics9060300
Montali A, Berini F, Brivio MF, Mastore M, Saviane A, Cappellozza S, Marinelli F, Tettamanti G. A Silkworm Infection Model for In Vivo Study of Glycopeptide Antibiotics. Antibiotics. 2020; 9(6):300. https://doi.org/10.3390/antibiotics9060300
Chicago/Turabian StyleMontali, Aurora, Francesca Berini, Maurizio Francesco Brivio, Maristella Mastore, Alessio Saviane, Silvia Cappellozza, Flavia Marinelli, and Gianluca Tettamanti. 2020. "A Silkworm Infection Model for In Vivo Study of Glycopeptide Antibiotics" Antibiotics 9, no. 6: 300. https://doi.org/10.3390/antibiotics9060300
APA StyleMontali, A., Berini, F., Brivio, M. F., Mastore, M., Saviane, A., Cappellozza, S., Marinelli, F., & Tettamanti, G. (2020). A Silkworm Infection Model for In Vivo Study of Glycopeptide Antibiotics. Antibiotics, 9(6), 300. https://doi.org/10.3390/antibiotics9060300













