Discontinuation of Glycopeptides in Patients with Culture Negative Severe Sepsis or Septic Shock: A Propensity-Matched Retrospective Cohort Study
Abstract
1. Introduction
2. Results
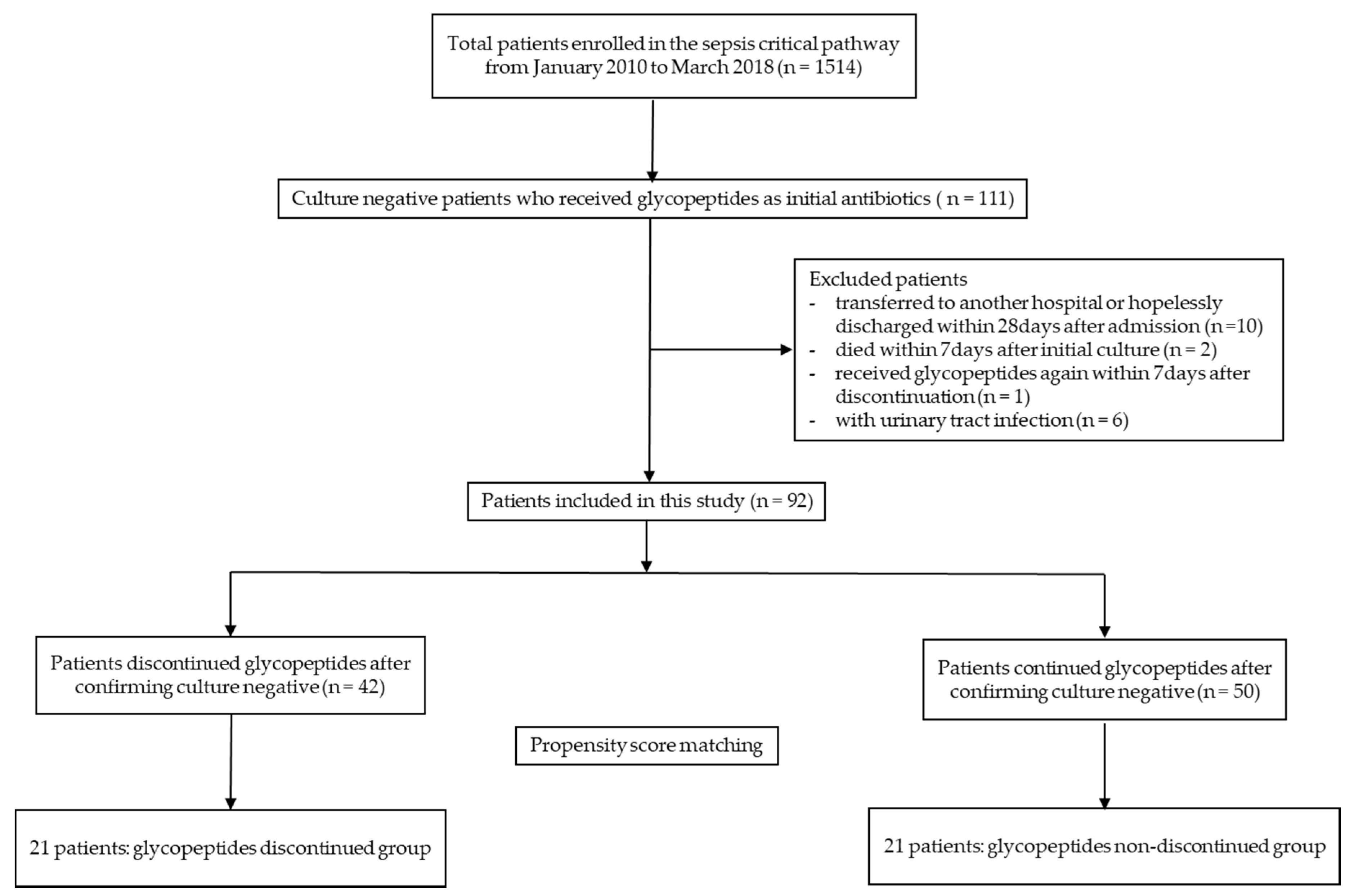
2.1. Study Population
2.2. Characteristics of Patients Before and After Propensity Score Matching
2.3. Outcomes
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Data Collection and Definition
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Rochwerg, B. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Baur, D.; Gladstone, B.P.; Burkert, F.; Carrara, E.; Foschi, F.; Döbele, S.; Tacconelli, E. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: A systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 990–1001. [Google Scholar] [CrossRef]
- Tabah, A.; Bassetti, M.; Kollef, M.H.; Zahar, J.R.; Paiva, J.A.; Timsit, J.F.; De Waele, J. Antimicrobial de-escalation in critically ill patients: A position statement from a task force of the European Society of Intensive Care Medicine (ESICM) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Critically Ill Patients Study Group (ESGCIP). Intensive Care Med. 2020, 46, 245–265. [Google Scholar] [PubMed]
- Leone, M.; Bechis, C.; Baumstarck, K.; Lefrant, J.Y.; Albanèse, J.; Jaber, S.; Allaouchiche, B. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: A multicenter non-blinded randomized noninferiority trial. Intensive Care Med. 2014, 40, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Garnacho-Montero, J.; Gutiérrez-Pizarraya, A.; Escoresca-Ortega, A.; Corcia-Palomo, Y.; Fernandez-Delgado, E.; Herrera-Melero, I.; Marquez-Vacaro, J.A. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014, 40, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Sadyrbaeva-Dolgova, S.; Aznarte-Padial, P.; Pasquau-Liaño, J.; Expósito-Ruiz, M.; Hernández, M.Á.C.; Hidalgo-Tenorio, C. Clinical outcomes of carbapenem de-escalation regardless of microbiological results: A propensity score analysis. Int. J. Infect. Dis. 2019, 85, 80–87. [Google Scholar] [CrossRef]
- Kim, D.; Ahn, J.Y.; Lee, C.H.; Jang, S.J.; Lee, H.; Yong, D.; Lee, K. Increasing Resistance to Extended-Spectrum Cephalosporins, Fluoroquinolone, and Carbapenem in Gram-Negative Bacilli and the Emergence of Carbapenem Non-Susceptibility in Klebsiella pneumoniae: Analysis of Korean Antimicrobial Resistance Monitoring System (KARMS) Data From 2013 to 2015. Ann. Lab. Med. 2017, 37, 231–239. [Google Scholar]
- Appelbaum, P.C. The emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 2006, 12 (Suppl. 1), 16–23. [Google Scholar] [CrossRef]
- Cowley, M.C.; Ritchie, D.J.; Hampton, N.; Kollef, M.H.; Micek, S.T. Outcomes Associated With De-escalating Therapy for Methicillin-Resistant Staphylococcus aureus in Culture-Negative Nosocomial Pneumonia. Chest 2019, 155, 53–59. [Google Scholar] [CrossRef]
- Bamgbola, O. Review of vancomycin-induced renal toxicity: An update. Ther. Adv. Endocrinol. Metab. 2016, 7, 136–147. [Google Scholar] [CrossRef]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Soper, D.E. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef] [PubMed]
- Looney, A.T.; Redmond, E.J.; Davey, N.M.; Daly, P.J.; Troy, C.; Carey, B.F.; Cullen, I.M. Methicillin-resistant Staphylococcus aureus as a uropathogen in an Irish setting. Medicine (Baltimore) 2017, 96, e4635. [Google Scholar] [CrossRef]
- Suetrong, B.; Walley, K.R. Lactic Acidosis in Sepsis: It’s Not All Anaerobic: Implications for Diagnosis and Management. Chest 2016, 149, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Song, J.E.; Kim, E.J.; Choi, H.; Jeong, W.Y.; Jung, I.Y.; Kim, J.M. A Simple Scoring System Using the Red Blood Cell Distribution Width, Delta Neutrophil Index, and Platelet Count to Predict Mortality in Patients With Severe Sepsis and Septic Shock. J. Intensive Care Med. 2018, 34, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Joung, K.M.; Lee, J.A.; Moon, S.Y.; Cheong, H.S.; Joo, E.J.; Ha, Y.E.; Song, J.H. Impact of de-escalation therapy on clinical outcomes for intensive care unit-acquired pneumonia. Crit. Care 2011, 15, R79. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.M.; Drozd, M.; Hall, M.; Patel, P.A.; Paton, M.; Lowry, J.; Witte, K.K. Prevalence and Predictors of Sepsis Death in Patients with Chronic Heart Failure and Reduced Left Ventricular Ejection Fraction. J. Am. Heart Assoc. 2018, 7, e009684. [Google Scholar] [CrossRef] [PubMed]
- Soo, K.B.; Ho, C.S.; Younsuck, K.; Jin-Won, H.; Sang-Bum, H.; Chae-Man, L. Safety of antimicrobial de-escalation for culture-negative severe pneumonia. J. Crit. Care 2019, 54, 14–19. [Google Scholar]
- Li, H.; Yang, C.H.; Huang, L.O.; Cui, Y.H.; Xu, D.; Wu, C.R.; Tang, J.G. Antibiotics De-Escalation in the Treatment of Ventilator-Associated Pneumonia in Trauma Patients: A Retrospective Study on Propensity Score Matching Method. Chin. Med. J. (Engl) 2018, 131, 1151–1157. [Google Scholar] [CrossRef]
- Gonzalez, L.; Cravoisy, A.; Barraud, D.; Conrad, M.; Nace, L.; Lemarié, J.; Gibot, S. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit. Care 2013, 17, R140. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.A.; Klugman, K.; Davies, S. Access to effective antimicrobials: A worldwide challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Carlet, J.M.; Bion, J.; Parker, M.M.; Jaeschke, R.; Calandra, T. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008, 34, 17–60. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Osborn, T.M. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Kim, M.H.; Song, J.E.; Ahn, J.Y.; Oh, D.H.; Kweon, O.M.; Ku, N.S. Trend of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia in an institution with a high rate of MRSA after the reinforcement of antibiotic stewardship and hand hygiene. Am. J. Infect. Control. 2013, 41, e39–e43. [Google Scholar] [CrossRef] [PubMed]
- Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work grouKDIGO clinical practice guideline for acute kidney injury. Kidney Int. Supp. 2012, 2, 138.
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control. 2008, 36, 309–332. [Google Scholar] [CrossRef]
| Variables | Total (n = 92) |
|---|---|
| Demographic | |
| Female, no. (%) | 36(39.13) |
| Age, years | 61.07(14.26) |
| Body mass index, kg/m2 | 23.96(10.98) |
| Laboratory | |
| White blood cell count per mm3 | 14,520(16,826) |
| Hematocrit, % | 34.2(8.26) |
| Platelet per mm3 | 183,745(123,401.5) |
| Blood urea nitrogen, mg/dL | 32.71(25.63) |
| Creatinine, mg/dL | 2.31(2.38) |
| Total bilirubin, mg/dL | 1.07(1.08) |
| Albumin, g/dL | 3.18(0.74) |
| C-reactive protein, mg/L | 137.58(112.75) |
| Lactate, mmol/L | 4.12(3.18) |
| Comorbidities | |
| Congestive heart failure, no. (%) | 6(6.52) |
| Hypertension, no. (%) | 50(54.34) |
| Pulmonary disease, no. (%) | 7(7.61) |
| Liver disease, no. (%) | 6(6.52) |
| Diabetes mellitus, no. (%) | 26(28.26) |
| Renal disease, no. (%) | 13(14.13) |
| Cancer, no. (%) | 38(41.3) |
| Risk factor for MRSA, no. (%) | 52(56.52) |
| Initial SOFA score | 8.68(2.86) |
| Admission to ICU from emergency department, no. (%) | 58(63.04) |
| SOFA score at day 5 | 4.1(4.25) |
| Primary focus of sepsis | |
| Primary sepsis, no. (%) | 19(20.65) |
| Pneumonia, no. (%) | 32(34.78) |
| Intra-abdominal, no. (%) | 22(23.91) |
| Skin and soft tissue, no. (%) | 8(8.7) |
| Others a, no. (%) | 11(11.96) |
| Outcomes | |
| Acute kidney injury, no. (%) | 16(17.39) |
| 28-day mortality, no. (%) | 14(15.22) |
| Overall mortality, no. (%) | 21(22.83) |
| Variables | Before Propensity Score Matching | After Propensity Score Matching | ||||
|---|---|---|---|---|---|---|
| Glycopeptides Discontinued Group (n = 42) | Glycopeptides Non-Discontinued Group (n = 50) | p-Value | Glycopeptides Discontinued Group (n = 21) | Glycopeptides Non-Discontinued Group (n = 21) | p-Value | |
| Demographic | ||||||
| Female, no. (%) | 17(40.48) | 19(38) | 0.809 | 9(42.86) | 8(38.10) | 0.706 |
| Age, years | 61.02 ± 12.9 | 61.12 ± 14.62 | 0.974 | 61.38 ± 13.75 | 63.14 ± 14.35 | 0.714 |
| Body mass index, kg/m2 | 24.59 ± 16.22 | 23.39 ± 3.39 | 0.656 | 26.24 ± 23.1 | 23.21 ± 2.84 | 0.557 |
| Laboratory | ||||||
| White blood cell count per mm3 | 11,727.14 ± 10,485.66 | 16,539.4 ± 21,144.89 | 0.161 | 13,250.48 ± 10,956.11 | 13,380 ± 12,844.07 | 0.968 |
| Hematocrit, % | 34.08 ± 7.47 | 35.26 ± 8.62 | 0.489 | 33.95 ± 8.05 | 32.98 ± 7.55 | 0.696 |
| Platelet per mm3 | 179,238.1 ± 131,796.19 | 185,660 ± 122,677.05 | 0.81 | 151,428.57 ± 87,436.59 | 189,380.95 ± 123,681.64 | 0.239 |
| Blood urea nitrogen, mg/dL | 32.35 ± 33.84 | 32.97 ± 18.04 | 0.915 | 34.18 ± 39.22 | 30.75 ± 17.1 | 0.725 |
| Creatinine, mg/dL | 2.05 ± 2.43 | 2.46 ± 2.44 | 0.421 | 2.4 ± 3.19 | 2.38 ± 2.89 | 0.981 |
| Total bilirubin, mg/dL | 0.91 ± 0.76 | 1.22 ± 1.33 | 0.168 | 0.97 ± 0.91 | 0.86 ± 0.53 | 0.642 |
| Albumin, g/dL | 3.19 ± 0.72 | 3.17 ± 0.78 | 0.93 | 3.11 ± 0.72 | 3.27 ± 0.65 | 0.419 |
| C-reactive protein, mg/L | 118.5 ± 87.18 | 152.78 ± 127.66 | 0.138 | 127.84 ± 93.94 | 167.4 ± 152.37 | 0.339 |
| Lactate, mmol/L | 3.3 ± 2.41 | 4.9 ± 4.18 | 0.029 | 3.23 ± 2.08 | 3.04 ± 1.96 | 0.576 |
| Comorbidity | ||||||
| Congestive heart failure, no. (%) | 0(0) | 6(12) | 0.03 | 0(0) | 0(0) | NA |
| Hypertension, no. (%) | 23(54.76) | 27(54) | 0.942 | 12(57.14) | 13(61.90) | 0.739 |
| Pulmonary disease, no. (%) | 4(9.52) | 3(6) | 0.698 | 2(9.52) | 0(0) | NA |
| Liver disease, no. (%) | 3(7.14) | 3(6) | >0.999 | 1(4.76) | 1(4.76) | >0.999 |
| Diabetes mellitus, no. (%) | 11(26.19) | 15(30) | 0.686 | 5(23.81) | 6(28.57) | 0.706 |
| Renal disease, no. (%) | 5(11.9) | 8(16) | 0.574 | 3(14.29) | 3(14.29) | >0.999 |
| Cancer, no. (%) | 18(42.86) | 20(40) | 0.782 | 9(42.86) | 9(42.86) | >0.999 |
| Risk factor for MRSA, no. (%) | 27(64.29) | 25(50) | 0.169 | 11(52.38) | 11(52.38) | >0.999 |
| Initial SOFA score | 7.95 ± 2.37 | 9.36 ± 3.17 | 0.02 | 8.05 ± 2.20 | 9.14 ± 3.73 | 0.169 |
| Admission to ICU from ED, no. (%) | 24(57.14) | 34(68) | 0.283 | 13(61.90) | 13(61.90) | >0.999 |
| SOFA score at day 5 | 3.5 ± 3.62 | 4.7 ± 4.67 | 0.178 | 3.33 ± 3.5 | 3.71 ± 4.6 | 0.527 |
| Primary focus of sepsis | 0.801 | 0.609 | ||||
| Primary sepsis, no. (%) | 9(21.43) | 10(20) | 3(14.29) | 6(28.57) | ||
| Pneumonia, no. (%) | 16(38.10) | 16(32) | 6(28.57) | 4(19.05) | ||
| Intra-abdominal, no. (%) | 10(23.81) | 12(24) | 9(42.86) | 4(19.05) | ||
| Skin and soft tissue, no. (%) | 2(4.76) | 6(12) | 1(4.76) | 4(19.05) | ||
| Others a, no. (%) | 5(11.9) | 6(12) | 2(9.52) | 3(14.29) | ||
| Outcomes | Before Propensity Score Matching | After Propensity Score Matching | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycopeptides Discontinuation Group (n = 42) | Glycopeptides Non-Discontinuation Group (n = 50) | OR | 95% CI | p-Value | Glycopeptides Discontinuation Group (n = 21) | Glycopeptides Non-Discontinuation Group (n = 21) | OR | 95% CI | p-Value | ||
| New AKI, no. (%) | 4.16 | 1.49–11.6 | 0.007 | 5.54 | 1.49–20.6 | 0.011 | |||||
| None | 38(90.1) | 37(74) | 20(95.2) | 16(76.2) | |||||||
| 1-Creatinine ≥1.3 mg/dL or ≥ 1.5 times than baseline | 0(0) | 4(8.16) | 0(0) | 1(5) | |||||||
| 2-≥ 2 times than baseline | 2(4.76) | 1(2.04) | 1(4.76) | 0(0) | |||||||
| 3-≥ 3 times than baseline | 1(2.38) | 1(2.04) | 0(0) | 0(0) | |||||||
| Hemodialysis needed | 1(2.38) | 6(12.24) | 0(0) | 3(15) | |||||||
| 7-day mortality, no. (%) | 1(2.38) | 1(2) | 0.84 | 0.05–13.8 | 0.901 | 1(4.76) | 1(4.76) | 1 | 0.06–15.99 | >0.999 | |
| 14-day mortality, no. (%) | 2(4.76) | 6(12) | 2.73 | 0.52–14.29 | 0.235 | 1(4.76) | 1(4.76) | 1 | 0.06–15.99 | >0.999 | |
| 28-day mortality, no. (%) | 4(9.52) | 10(20) | 2.38 | 0.69–8.22 | 0.172 | 2(9.52) | 4(19.05) | 2 | 0.37–10.92 | 0.427 | |
| Outcomes | Before propensity score matching | After propensity score matching | |||||||||
| Glycopeptides discontinuation group (n = 42) | Glycopeptides Non-discontinuation group(n = 50) | β | Standard error | p-value | Glycopeptides discontinuation group (n = 21) | Glycopeptides Non-discontinuation group (n = 21) | β | Standard error | p-value | ||
| Hospital day | 17.79 ± 18.65 | 27.62 ± 21.71 | 9.834 | 4.264 | 0.023 | 16.33 ± 17.11 | 25.05 ± 14.37 | 8.714 | 4.876 | 0.082 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.C.; Kim, J.H.; Ahn, J.Y.; Jeong, S.J.; Ku, N.S.; Choi, J.Y.; Yeom, J.-S.; Park, Y.S.; Song, Y.G.; Kim, H.Y. Discontinuation of Glycopeptides in Patients with Culture Negative Severe Sepsis or Septic Shock: A Propensity-Matched Retrospective Cohort Study. Antibiotics 2020, 9, 250. https://doi.org/10.3390/antibiotics9050250
Kim YC, Kim JH, Ahn JY, Jeong SJ, Ku NS, Choi JY, Yeom J-S, Park YS, Song YG, Kim HY. Discontinuation of Glycopeptides in Patients with Culture Negative Severe Sepsis or Septic Shock: A Propensity-Matched Retrospective Cohort Study. Antibiotics. 2020; 9(5):250. https://doi.org/10.3390/antibiotics9050250
Chicago/Turabian StyleKim, Yong Chan, Jung Ho Kim, Jin Young Ahn, Su Jin Jeong, Nam Su Ku, Jun Yong Choi, Joon-Sup Yeom, Yoon Soo Park, Young Goo Song, and Ha Yan Kim. 2020. "Discontinuation of Glycopeptides in Patients with Culture Negative Severe Sepsis or Septic Shock: A Propensity-Matched Retrospective Cohort Study" Antibiotics 9, no. 5: 250. https://doi.org/10.3390/antibiotics9050250
APA StyleKim, Y. C., Kim, J. H., Ahn, J. Y., Jeong, S. J., Ku, N. S., Choi, J. Y., Yeom, J.-S., Park, Y. S., Song, Y. G., & Kim, H. Y. (2020). Discontinuation of Glycopeptides in Patients with Culture Negative Severe Sepsis or Septic Shock: A Propensity-Matched Retrospective Cohort Study. Antibiotics, 9(5), 250. https://doi.org/10.3390/antibiotics9050250





