Assessment of the Usefulness of Cefapirin and Cefalonium Disks for Susceptibility Testing of Staphylococcus aureus Isolates from Bovine Mastitis
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ashraf, A.; Imran, M. Diagnosis of bovine mastitits: From laboratory to farm. Trop. Anim. Health Prod. 2018, 50, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Jamali, H.; Barkema, H.W.; Jacques, M.; Lavallée-Bourget, E.M.; Malouin, F.; Saini, V.; Stryhn, H.; Dufour, S. Invited review: Incidence, risk factors, and effects of clinical mastitis recurrence in dairy cows. J. Dairy Sci. 2018, 101, 4729–4746. [Google Scholar] [CrossRef] [PubMed]
- Rainard, P.; Foucras, G.; Fitzgerald, J.R.; Watts, J.L.; Koop, G.; Middleton, J.R. Knowledge gaps and research priorities in Staphylococcus aureus mastitis control. Transbound. Emerg. Dis. 2018, 65 (Suppl 1), 149–165. [Google Scholar] [CrossRef] [PubMed]
- Ruegg, P.L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef] [PubMed]
- Taponen, S.; Pyörälä, S. Coagulase-negative staphylococci as cause of bovine mastitis-Not so different from Staphylococcus aureus? Vet. Microbiol. 2009, 134, 29–36. [Google Scholar] [CrossRef]
- Gomes, F.; Henriques, M. Control of bovine mastitis: Old and recent therapeutic approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef]
- White, D.G.; McDermott, P.F. Emergence and transfer of antibacterial resistance. J. Dairy Sci. 2001, 84 (Suppl. E), E151–E155. [Google Scholar] [CrossRef]
- Barkema, H.W.; Schukken, Y.H.; Zadoks, R.N. Invited Review: The role of cow, pathogen, and treatment regimen in the therapeutic success of bovine Staphylococcus aureus mastitis. J. Dairy Sci. 2006, 89, 1877–1895. [Google Scholar] [CrossRef]
- Apparao, M.D.; Ruegg, P.L.; Lago, A.; Godden, S.; Bey, R.; Leslie, K. Relationship between in vitro susceptibility test results and treatment outcomes for gram-positive mastitis pathogens following treatment with cephapirin sodium. J. Dairy Sci. 2009, 92, 2589–2597. [Google Scholar] [CrossRef]
- Tomazi, T.; Lopes, T.A.F.; Masson, V.; Swinkels, J.M.; Santos, M.V. Randomized noninferiority field trial evaluating cephapirin sodium for treatment of nonsevere clinical mastitis. J. Dairy Sci. 2018, 101, 7334–7347. [Google Scholar] [CrossRef]
- Berry, E.A.; Hillerton, J.E. Effect of an intramammary test seal and dry cow antibiotic in relation to dry period length on postpartum mastitis. J. Dairy Sci. 2007, 90, 760–765. [Google Scholar] [CrossRef]
- Brunton, L.A.; Duncan, D.; Coldham, N.G.; Snow, L.C.; Jones, J.R. A survey of antimicrobial usage on dairy farms and waste milk feeding practices in England and Wales. Vet. Rec. 2012, 171, 296. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.A.; Zadoks, R.N. Methicillin resistant S. aureus in human and bovine mastitis. J. Mammary Gland Biol. Neoplasia 2011, 16, 373–382. [Google Scholar] [CrossRef]
- Aires-de-Sousa, M. Methicillin-resistant Staphylococcus aureus among animals: Current overview. Clin. Microbiol. Infect. 2017, 23, 373–380. [Google Scholar] [CrossRef]
- Joneberg, J.; Rylander, M.; Galas, M.F.; Carlos, C.; Kronvall, G. Analysis of parameters and validation of method for normalized interpretation of antimicrobial resistance. Int. J. Antimicrob. Agents 2003, 21, 525–535. [Google Scholar] [CrossRef]
- Kronvall, G.; Giske, C.G.; Kahlmeter, G. Setting interpretive breakpoints for antimicrobial susceptibility testing using disk diffusion. Int. J. Antimicrob. Agents 2011, 38, 281–290. [Google Scholar] [CrossRef] [PubMed]
- National Mastitis Council. Laboratory Handbook on Bovine Mastitis, Revised edition; National Mastitis Council: Madison, WI, USA, 1999. [Google Scholar]
- Moroni, P.; Vellere, F.; Antonini, M.; Pisoni, G.; Ruffo, G.; Carli, S. Antibiotic susceptibility of coagulase-negative staphylococci isolated from goats’ milk. Int. J. Anticmicrob. Agents 2004, 23, 637–640. [Google Scholar] [CrossRef]
- Hata, E.; Katsuda, K.; Kobayashi, H.; Ogawa, T.; Endo, T.; Eguchi, M. Characteristics and epidemiologic genotyping of Staphylococcus aureus isolates from bovine mastitis milk in Hokkaido, Japan. J. Vet. Med. Sci. 2006, 68, 165–170. [Google Scholar] [CrossRef]
- Nakatomi, Y.; Sugiyama, J. A rapid latex agglutination assay for the detection of penicillin-binding protein 2’. Microbiol. Immunol. 1998, 42, 739–743. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 5th ed.; CLSI standard VET01: Wayne, PA, USA, 2018. [Google Scholar]
- Fujisaki, M.; Sadamoto, S.; Ikedo, M.; Totsuka, K.; Kaku, M.; Tateda, K.; Hirakata, Y.; Yamaguchi, K. Development of interpretive criteria for tebipenem disk diffusion susceptibility testing with Staphylococcus spp. and Haemophilus influenzae. J. Infect. Chemother. 2011, 17, 17–23. [Google Scholar] [CrossRef]
- Silley, P. Susceptibility testing methods, resistance and breakpoints: What do these terms really mean? Rev. Sci. Off. Int. Epiz. 2012, 31, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Bolte, J.; Zhang, Y.; Wente, N.; Krömker, V. In vitro susceptibility of mastitis pathogens isolated from clinical mastitis cases on northern German dairy farms. Vet. Sci. 2020, 7, 10. [Google Scholar] [CrossRef] [PubMed]
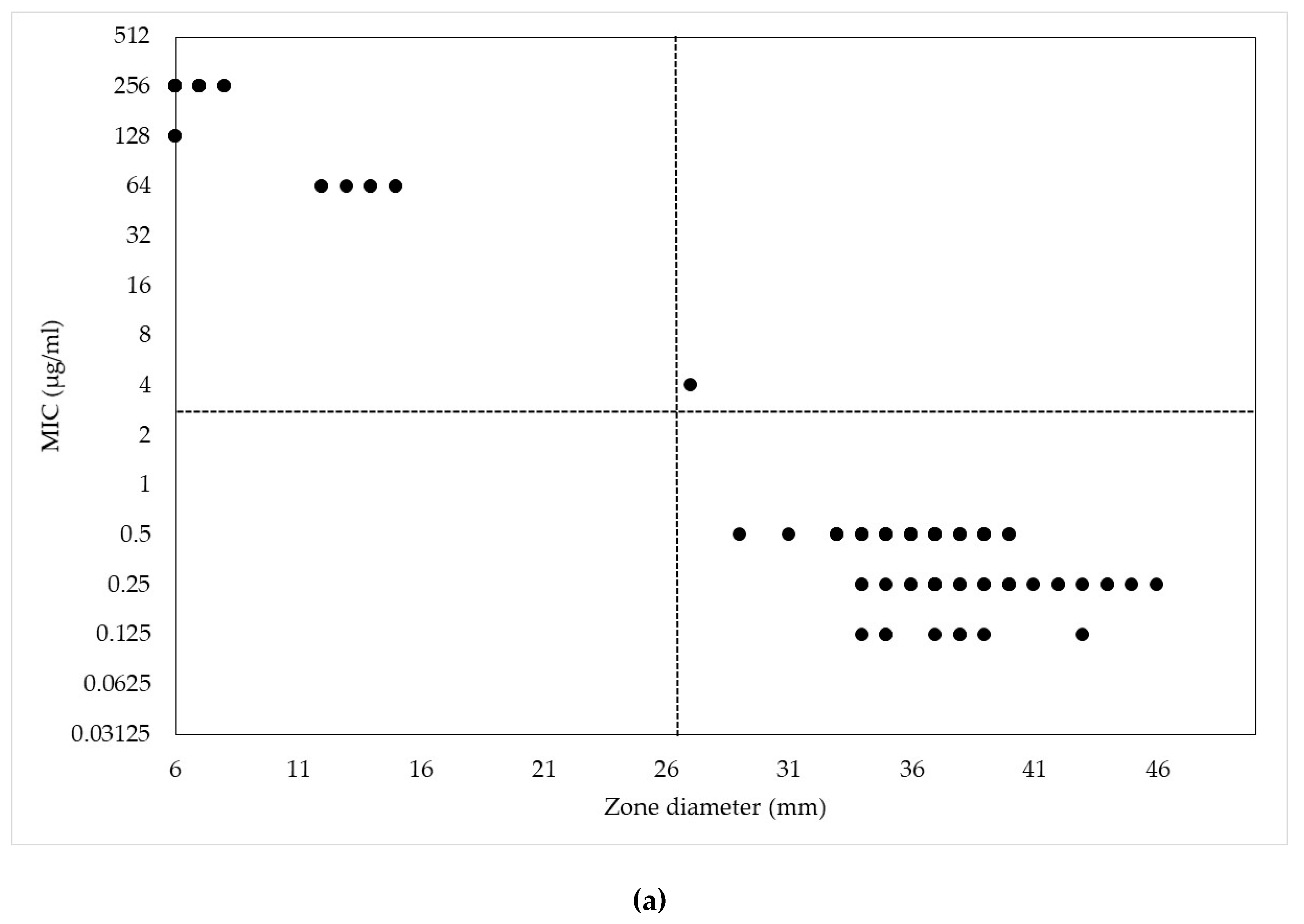
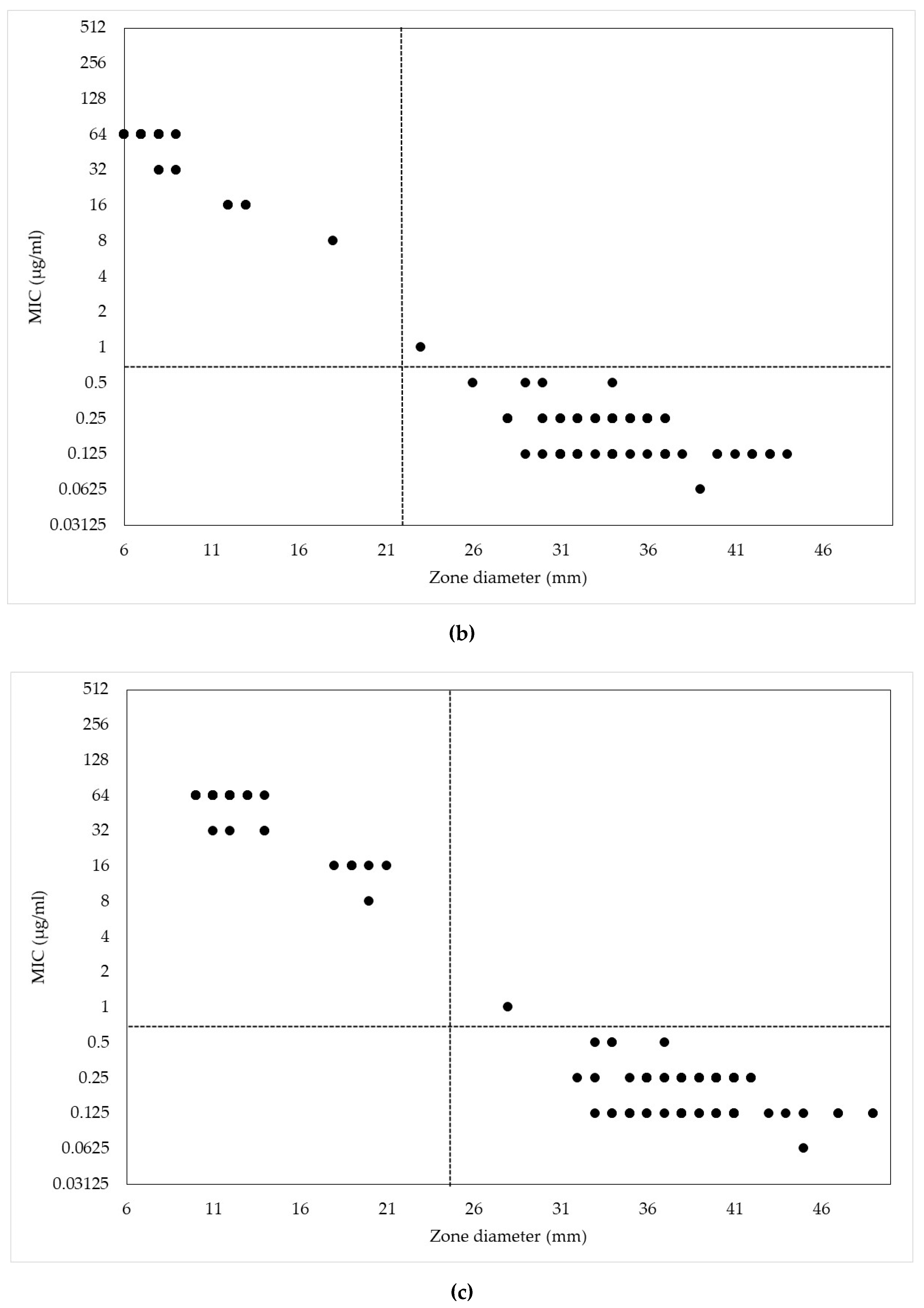
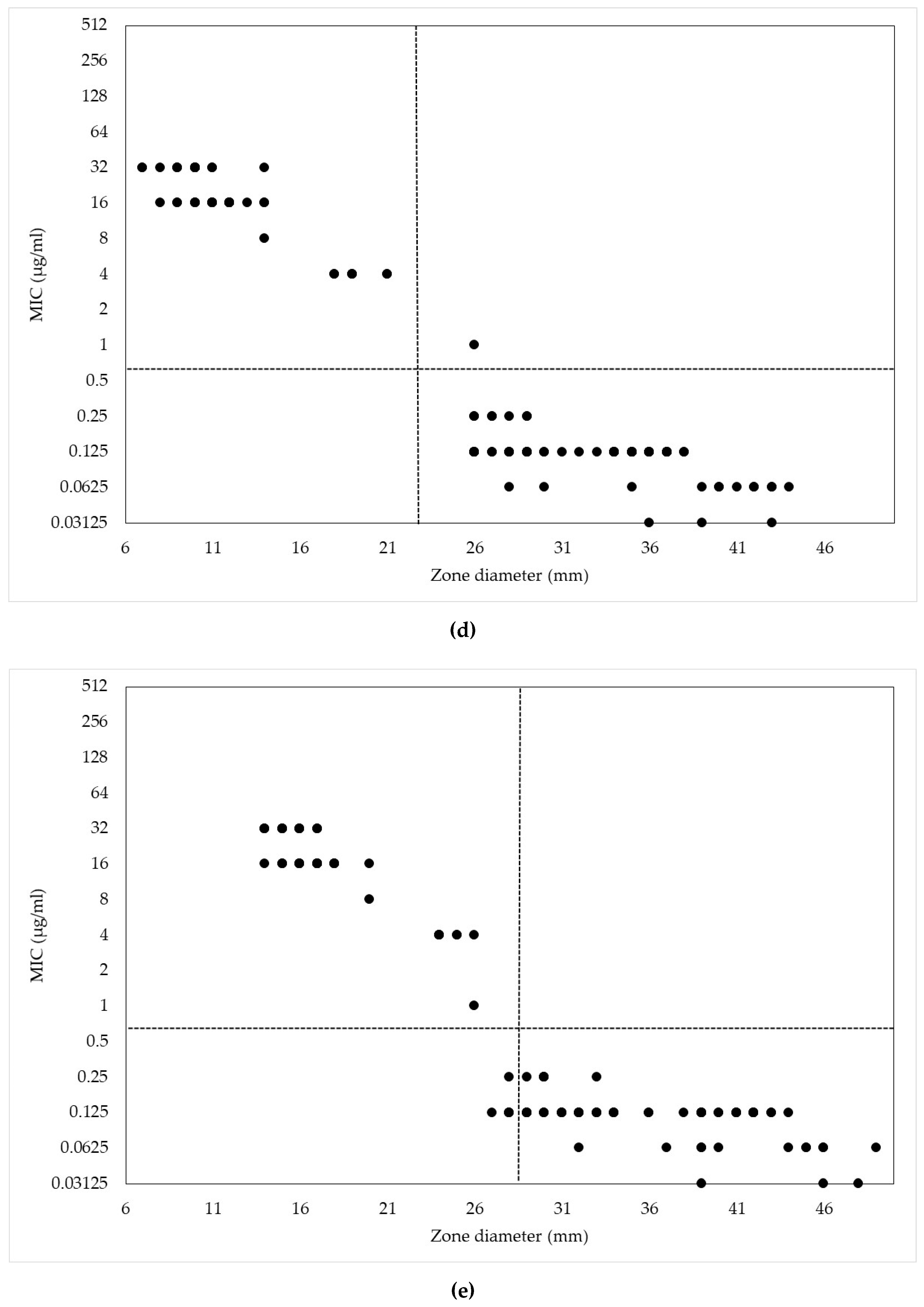
| Antimicrobials | MIC (µg/mL) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.031 | 0.063 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | |
| Cefazolin | − | − | 8 | 28 (1) | 43 (2) | − | − | 1 (1) | − | − | − | 7 (7) | 3 (3) | 40 (40) |
| Cefapirin | − | 1 | 34 (2) | 40 (1) | 4 | 1 (1) | 1 (1) | 6 (6) | 4 (4) | 39 (39) | − | − | ||
| Cefalonium | 3 | 12 (1) | 57 (1) | 7 (1) | 1 (1) | 7 (7) | 1 (1) | 25 (25) | 17 (17) | − | − | − | ||
| MIC (μg/mL) | Range of Zone Diameters (mm) | ||||
|---|---|---|---|---|---|
| CEZ30 | CEP10 | CEP30 | CNM10 | CNM30 | |
| 256 | 6–8 | − | − | − | − |
| 128 | 6 | − | − | − | − |
| 64 | 12–15 | 6–9 | 10–14 | − | − |
| 32 | − | 8–9 | 12–14 | 7–14 | 14–17 |
| 16 | − | 12–13 | 18–21 | 8–14 | 14–19 |
| 8 | − | 18 | 20 | 14 | 20 |
| 4 | 27 | − | − | 18–21 | 24–26 |
| 2 | − | − | − | − | − |
| 1 | − | 23 | 28 | 26 | 26 |
| 0.5 | 29–40 | 26–34 | 33–37 | − | − |
| 0.25 | 34–46 | 28–37 | 32–42 | 26–29 | 28–33 |
| 0.125 | 34–43 | 29–44 | 33–51 | 26–38 | 27–44 |
| 0.063 | − | 39 | 45 | 28–44 | 32–51 |
| 0.031 | − | − | − | 36–43 | 39–48 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harada, K.; Irie, S.; Ohnishi, M.; Kataoka, Y. Assessment of the Usefulness of Cefapirin and Cefalonium Disks for Susceptibility Testing of Staphylococcus aureus Isolates from Bovine Mastitis. Antibiotics 2020, 9, 197. https://doi.org/10.3390/antibiotics9040197
Harada K, Irie S, Ohnishi M, Kataoka Y. Assessment of the Usefulness of Cefapirin and Cefalonium Disks for Susceptibility Testing of Staphylococcus aureus Isolates from Bovine Mastitis. Antibiotics. 2020; 9(4):197. https://doi.org/10.3390/antibiotics9040197
Chicago/Turabian StyleHarada, Kazuki, Shieri Irie, Mamoru Ohnishi, and Yasushi Kataoka. 2020. "Assessment of the Usefulness of Cefapirin and Cefalonium Disks for Susceptibility Testing of Staphylococcus aureus Isolates from Bovine Mastitis" Antibiotics 9, no. 4: 197. https://doi.org/10.3390/antibiotics9040197
APA StyleHarada, K., Irie, S., Ohnishi, M., & Kataoka, Y. (2020). Assessment of the Usefulness of Cefapirin and Cefalonium Disks for Susceptibility Testing of Staphylococcus aureus Isolates from Bovine Mastitis. Antibiotics, 9(4), 197. https://doi.org/10.3390/antibiotics9040197




