Is Blood a Good Indicator for Detecting Antimicrobials in Meat? Evidence for the Development of In Vivo Surveillance Methods
Abstract
1. Introduction
2. Results and Discussion
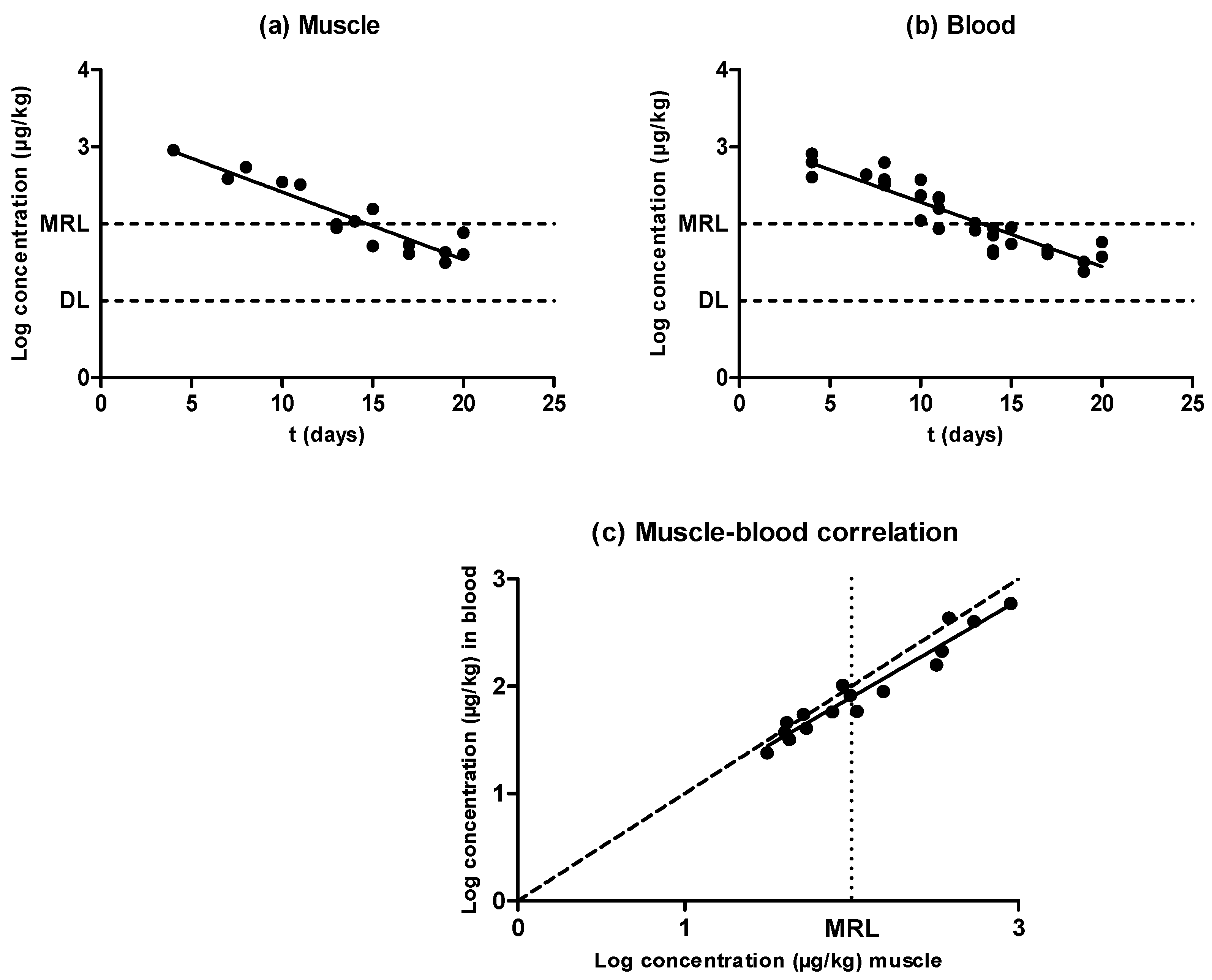
2.1. Oxytetracycline
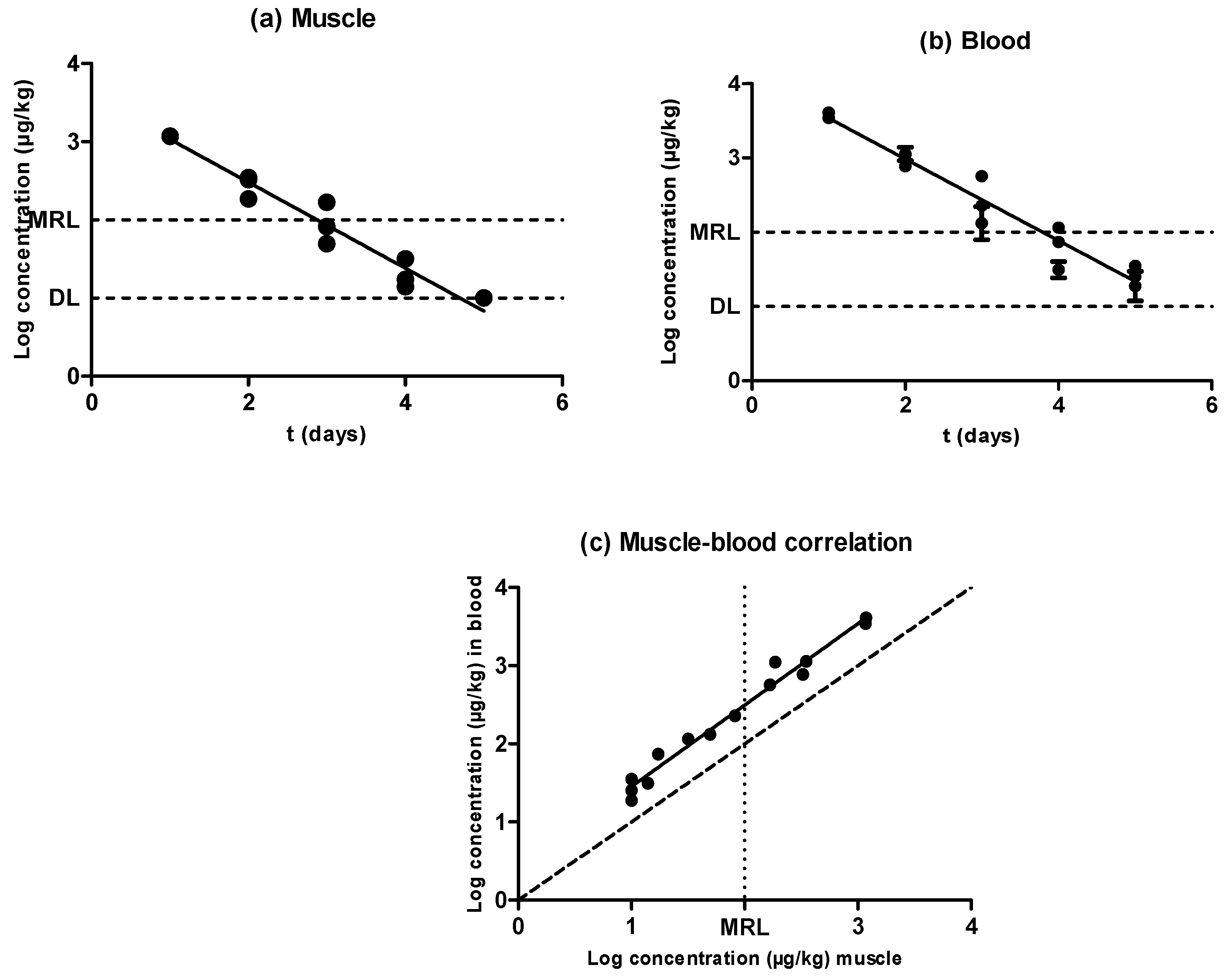
2.2. Sulfamethoxypyridazine
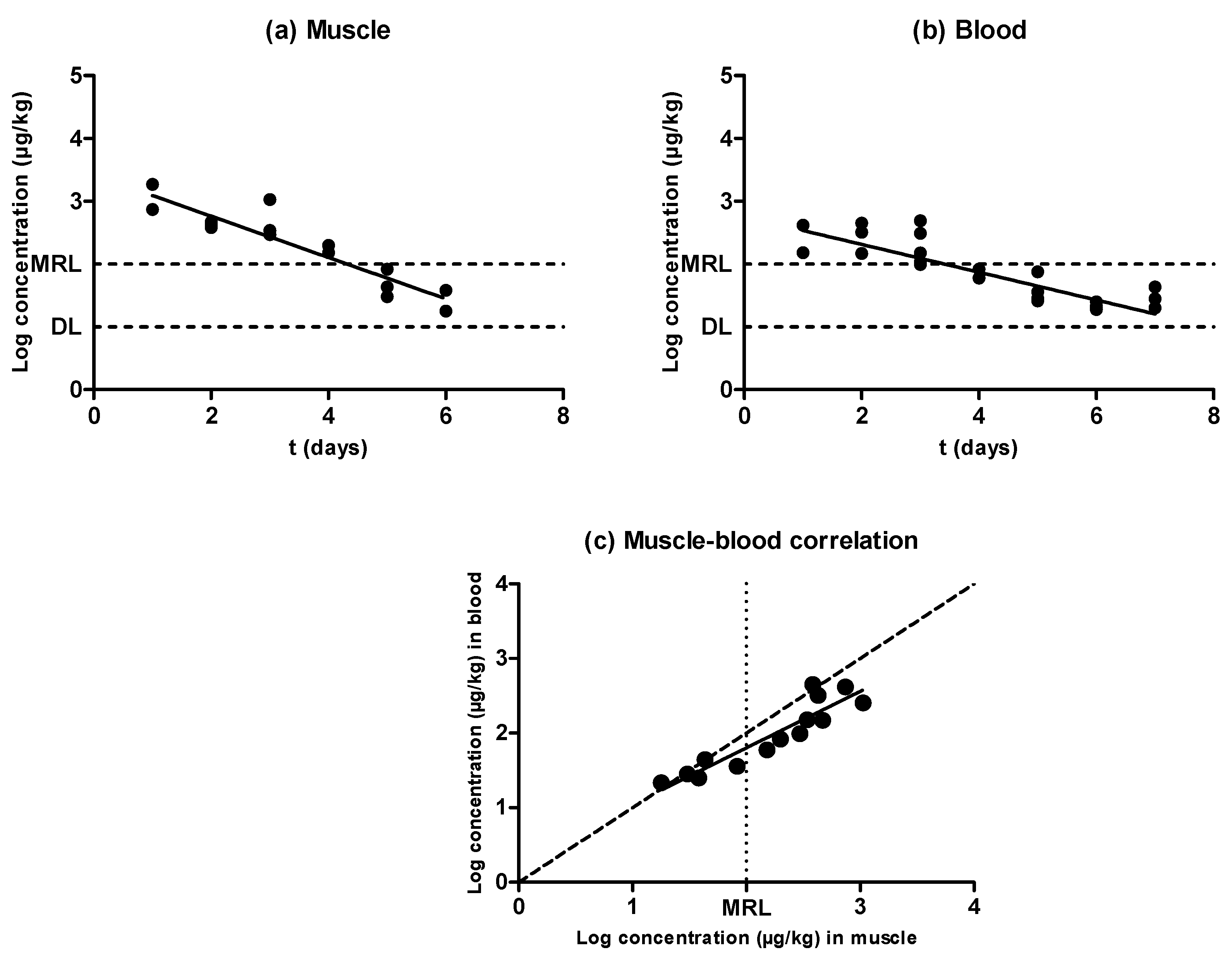
2.3. Enrofloxacin
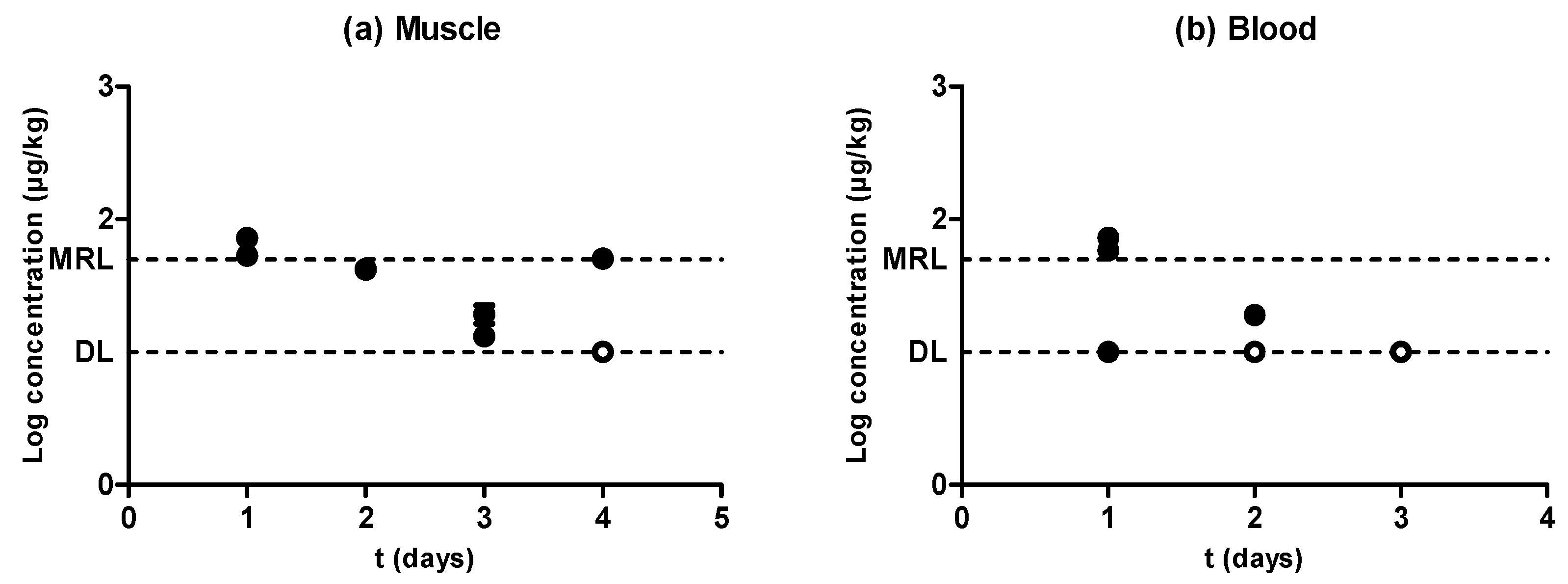
2.4. Amoxicillin
2.5. Evidences for the Development of In Vivo Surveillance Methods
3. Materials and Methods
3.1. Chemicals and Reagents
3.1.1. Antimicrobials
3.1.2. HPLC Reagents and Standards
3.2. Experimental Sample Bank with Antimicrobials Administered In Vivo
3.2.1. Muscle Tissue
3.2.2. Blood
3.3. Ethical Approval
3.4. Sample Extraction
3.4.1. Muscle Tissue
3.4.2. Blood
3.5. HPLC-FLD and LC–MS/MS Analyses
3.6. Pharmacokinetic Parameters and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. London: Review on Antimicrobial Resistance; 2014. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 23 October 2019).
- ECDC (European Centre for Disease Prevention and Control); EFSA (European Food Safety Authority) and EMA (European Medicines Agency). ECDC/EFSA/EMA Second Joint Report on the Integrated Analysis of the Consumption of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Humans and Food-Producing Animals. Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA) Report. EFSA J. 2017, 15, e04872. Available online: https://ecdc.europa.eu/sites/portal/files/documents/efs2_4872_final.pdf (accessed on 10 November 2019).
- Council of the European Communities. Regulation 2377/90 of 26 June 1990 Laying down a Community Procedure for the Establishment of Maximum Residue Limits of Veterinary Medicinal Products in Foodstuffs of Animal Origin. Off. J. Eur. Commun. 1990, L224, 1–8. Available online: https://ec.europa.eu/health//sites/health/files/files/eudralex/vol-5/reg_1990_2377/reg_1990_2377_en.pdf (accessed on 11 November 2019).
- European Parliament and Council. Directive 2001/82/EC of November 28 2001 on the Community Code Relating to Veterinary Medicinal Products. Off. J. Eur. Commun. 2001, L311, 1–110. Available online: https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-5/dir_2001_82_cons2009/dir_2001_82_cons2009_en.pdf (accessed on 11 November 2019).
- EMA (European Medicines Agency). Guideline on Determination of Withdrawal Periods for Edible Tissues; EMA/CVMP/SWP/735325/2012; Committee for Medicinal Products for Veterinary Use (CVMP): London, UK, 2018; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-determination-withdrawal-periods-edible-tissues-revision-1_en.pdf (accessed on 8 November 2019).
- Mitchell, J.M.; Griffiths, M.W.; McEwen, S.A.; McNab, W.B.; Yee, A.J. Antimicrobial drug residues in milk and meat: Causes, concerns, prevalence, regulations, tests, and test performance. J. Food Prot. 1998, 61, 742–756. [Google Scholar] [CrossRef] [PubMed]
- Pikkemaat, M.G. Microbial screening methods for detection of antibiotic residues in slaughter animals. Anal. Bioanal. Chem. 2009, 395, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, V. Advances in biosensor development for the screening of antibiotic residues in food products of animal origin—A comprehensive review. Biosens. Bioelectron. 2017, 90, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Council of the European Union. Directive 96/23/EC of 29 April 1996 on Measures to Monitor Certain Substances and Residues Thereof in Live Animals and Animal Products and Repealing Directives 85/358/EEC and 86/469/EEC and Decisions 89/187/EEC and 91/664/EEC. Off. J. Eur. Commun. 1996, L125, 10–31. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31996L0023&from=EN (accessed on 11 November 2019).
- Djekic, I.; Radović, Č.; Lukić, M.; Stanišić, N.; Lilić, S. Environmental life-cycle assessment in production of pork products. Meso 2015, 17. [Google Scholar]
- Reyes-Herrera, I.; Schneider, M.J.; Cole, K.; Farnell, M.B.; Blore, P.J.; Donoghue, D.J. Concentrations of antibiotic residues vary between different edible muscle tissues in poultry. Research note. J. Food Prot. 2005, 68, 2217–2219. [Google Scholar] [CrossRef]
- Hernández, E.; Rey, R.; Puig, M.; Garcia, M.A.; Solans, C.; Bregante, M.A. Pharmacokinetics and residues of a new oral amoxicillin formulation in piglets: A preliminary study. Vet. J. 2005, 170, 237–242. [Google Scholar] [CrossRef]
- Ziółkowski, H.; Grabowski, T.; Jasiecka, A.; Zuśka-Prot, M.; Barski, D.; Jaroszewski, J.J. Pharmacokinetics of oxytetracycline in broiler chickens following different routes of administration. Vet. J. 2016, 208, 96–98. [Google Scholar] [CrossRef]
- Castellari, M.; Gratacos-Cubarsi, M.; Garcia-Regueiro, J.A. Detection of tetracycline and oxytetracycline residues in pig and calf hair by ultra-high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 8096–8100. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Nobile, M.; Panseri, S.; Arioli, F. Antibiotic use in heavy pigs: Comparison between urine and muscle samples from food chain animals analysed by HPLC-MS/MS. Food Chem. 2017, 235, 111–118. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Nobile, M.; Panseri, S.; Arioli, F. Suitability of feathers as control matrix for antimicrobial treatments detection compared to muscle and liver of broilers. Food Control. 2018, 91, 268–275. [Google Scholar] [CrossRef]
- Riviere, J.E. 2. Absorption, distribution, metabolism, and elimination. In Veterinary Pharmacology and Therapeutics, 9th ed.; Riviere, J.E., Papich, M.G., Eds.; Willey Blackwell: Ames, IA, USA, 2009; pp. 11–46. [Google Scholar]
- De Vos, S.; Maervoet, J.; Schepens, P.; De Schrijver, R. Polychlorinated biphenyls in broiler diets: Their digestibility and incorporation in body tissues. Chemosphere 2003, 51, 7–11. [Google Scholar] [CrossRef]
- Reyes-Herrera, I.; Donoghue, D.J. Antibiotic residues distribute uniformly in broiler chicken breast muscle tissue. J. Food Prot. 2008, 71, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Riviere, J.E.; Spoo, J.W. Chapter 42. Tetracycline antibiotics. In Veterinary Pharmacology and Therapeutics, 8th ed.; Adams, R.H., Ed.; Iowa State University Press: Ames, IA, USA, 2001; pp. 828–940. [Google Scholar]
- Moreno, L.; Serrano, J.M.; Guimera, M.E.; Cárceles, C.M. Pharmacokinetics of oxytetracycline after intramuscular administration with lidocaine in sheep, comparison with a conventional formulation. Res. Vet. Sci. 1998, 65, 209–213. [Google Scholar] [CrossRef]
- Berninger, J.P.; Brooks, B.W. Leveraging mammalian pharmaceutical toxicology and pharmacology data to predict chronic fish responses to pharmaceuticals. Toxicol. Lett. 2010, 193, 69–78. [Google Scholar] [CrossRef]
- Cars, O.; Ryan, D.M. Determination of extravascular concentrations of doxycycline. J. Antimicrob. Chemother. 1983, 12, 277–279. [Google Scholar] [CrossRef]
- Spoo, J.W.; Riviere, J.E. Chapter 40. Sulfonamides. In Veterinary Pharmacology and Therapeutics, 8th ed.; Adams, R.H., Ed.; Iowa State University Press: Ames, IA, USA, 2001; pp. 796–817. [Google Scholar]
- Papich, M.G.; Riviere, J.E. Chapter 45. Fluoroquinolone antimicrobial drugs. In Veterinary Pharmacology and Therapeutics, 8th ed.; Adams, R.H., Ed.; Iowa State University Press: Ames, IA, USA, 2001; pp. 898–917. [Google Scholar]
- Cars, O. Efficacy of beta-lactam antibiotics: Integration of pharmacokinetics and pharmacodynamics. Diagn. Microbiol. Infect. Dis. 1997, 27, 29–33. [Google Scholar] [CrossRef]
- Vaden, S.L.; Riviere, J.E. Chapter 41. Penicillins and related β-lactam antibiotics. In Veterinary Pharmacology and Therapeutics, 8th ed.; Adams, R.H., Ed.; Iowa State University Press: Ames, IA, USA, 2001; pp. 818–827. [Google Scholar]
- Burmańczuk, A.; Tomasz, G.; Gbylik-Sikorska, M.; Gajda, A.; Kowalski, C. Withdrawal of amoxicillin and penicillin G procaine from milk after intramammary administration in dairy cows with mastitis. J. Vet. Res. 2017, 61, 37–43. [Google Scholar] [CrossRef]
- Hung, Y.W.; Lin, Y.H.; Chan, C.Y.; Wang, W.S.; Chiu, C.F.; Chiu, C.C.; Chiu, H.W.; Tsai, W.H.; Hung, S.W. Pharmacokinetic study of amoxicillin in Japanese eel Anguilla japonica by high performance liquid chromatography with fluorescence detection. Aquac. Rep. 2019, 13, 100184. [Google Scholar] [CrossRef]
- Delis, G.A.; Koutsoviti-Papadopoulou, M.; Theodosiadou, E.; Kounenis, G.; Batzias, G.C. Peripheral distribution of amoxicillin in sheep and influence of local inflammation. Vet. J. 2010, 185, 310–316. [Google Scholar] [CrossRef]
- Rosa, J.; Leston, S.; Freitas, A.; Barbosa, J.; Rema, P.; Dias, J.; Lemos, M.F.L.; Pardal, M.A.; Ramos, F. Tissue depletion of five antibiotic residues in farmed European seabass (Dicentrarchus labrax). Aquaculture 2019, 498, 413–421. [Google Scholar] [CrossRef]
- Mello, L.D.; Kubota, L.T. Review of the use of biosensors as analytical tools in the food and drink industries. Food Chem. 2002, 77, 237–256. [Google Scholar] [CrossRef]
- Sanz, D.; Mata, L.; Condón, S.; Sanz, M.Á.; Razquin, P. Performance of a new microbial test for quinolone residues in muscle. Food Anal. Methods 2011, 4, 212–220. [Google Scholar] [CrossRef]
- Suzuki, S.; Hoa, P.T.P. Distribution of quinolones, sulfonamides, tetracyclines in aquatic environment and antibiotic resistance in Indochina. Front. Microbiol. 2012, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- IDF (International Dairy Federation). Current Situation and Compilation of Commercially Available Screening Methods for the Detection of Inhibitors/Antibiotic Residues in Milk; IDF Bulletin 442; International Dairy Federation: Brussels, Belgium, 2010. [Google Scholar]
- Sanz, D.; Razquin, P.; Condón, S.; Juan, T.; Herráiz, B.; Mata, L. Incidence of antimicrobial residues in meat using a broad spectrum screening strategy. Eur. J. Nutr. Food Saf. 2015, 5, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Scortichini, G.; Annunziata, L.; Di Girolamo., V.; Buratti, R.; Galarini, R. Validation of an enzyme-linked immunosorbent assay screening for quinolones in egg, poultry muscle and feed samples. Anal. Chim. Acta 2009, 637, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Roybal, J.E.; Walker, C.C.; Pfenning, A.P.; Turnipseed, S.B.; Storey, J.M.; Gonzales, S.A.; Hurlbut, J.A. Concurrent determination of four fluoroquinolones in catfish, shrimp, and salmon by liquid chromatography with fluorescence detection. J. AOAC Int. 2002, 85, 1293–1301. [Google Scholar] [CrossRef]
- Council of the European Union. Regulation (EC) No. 1099/2009 of 24 September 2009 on the protection of animals at the time of killing. Off. J. Eur. Commun. 2009, L303, 1. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009R1099&from=EN (accessed on 13 November 2019).
- Chico, J.; Rúbies, A.; Centrich, F.; Companyó, R.; Prat, M.; Granados, M.D. High-throughput multiclass method for antibiotic residue analysis by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2008, 1213, 189–199. [Google Scholar] [CrossRef]
- General Requirements for the Competence of Testing and Calibration Laboratories; ISO/IEC 17025:2017; International Organization for Standardization ISO: Vernier, Geneva, Switzerlan, 2017.
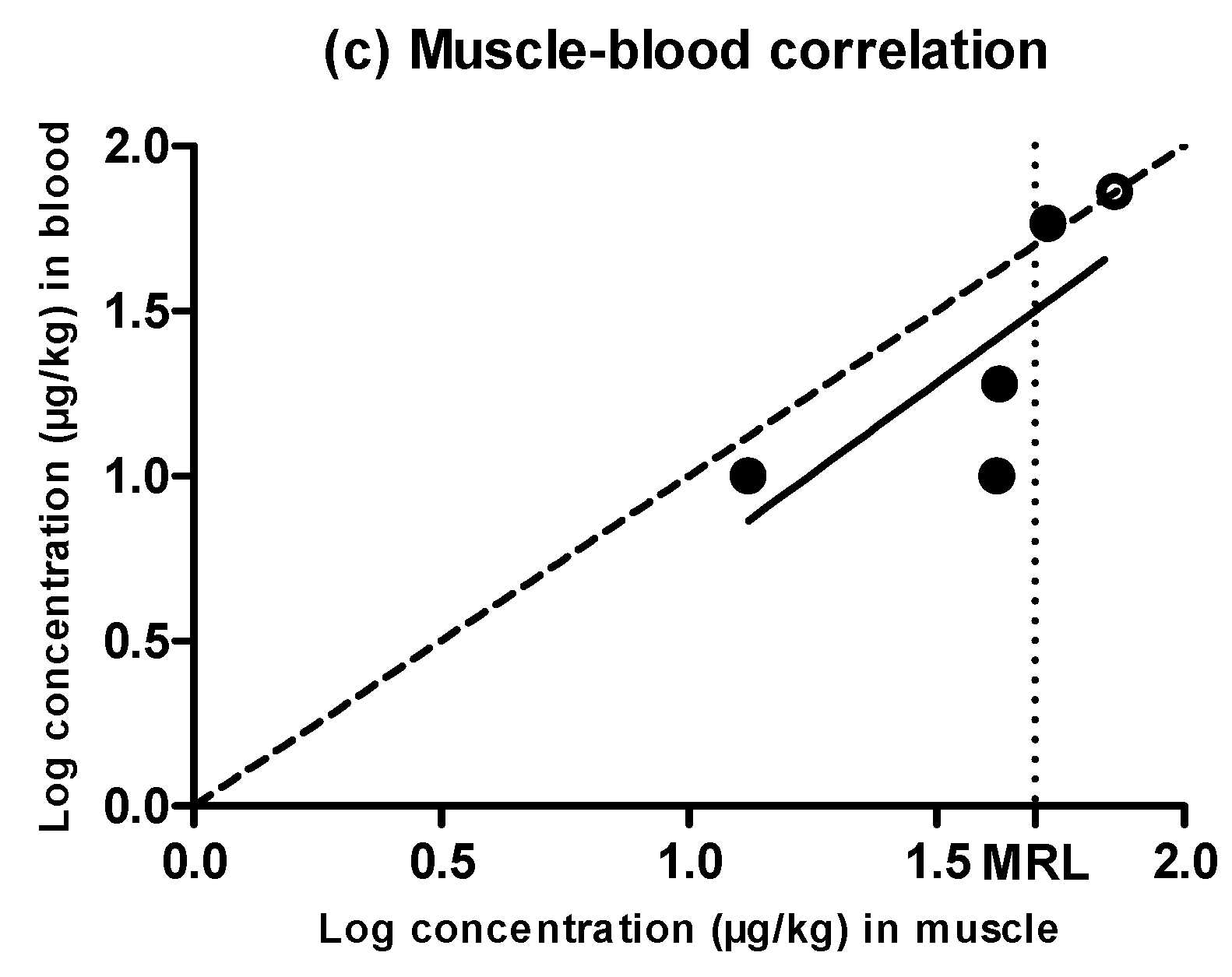
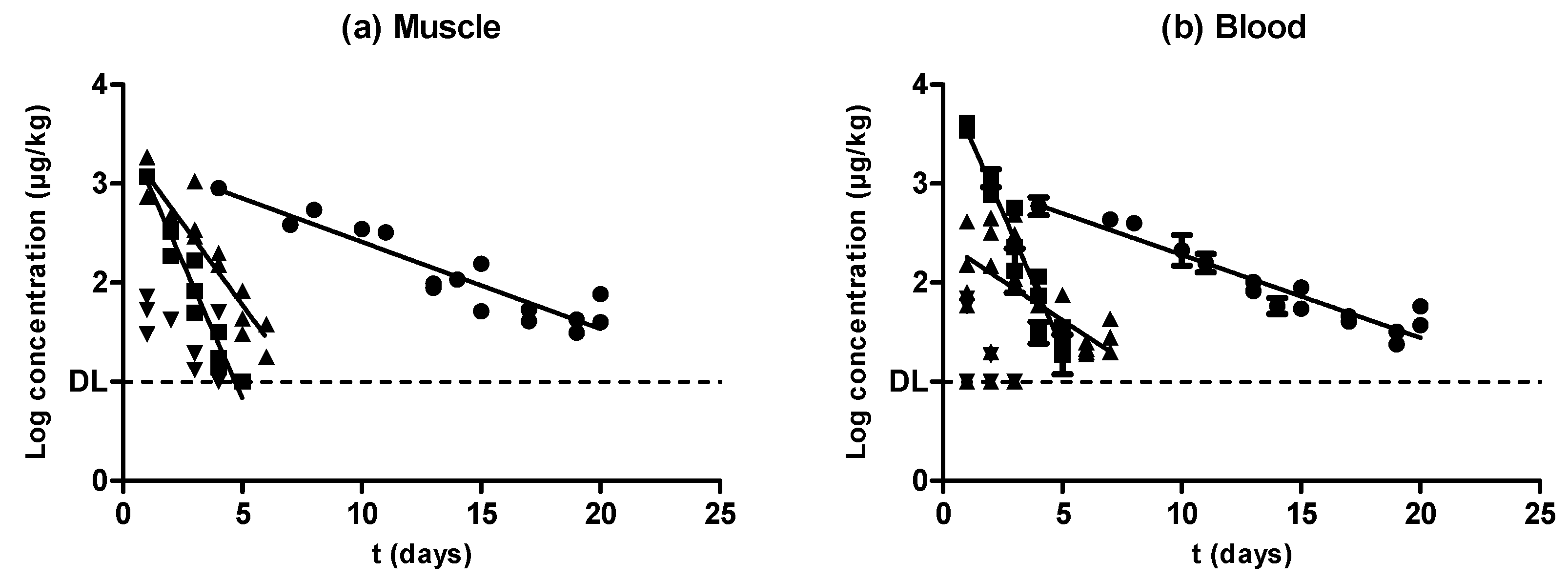
| MUSCLE | BLOOD | |||||||
|---|---|---|---|---|---|---|---|---|
| Slope | R2 | T1/2 | Slope | R2 | T1/2 | |||
| Oxytetracycline | −0.08784 ± 0.005403 a | 0.85 | 4.94 | 3.43 | −0.08879 ± 0.007069 a | 0.85 | 5.18 | 3.59 |
| Sulfamethoxypyridazine | −0.5494 ± 0.02370 a | 0.95 | 0.79 | 0.55 | −0.5496 ± 0.02589 a | 0.92 | 0.79 | 0.55 |
| Enrofloxacin | −0.3289 ± 0.01929 a | 0.87 | 1.32 | 0.92 | −0.2213 ± 0.01639 b | 0.80 | 2.73 | 1.90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, M.J.; Mitjana, O.; Bonastre, C.; Laborda, A.; Falceto, M.V.; García-Gonzalo, D.; Abilleira, E.; Elorduy, J.; Bousquet-Melou, A.; Mata, L.; et al. Is Blood a Good Indicator for Detecting Antimicrobials in Meat? Evidence for the Development of In Vivo Surveillance Methods. Antibiotics 2020, 9, 175. https://doi.org/10.3390/antibiotics9040175
Serrano MJ, Mitjana O, Bonastre C, Laborda A, Falceto MV, García-Gonzalo D, Abilleira E, Elorduy J, Bousquet-Melou A, Mata L, et al. Is Blood a Good Indicator for Detecting Antimicrobials in Meat? Evidence for the Development of In Vivo Surveillance Methods. Antibiotics. 2020; 9(4):175. https://doi.org/10.3390/antibiotics9040175
Chicago/Turabian StyleSerrano, María Jesús, Olga Mitjana, Cristina Bonastre, Alicia Laborda, María Victoria Falceto, Diego García-Gonzalo, Eunate Abilleira, Janire Elorduy, Alain Bousquet-Melou, Luis Mata, and et al. 2020. "Is Blood a Good Indicator for Detecting Antimicrobials in Meat? Evidence for the Development of In Vivo Surveillance Methods" Antibiotics 9, no. 4: 175. https://doi.org/10.3390/antibiotics9040175
APA StyleSerrano, M. J., Mitjana, O., Bonastre, C., Laborda, A., Falceto, M. V., García-Gonzalo, D., Abilleira, E., Elorduy, J., Bousquet-Melou, A., Mata, L., Condón, S., & Pagán, R. (2020). Is Blood a Good Indicator for Detecting Antimicrobials in Meat? Evidence for the Development of In Vivo Surveillance Methods. Antibiotics, 9(4), 175. https://doi.org/10.3390/antibiotics9040175






