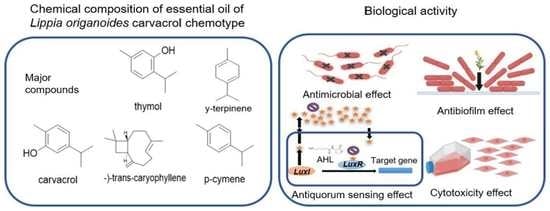
Essential Oils of Aromatic Plants with Antibacterial, Anti-Biofilm and Anti-Quorum Sensing Activities against Pathogenic Bacteria
Abstract
1. Introduction
2. Results
2.1. Chemical Analysis of the Essential Oils (EOs)
2.2. Antibacterial Activity of EOs
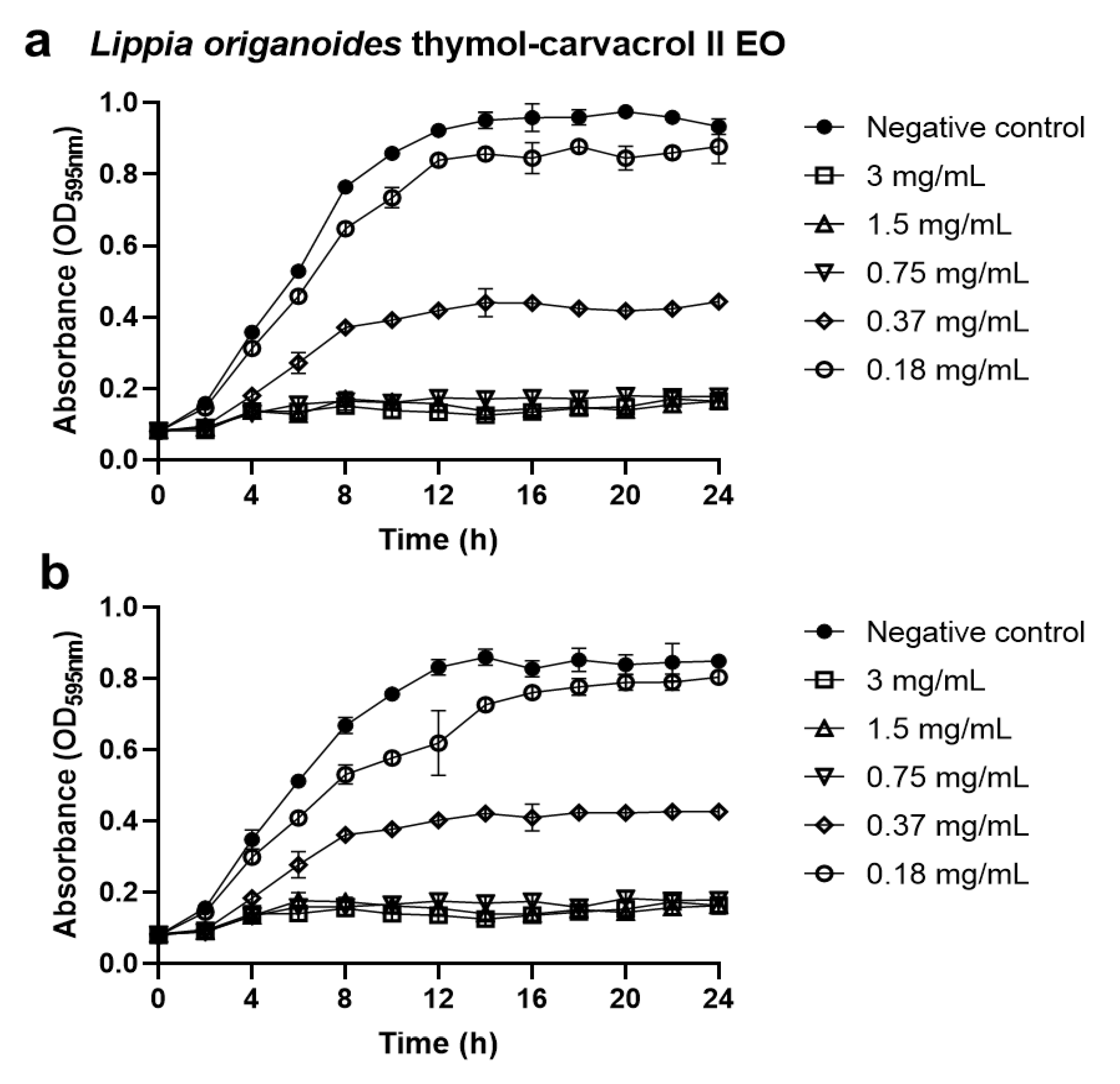
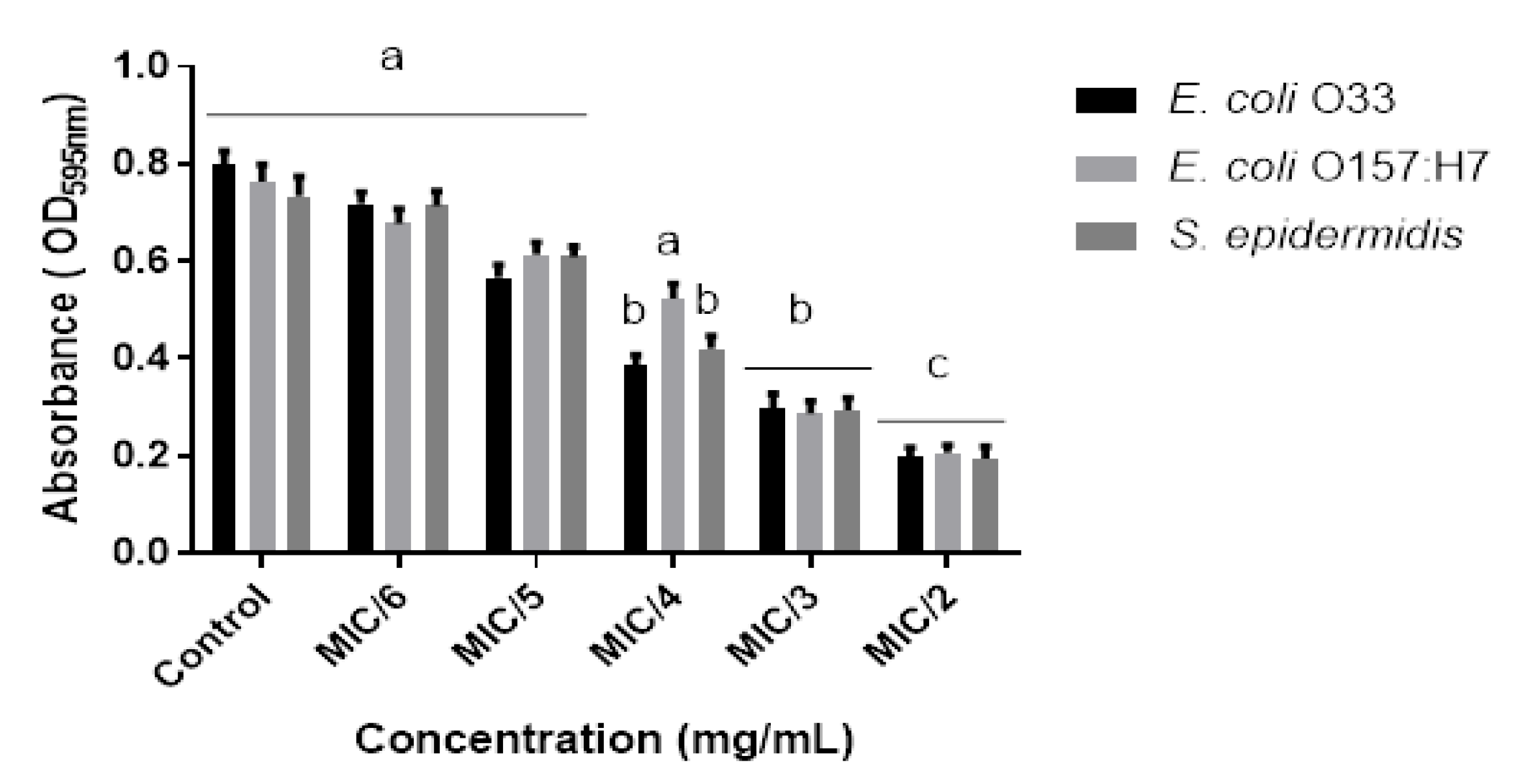
2.3. Anti-Biofilms Activity of the Essential Oils
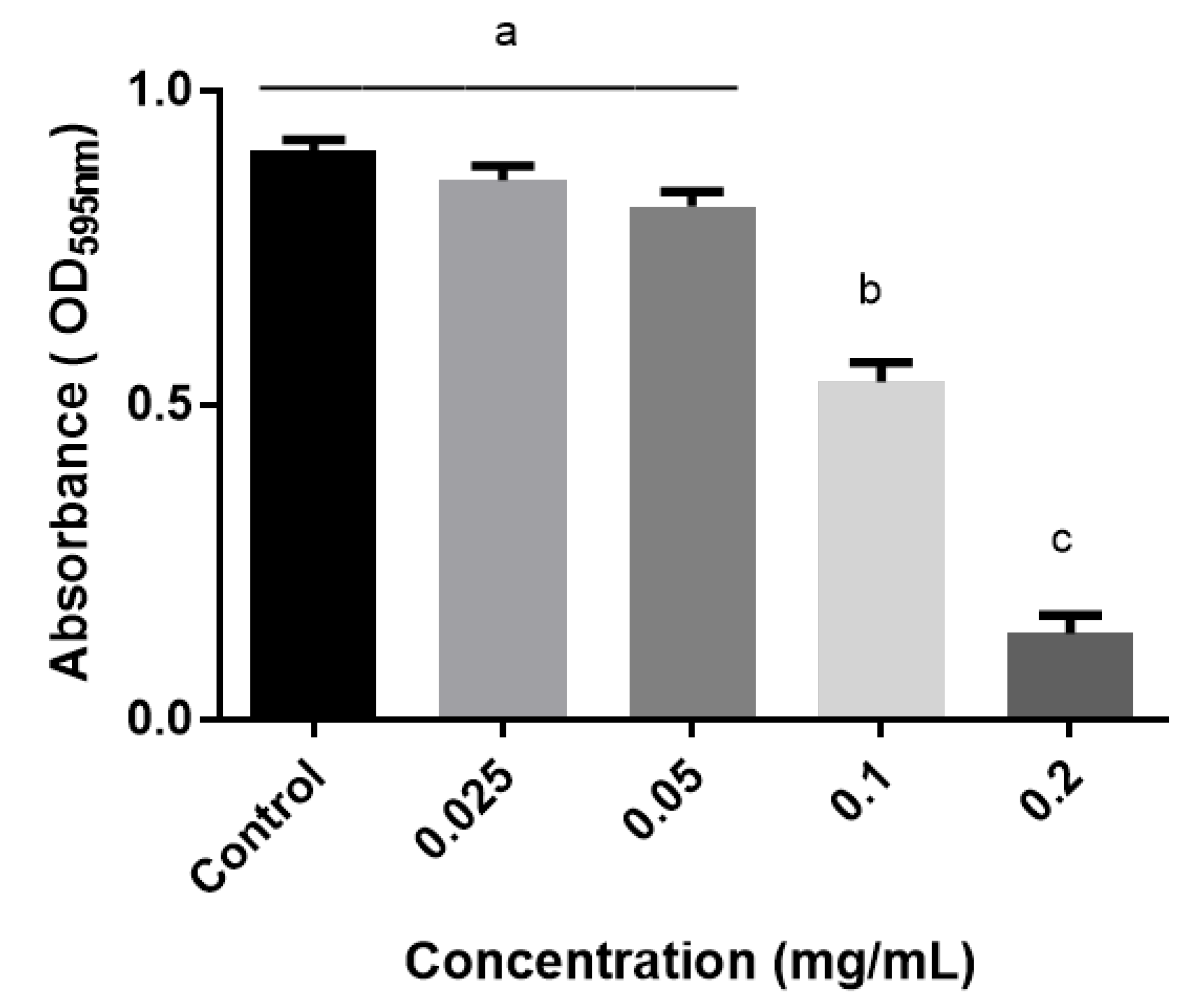
2.4. Anti-Quorum (AQ) Sensing Activity
2.5. Cellular Viability Assays
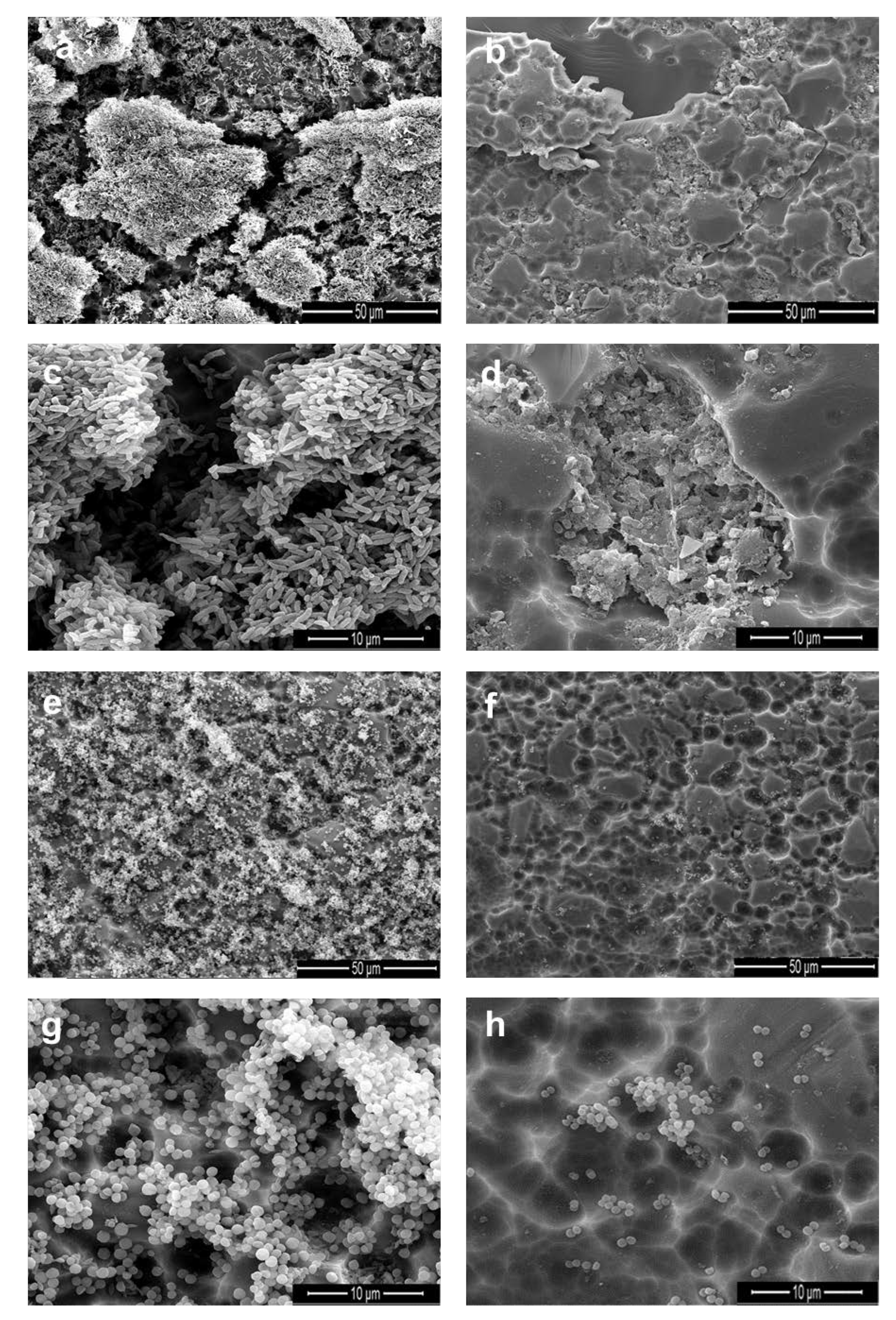
2.6. Scanning Electron Microscope (SEM) Analysis
3. Discussion
4. Materials and Methods
4.1. Essential Oils Analysis
4.2. GC-MS Analysis of Essential Oil
4.3. Determination of Antimicrobial Activity
4.4. Effect on Biofilm Formation
4.5. Violacein Inhibition Assay
4.6. Cellular Viability Assays
4.7. Scanning Electron Microscope Analysis
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dugassa, J.; Shukuri, N. Review on antibiotic resistance and its mechanism of development. J. Health Med. Nurs. 2017, 1, 1–17. [Google Scholar]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Gunn, J.S.; Bakaletz, L.O.; Wozniak, D.J. What’s on the outside matters: The role of the extracellular polymeric substance of gram-negative biofilms in evading host immunity and as a target for therapeutic intervention. J. Biol. Chem. 2016, 291, 12538–12546. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.W.; He, Y.; Ren, Y.; Zerdoum, A.; Libera, M.R.; Sharma, P.K.; van Winkelhoff, A.J.; Neut, D.; Stoodley, P.; van der Mei, H.C.; et al. Viscoelasticity of biofilms and their recalcitrance to mechanical and chemical challenges. FEMS Microbiol. Rev. 2015, 39, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhu, M.J. Suppressing autophagy: A strategy by Escherichia coli O157:H7 for its survival on host epithelial cells comment. Cell Death Dis. 2018, 9, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Coombes, B.K.; Gilmour, M.W.; Goodman, C.D. The evolution of virulence in non-O157 shiga toxin-producing Escherichia coli. Front. Microbiol. 2011, 2, 1–3. [Google Scholar] [CrossRef]
- Nagy, A.; Mowery, J.; Bauchan, G.R.; Wang, L.; Nichols-Russell, L.; Nou, X. Role of extracellular structures of Escherichia coli O157:H7 in initial attachment to biotic and abiotic surfaces. Appl. Environ. Microbiol. 2015, 81, 4720–4727. [Google Scholar] [CrossRef]
- Linnes, J.C.; Ma, H.; Bryers, J.D. Giant extracellular matrix binding protein expression in Staphylococcus epidermidis is regulated by biofilm formation and osmotic pressure. Curr. Microbiol. 2013, 66, 627–633. [Google Scholar] [CrossRef]
- Morgenstern, M.; Post, V.; Erichsen, C.; Hungerer, S.; Bühren, V.; Militz, M.; Richards, R.G.; Moriarty, T.F. Biofilm formation increases treatment failure in Staphylococcus epidermidis device-related osteomyelitis of the lower extremity in human patients. J. Orthop. Res. 2016, 34, 1905–1913. [Google Scholar] [CrossRef]
- Rogers, K.L.; Fey, P.D.; Rupp, M.E. Coagulase-Negative Staphylococcal Infections. Infect. Dis. Clin. N. Am. 2009, 23, 73–98. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harbor Perspect. Med. 2012, 2, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Papenfort, K.; Bassler, B.; Chase, C. Quorum sensing Signal- Response Systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2017, 14, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.K.; Vinothkumar, K.; Rajpara, N. Bacterial quorum sensing inhibitors: attractive alternatives for control of infectious pathogens showing multiple drug resistance. Recent Pat. Anti-Infect. Drug Discov. 2013, 8, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Vadakkan, K.; Choudhury, A.A.; Gunasekaran, R.; Hemapriya, J.; Vijayanand, S. Quorum sensing intervened bacterial signaling: Pursuit of its cognizance and repression. J. Genet. Eng. Biotechnol. 2018, 16, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Kai, K. Bacterial quorum sensing in symbiotic and pathogenic relationships with hosts. Biosci. Biotechnol. Biochem. 2018, 82, 363–371. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2017, 7. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.; Ademiluyi, A.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Boudjedjou, L.; Ramdani, M.; Zeraib, A.; Benmeddour, T.; Fercha, A. Chemical composition and biological activities of Algerian Santolina africana essential oil. Sci. Afr. 2019, 4, e00090. [Google Scholar] [CrossRef]
- Nunes, B.C.; Martins, M.M.; Chang, R.; Morais, S.A.L.; Nascimento, E.A.; de Oliveira, A.; Cunha, L.C.S.; da Silva, C.V.; Teixeira, T.L.; Ambrósio, M.A.L.V.; et al. Antimicrobial activity, cytotoxicity and selectivity index of Banisteriopsis laevifolia (A. Juss.) B. Gates leaves. Ind. Crops Prod. 2016, 92, 277–289. [Google Scholar] [CrossRef]
- Case, R.J.; Franzblau, S.G.; Wang, Y.; Cho, S.H.; Soejarto, D.D.; Pauli, G.F. Ethnopharmacological evaluation of the informant consensus model on anti-tuberculosis claims among the Manus. J. Ethnopharmacol. 2006, 106, 82–89. [Google Scholar] [CrossRef]
- Burt, S.A.; Ojo-Fakunle, V.T.A.; Woertman, J.; Veldhuizen, E.J.A. The natural antimicrobial carvacrol inhibits quorum sensing in Chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoS ONE 2014, 9, 1–6. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complementary Altern. Med. 2016, 2016, 1–21. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review: Carvacrol and Human Health. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.J.; Deans, S.G. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Assessment of factors influencing antimicrobial activity of carvacrol and cymene against Vibrio cholerae in food. J. Biosci. Bioeng. 2010, 110, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Ochoa, S.; Nevárez-Moorillón, G.V.; Sánchez-Torres, L.E.; Villanueva-García, M.; Sánchez-Ramírez, B.E.; Rodríguez-Valdez, L.M.; Rivera-Chavira, B.E. Quantitative structure-activity relationship of molecules constituent of different essential oils with antimycobacterial activity against Mycobacterium tuberculosis and Mycobacterium bovis. BMC Complementary Altern. Med. 2015, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, T.; Li, F.-Y.; Yao, Y.; Sun, Z.-M. Antibacterial activity and mechanism of action of Monarda punctata essential oil and its main components against common bacterial pathogens in respiratory tract. Int. J. Clin. Exp. Pathol. 2014, 7, 7389–7398. [Google Scholar] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Valdramidis, V.P.; O’Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef] [PubMed]
- Picone, G.; Laghi, L.; Gardini, F.; Lanciotti, R.; Siroli, L.; Capozzi, F. Evaluation of the effect of carvacrol on the Escherichia coli 555 metabolome by using1H-NMR spectroscopy. Food Chem. 2013, 141, 4367–4374. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G.; Grazia, M. Mechanisms of antibacterial action of three monoterpenes mechanisms of antibacterial action of three monoterpenes. J. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Lee, J.H.; Gwon, G.; Kim, S., II; Park, J.G.; Lee, J. Essential Oils and Eugenols Inhibit Biofilm Formation and the Virulence of Escherichia coli O157:H7. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, H.S.; Joo, S.W.; Chandra Regmi, S.; Kim, J.A.; Ryu, C.M.; Ryu, S.Y.; Cho, M.H.; Lee, J. Diverse plant extracts and trans-resveratrol inhibit biofilm formation and swarming of Escherichia coli O157:H7. Biofouling 2013, 29, 1189–1203. [Google Scholar] [CrossRef]
- Okoh, S.O.; Iweriegbor, B.C.; Okoh, O.O.; Nwodo, U.U.; I.Okoh, A. Bactericidal and antioxidant properties of essential oils from the fruits Dennettia tripetala G. Baker. BMC Complementary Altern. Med. 2016, 16, 1–12. [Google Scholar] [CrossRef]
- Upadhyay, A.; Upadhyaya, I.; Kollanoor-Johny, A.; Venkitanarayanan, K. Antibiofilm effect of plant derived antimicrobials on Listeria monocytogenes. Food Microbiol. 2013, 36, 79–89. [Google Scholar] [CrossRef]
- El abed, S.; Ibnsouda koraichi, S.; Latrache, H.; Zineb, G.; Mouradi, H.; Remmal, A. Carvacrol and thymol components inhibiting Pseudomonas aeruginosa adherence and biofilm formation. Afr. J. Microbiol. Res. 2011, 5, 3229–3232. [Google Scholar]
- Faleiro, M.L. The mode of antibacterial action of essential oils. Sci. Against Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 3, 1143–1156. [Google Scholar]
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Ceballos, L.; Caballero-Gallardo, K.; Olivero-Verbel, J. Repellent and anti-quorum sensing activity of six aromatic plants occurring in Colombia. Nat. Prod. Commun. 2015, 10, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Viljoen, A.M.; Chenia, H.Y. The impact of plant volatiles on bacterial quorum sensing. Lett. Appl. Microbiol. 2015, 60, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, E.E.; Ruı, C.A.; Salgar, W. Lippia origanoides chemotype differentiation based on essential oil GC-MS and principal component analysis. J. Sep. Sci. 2010, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40. [Google Scholar] [CrossRef]
- Davies, N.W.; Hons, B.S.A.; Davies, N.W. Mass Spectrometry and Gas Chromatography in Chemical Analysis and identIfication: Experimental Factors and Case Studies by. Ph.D. Thesis, University of Tasmania, Hobart, Australia, 1993. [Google Scholar]
- Cruz, J.; Ortiz, C.; Guzman, F.; Cardenas, C.; Fernandez-Lafuente, R.; Torres, R. Design and activity of novel lactoferrampin analogues against O157:H7 enterohemorrhagic Escherichia coli. Biopolymers 2014, 101, 319–328. [Google Scholar] [CrossRef]
- Blosser, R.S.; Gray, K.M. Extraction of violacein from Chromobacterium violaceum provides a new quantitative bioassay for N -acyl homoserine lactone autoinducers. J. Microbiol. Methods 2000, 40, 47–55. [Google Scholar] [CrossRef]
- Choo, J.H.; Rukayadi, Y.; Hwang, J.K. Inhibition of bacterial quorum sensing by vanilla extract. Lett. Appl. Microbiol. 2006, 42, 637–641. [Google Scholar] [CrossRef]
- Portilla-Pulido, J.S.; Castillo-Morales, R.M.; Barón-Rodríguez, M.A.; Duque, J.E.; Mendez-Sanchez, S.C. Design of a repellent against Aedes aegypti (Diptera: culicidae) using in silico simulations with AaegOBP1 Protein. J. Med. Entomol. 2019, 57, 463–476. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]

| Code | Plant Species | Identified Metabolites |
|---|---|---|
| LACA | Lippia alba | Limonene (29%), β-bourbonone (2.4%), germacrene D (12.2%), carvone (31.3%), and piperitenone (1.5%) |
| LACI | Lippia alba | Limonene (3.9%), trans-β-caryophyllene (11.8%), neral (15.4%), geranial (18.9%), and geraniol (6.1%) |
| CN | Cymbopogon nardus | Citronellal (11.6%), 2,6-dimethyl-2,6-octadiene (6.1%), β-citronellol (16.9%), and geraniol (17.8%) |
| CM | Cymbopogon martini | trans-β-Ocimene (1.9%), linalool (3.2%), geranyl acetate (1.3%), and geraniol (38.7%) |
| CF | Cymbopogon flexuosos | Neral (24.5%), geranial (33%), geraniol (7.9%), and geranyl acetate (0.5%) |
| LTC I | Lippia origanoides | p-Cymene (3.7%), thymyl methyl ether (4.6%), trans-β-caryophyllene (7.9%), thymol (22.1%), carvacrol (10.7%), and thymyl acetate (3.9%) |
| LTC II | Lippia origanoides | γ-Terpinene (5.2%), p-cymene (1.1%), thymol (32,7%), carvacrol (18.8%), and trans-β-caryophyllene (6.4%) |
| LOF | Lippia origanoides | α-Phellandrene (5.7%), 1,8-cineole (11.6%), p-cymene (5.7%), trans-β-caryophyllene (10.4%), and α-humulene (6.2%). |
| RO | Rosmarinus offiicinalis | α-Pinene (12.7%), camphene (7.7%), 1,8-cineole (17.5%), camphor (14.8%), and trans-β-caryophyllene (7.8%). |
| SO | Salvia officinalis | 1,8-Cineole (5.3%), trans-thujone (20.4%), cis-thujone (5.8%), camphor (8.5%), and α-humulene (9.8%). |
| SG | Swinglea glutinosa | α-Pinene (2.6%), trans-β-caryophyllene (36.6%), germacrene D (15.4%), germacrene B (10.8%), and trans-nerolidol (24.0%). |
| TL | Tagetes lucida | Estragole (79.9%) y β-myrcene (0.9%). |
| TV | Thymus vulgaris | γ-Terpinene (9.5%), p-cymene (20%), linalool (4.7%), trans-β-caryophyllene (9.5%), and thymol (23%). |
| SV | Satureja viminea | 1-Isopropenyl-4-methyl-1-ciclohexane (24.4%), trans-β-caryophyllene (11.8%), pulegone (11.1%), and cis-pulegol (7.1%). |
| CO | Cananga odorata | Linalool (11.7%), methyl benzoate (3.7%), benzyl acetate (10.3%), (Z)-cinnamyl acetate (5.4%), and benzyl benzoate (20.8%). |
| EC | E. cardamomum | 1,8-Cineole (8.9%), linalool (6.1%), linalyl butyrate (9.9%), α-terpinyl acetate (45.5%), and cis-nerolidol (3.1%). |
| CS | Citrus sinensis | Limonene (57.5%), linalool (7.9%), 1-octanol (2.1%), 4-terpineol (1.7%) y valencene (1.6%). |
| Essential Oil | E. coli O33 MIC50—MBC | E. coli O157:H7 MIC50—MBC | S. epidermidis MIC50—MBC | cv026 MIC50—MBC |
|---|---|---|---|---|
| Lippia alba (carvona) | >3–>3 | >3–>3 | >3–>3 | >3–>3 |
| Lippia alba (citral) | >3–>3 | >3–>3 | >3–>3 | >3–>3 |
| cymbopogon nardus | >3–>3 | >3–>3 | >3–>3 | >3–>3 |
| Cymbopogon martini | 3 ± 0.22 a–>3 | 3 ± 0.14 a–>3 | 3 ± 0.12 a–>3 | 1,5 ± 0,12 b–3 ± 0.24 a |
| Cymbopogon flexuosus | >3–>3 | >3–>3 | >3–>3 | 3 ± 0.21 a–>3 |
| Lippia origanoides (thymol-carvacrol I) | 0.75 ± 0.14 b–1.5 ± 0.14 b | 0.75 ± 0.10 b -1.5 ± 0.22 b | 0.37 ± 0.04 c–0.75 ± 0.02 b | 0.75 ± 0.03 b–0.75 ± 0.02 b |
| Lippia origanoides (thymol-carvacrol II) | 0.37 ± 0.03 c–0.75 ± 0.02 b | 0.75 ± 0.03 b–0.75 ± 0.03 b | 0.37 ± 0.03 c–0.75 ± 0.04 b | 0.37 ± 0.05 c–0.75 ± 0.02 b |
| Lippia origanoides (felandreno) | 3 ± 0.22 a–>3 | >3–>3 | 3 ± 0.31 a–>3 | 3 ± 0.24 a–3 |
| Rosmarinus officinalis | >3–>3 | >3–>3 | >3–>3 | 3 ± 0.26 a–>3 |
| Salvia officinalis | >3–>3 | >3–>3 | >3–>3 | 3 ± 0.28 a–>3 |
| Swinglea glutinosa | >3–>3 | >3–>3 | >3–>3 | >3–>3 |
| Tagetes lucida | 3 ± 0.23 a–>3 | 3 ± 0.21 a–>3 | 3 ± 0.24 a- >3 | 1.5 ± 0.16 b–3 ± 0.24 a |
| Thymus vulgaris | 0.75 ± 0.01 b –1.5 ± 0.12 b | 0.75 ± 0.02 b–1.5 ± 0.12 b | 0.75 ± 0.10 b–0.75 ± 0.12 b | 0.37 ± 0.05 c–0.75± 0.14 b |
| Satureja viminea | >3–>3 | >3–>3 | >3–>3 | 3 ± 0.22 a–>3 |
| Cananga odorata | >3–>3 | >3–>3 | >3–>3 | >3–>3 |
| Citrus sinensis | >3–>3 | <3–>3 | >3–>3 | 3 ± 0.32 a–>3 |
| Elettaria cardamomum | >3–>3 | >3–>3 | >3–>3 | 3 ± 0.26 a–>3 |
| Essential oils | Vero cell line | SI | ||||
|---|---|---|---|---|---|---|
| IC50 (mg/mL) ± SD a | R2 b | E. coli O33 | E. coli O157:H7 | S. epidermidis | CV 026 | |
| Cymbopogon martini | 0,86 ± 0.12 | 0.97 | −0.54 | −0.54 | −0.54 | −0.24 |
| Lippia origanoides thymol-carvacrol (I) | 0.48 ± 0.02 | 0.99 | −0.19 | −0.19 | −0.19 | −0.19 |
| Lippia origanoides thymol-carvacrol (II) | 0.83 ± 0.02 | 0.98 | 0.35 | 0.04 | 0.04 | 0.35 |
| Thymus vulgaris | 1.76 ± 0.36 | 0.94 | 0.37 | 0.37 | 0.37 | 0.68 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cáceres, M.; Hidalgo, W.; Stashenko, E.; Torres, R.; Ortiz, C. Essential Oils of Aromatic Plants with Antibacterial, Anti-Biofilm and Anti-Quorum Sensing Activities against Pathogenic Bacteria. Antibiotics 2020, 9, 147. https://doi.org/10.3390/antibiotics9040147
Cáceres M, Hidalgo W, Stashenko E, Torres R, Ortiz C. Essential Oils of Aromatic Plants with Antibacterial, Anti-Biofilm and Anti-Quorum Sensing Activities against Pathogenic Bacteria. Antibiotics. 2020; 9(4):147. https://doi.org/10.3390/antibiotics9040147
Chicago/Turabian StyleCáceres, Marlon, William Hidalgo, Elena Stashenko, Rodrigo Torres, and Claudia Ortiz. 2020. "Essential Oils of Aromatic Plants with Antibacterial, Anti-Biofilm and Anti-Quorum Sensing Activities against Pathogenic Bacteria" Antibiotics 9, no. 4: 147. https://doi.org/10.3390/antibiotics9040147
APA StyleCáceres, M., Hidalgo, W., Stashenko, E., Torres, R., & Ortiz, C. (2020). Essential Oils of Aromatic Plants with Antibacterial, Anti-Biofilm and Anti-Quorum Sensing Activities against Pathogenic Bacteria. Antibiotics, 9(4), 147. https://doi.org/10.3390/antibiotics9040147