The Use of a Non-Absorbable Membrane as an Occlusive Barrier for Alveolar Ridge Preservation: A One Year Follow-Up Prospective Cohort Study
Abstract
1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Study Design and Patient Selection
5.2. Clinical Parameters
5.3. Secondary Variables
5.3.1. Implant Stability
5.3.2. Marginal Bone Loss
5.3.3. Peri-Implant Clinical Parameters
- Probing Pocket Depth (PPD). Measured in millimeters, is the distance from the mucosal margin to the bottom of the probable pocket
- Plaque Index (PI) recorded with dichotomic values (present/absent)
- Bleeding on probing recorded with dichotomic values (present/absent)
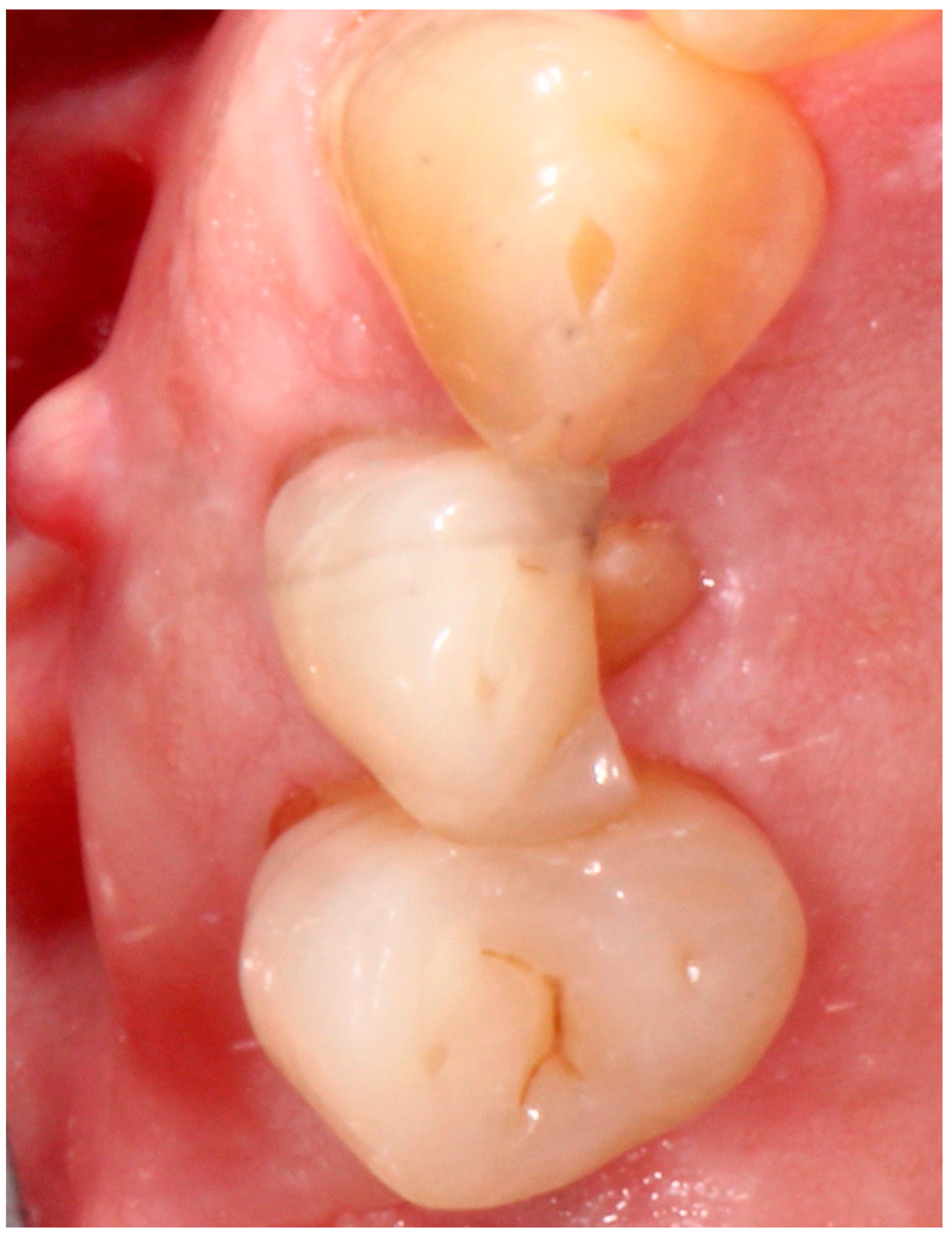
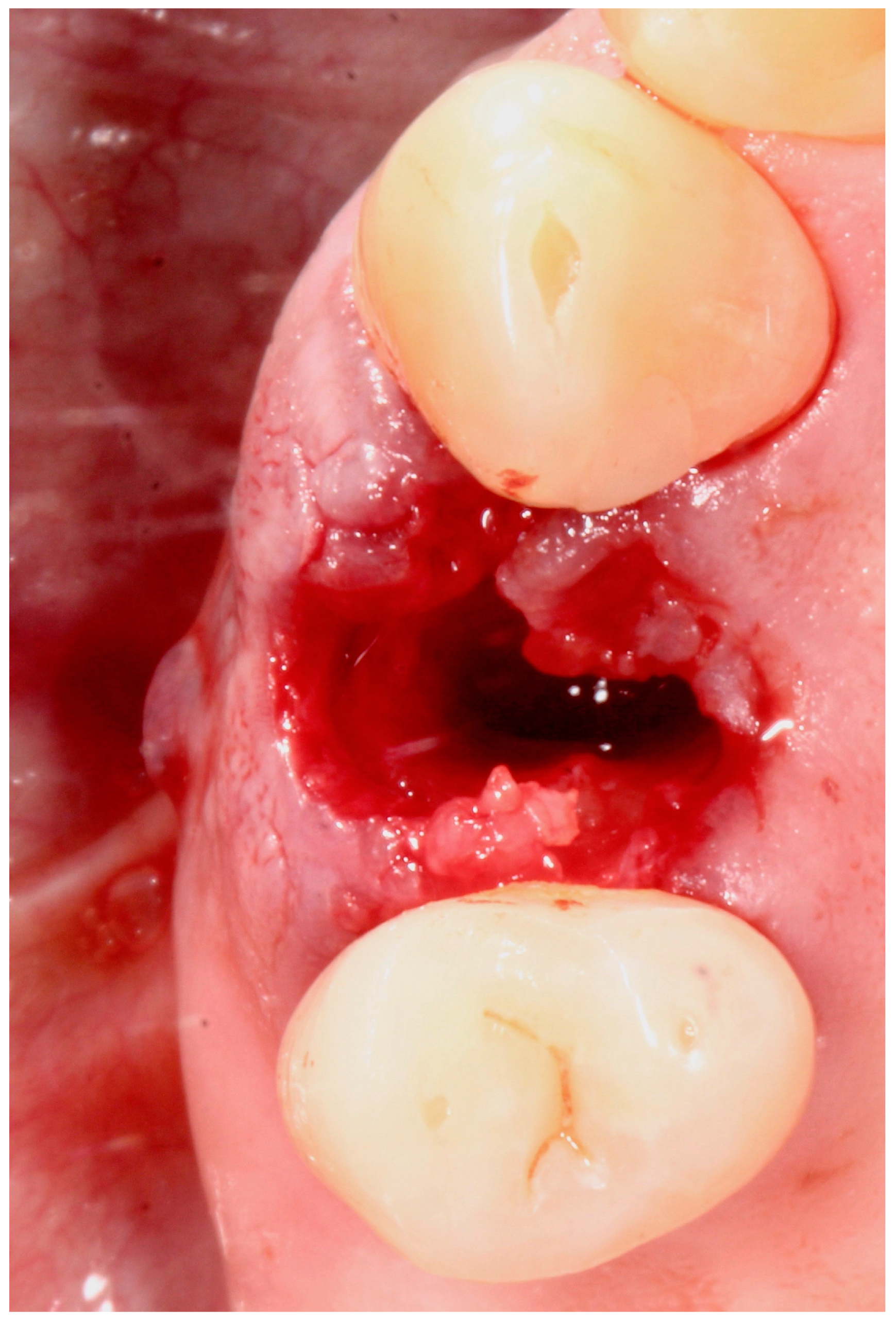
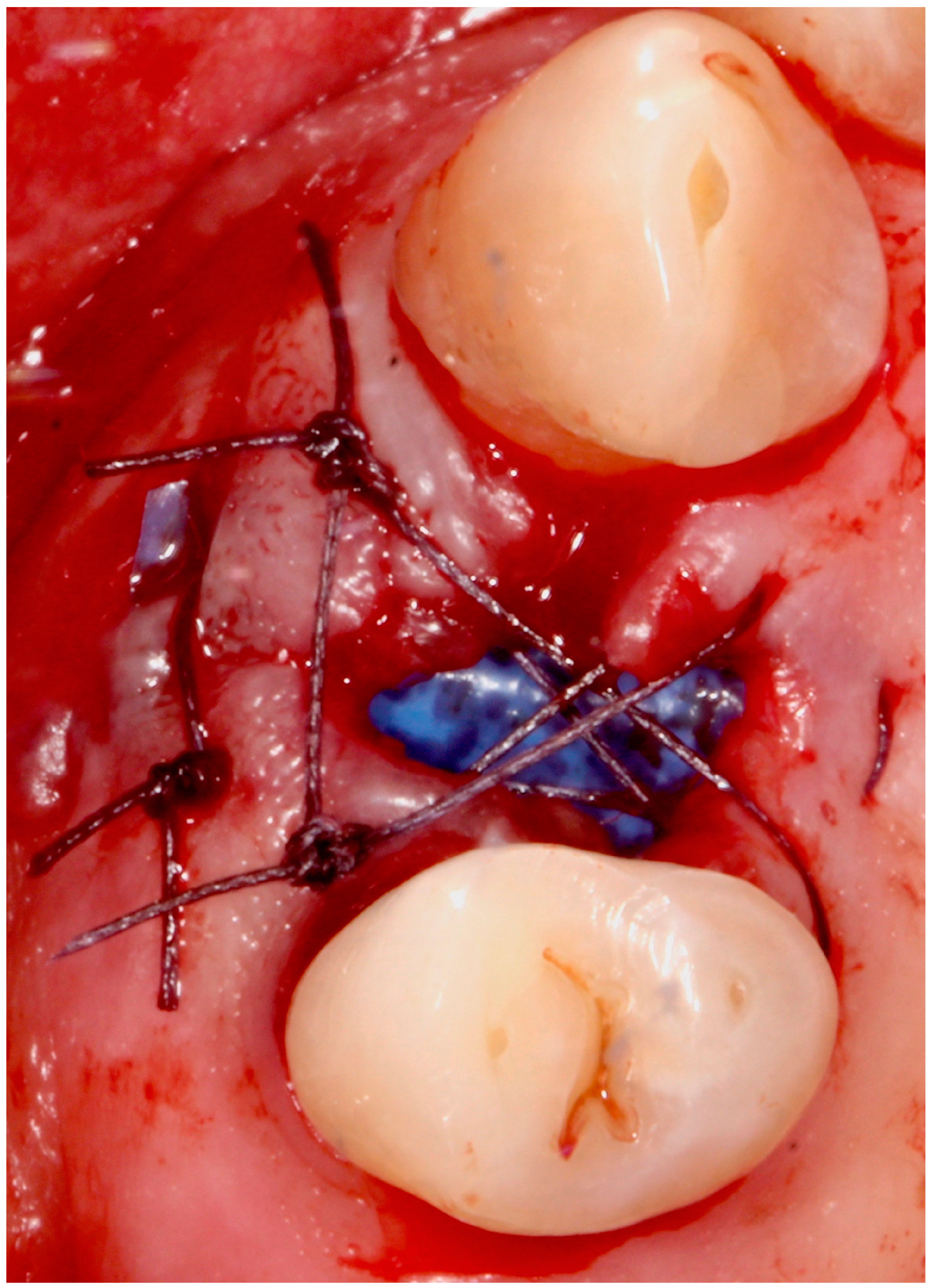
5.4. Surgical Technique
5.5. Implant Placement
5.6. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Pietrokovski, J.; Massler, M. Alveolar ridge resorption following tooth extraction. J. Prosthet. Dent. 1967, 17, 21–27. [Google Scholar] [CrossRef]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Kar-ring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Araujo, M.G.; Sukekava, F.; Wennstrom, J.L.; Lindhe, J. Ridge alterations following im-plant placement in fresh extraction sockets: An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Nevins, M.; Camelo, M.; De Paoli, S.; Friedland, B.; Schenk, R.; Parma-Benfenati, S. A study of the fate of the buccal wall of extraction sockets of teeth with prominent roots. Prim. Dent. Care 2006, 13, 90. [Google Scholar] [CrossRef]
- Braut, V.; Bornstein, M.M.; Belser, U.; Buser, D. Thickness of the anterior maxillary facial bone wall-a retrospective radiographic study using cone beam computed tomography. Int. J. Periodontics Restor. Dent. 2011, 31, 125–131. [Google Scholar]
- Chen, S.T.; Darby, I. The relationship between facial bone wall defects and dimensional alterations of the ridge following flapless tooth extraction in the anterior maxilla. Clin. Oral Implant. Res. 2016, 28, 931–937. [Google Scholar] [CrossRef]
- Januário, A.L.; Duarte, W.R.; Barriviera, M.; Mesti, J.C.; Araújo, M.G.; Lindhe, J. Dimension of the facial bone wall in the anterior maxilla: A cone-beam computed tomography study. Clin. Oral Implant. Res. 2011, 22, 1168–1171. [Google Scholar] [CrossRef]
- Chappuis, V.; Araújo, M.G.; Buser, D. Clinical relevance of dimensional bone and soft tissue alterations post-extraction in esthetic sites. Periodontol. 2000 2016, 73, 73–83. [Google Scholar] [CrossRef]
- Irinakis, T.; Tabesh, M. Preserving the Socket Dimensions With Bone Grafting in Single Sites: An Esthetic Surgical Approach When Planning Delayed Implant Placement. J. Oral Implant. 2007, 33, 156–163. [Google Scholar] [CrossRef]
- Brügger, O.; Bornstein, M.M.; Kuchler, U.; Janner, S.; Chappuis, V.; Buser, D. Implant Therapy in a Surgical Specialty Clinic: An Analysis of Patients, Indications, Surgical Procedures, Risk Factors, and Early Failures. Int. J. Oral Maxillofac. Implant. 2015, 30, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Khzam, N.; Arora, H.; Kim, P.; Fisher, A.; Mattheos, N.; Ivanovski, S. Systematic Review of Soft Tissue Alterations and Esthetic Outcomes Following Immediate Implant Placement and Restoration of Single Implants in the Anterior Maxilla. J. Periodontol. 2015, 86, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, V.; Engel, O.; Shahim, K.; Reyes, M.; Katsaros, C.; Buser, D. Soft Tissue Alterations in Esthetic Postextraction Sites. J. Dent. Res. 2015, 94, 187S–193S. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Naenni, N.; Figuero, E.; Hämmerle, C.; Schwarz, F.; Jung, R.E.; Sanz-Sánchez, I. Effects of soft tissue augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29, 32–49. [Google Scholar] [CrossRef]
- Perussolo, J.; De Souza, A.B.; Matarazzo, F.; De Oliveira, R.P.; Araújo, M.G. Influence of the keratinized mucosa on the stability of peri-implant tissues and brushing discomfort: A 4-year follow-up study. Clin. Oral Implant. Res. 2018, 29, 1177–1185. [Google Scholar] [CrossRef]
- Bonino, F.; Steffensen, B.; Natto, Z.S.; Hur, Y.; Holtzman, L.P.; Weber, H.-P. Prospective study of the impact of peri-implant soft tissue properties on patient-reported and clinically assessed outcomes. J. Periodontol. 2018, 89, 1025–1032. [Google Scholar] [CrossRef]
- Papi, P.; Pompa, G. The Use of a Novel Porcine Derived Acellular Dermal Matrix (Mucoderm) in Peri-Implant Soft Tissue Augmentation: Preliminary Results of a Prospective Pilot Cohort Study. BioMed Res. Int. 2018, 2018, 6406051. [Google Scholar] [CrossRef]
- Mencio, F.; De Angelis, F.; Papi, P.; Rosella, D.; Pompa, G.; Di Carlo, S. A randomized clinical trial about presence of pathogenic microflora and risk of peri-implantitis: Comparison of two different types of implant-abutment connections. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1443–1451. [Google Scholar]
- Del Amo, F.S.L.; Lin, G.-H.; Monje, A.; Galindo-Moreno, P.; Wang, H.-L. Influence of Soft Tissue Thickness on Peri-Implant Marginal Bone Loss: A Systematic Review and Meta-Analysis. J. Periodontol. 2016, 87, 690–699. [Google Scholar] [CrossRef]
- De Angelis, P.; De Angelis, S.; Passarelli, P.C.; Liguori, M.G.; Manicone, P.F.; D’Addona, A. Hard and Soft Tissue Evaluation of Different Socket Preservation Procedures Using Leukocyte and Platelet-Rich Fibrin: A Retrospective Clinical and Volumetric Analysis. J. Oral Maxillofac. Surg. 2019, 77, 1807–1815. [Google Scholar] [CrossRef]
- Caiazzo, A.; Brugnami, F.; Mehra, P. Buccal Plate Augmentation: A New Alternative to Socket Preservation. J. Oral Maxillofac. Surg. 2010, 68, 2503–2506. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Ioannidis, A.; Hämmerle, C.; Thoma, D.S. Alveolar ridge preservation in the esthetic zone. Periodontol. 2000 2018, 77, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Toti, P.; Piattelli, A.; Iezzi, G.; Derchi, G.; Covani, U. Extraction Socket Healing in Humans After Ridge Preservation Techniques: Comparison Between Flapless and Flapped Procedures in a Randomized Clinical Trial. J. Periodontol. 2014, 85, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Fickl, S.; Zuhr, O.; Wachtel, H.; Stappert, C.F.J.; Stein, J.M.; Hürzeler, M.B. Dimensional changes of the alveolar ridge contour after different socket preservation techniques. J. Clin. Periodontol. 2008, 35, 906–913. [Google Scholar] [CrossRef]
- Bassir, S.; Alhareky, M.; Wangsrimongkol, B.; Jia, Y.; Karimbux, N. Systematic Review and Meta-Analysis of Hard Tissue Outcomes of Alveolar Ridge Preservation. Int. J. Oral Maxillofac. Implant. 2018, 33, 979–994. [Google Scholar] [CrossRef]
- Avila-Ortiz, G.; Chambrone, L.; Vignoletti, F. Effect of alveolar ridge preservation interventions following tooth extraction: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 195–223. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Bucchi, C.; Lolato, A.; Corbella, S.; Testori, T.; Taschieri, S. Healing of Postextraction Sockets Preserved With Autologous Platelet Concentrates. A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2017, 75, 1601–1615. [Google Scholar] [CrossRef]
- Jonker, B.P.; Roeloffs, M.W.K.; Wolvius, E.B.; Pijpe, J. The clinical value of membranes in bone augmentation procedures in oral implantology: A systematic review of randomised controlled trials. Eur. J. Oral Implant. 2016, 9, 335–365. [Google Scholar]
- Iocca, O.; Farcomeni, A.; Talib, H.S.; Pardiñas-Lopez, S. Alveolar Ridge Preservation after tooth extraction: A Bayesian Network meta-analysis of grafting materials efficacy on prevention of bone height and width reduction. J. Clin. Periodontol. 2016, 44, 104–114. [Google Scholar] [CrossRef]
- De Risi, V.; Clementini, M.; Vittorini, G.; Mannocci, A.; De Sanctis, M. Alveolar ridge preservation techniques: A systematic review and meta-analysis of histological and histomorphometrical data. Clin. Oral Implant. Res. 2013, 26, 50–68. [Google Scholar] [CrossRef]
- Macbeth, N.; Trullenque-Eriksson, A.; Donos, N.; Mardas, N. Hard and soft tissue changes following alveolar ridge preservation: A systematic review. Clin. Oral Implant. Res. 2016, 28, 982–1004. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kim, -Y.; Kim, K.-H.; Ku, Y.; Rhyu, I.-C.; Lee, Y.-M. The efficacy of a double-layer collagen membrane technique for overlaying block grafts in a rabbit calvarium model. Clin. Oral Implant. Res. 2009, 20, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- AlKanan, A.; Greenwell, H.; Patel, A.; Hill, M.; Shumway, B.; Lowy, J. Ridge Preservation Comparing the Clinical and Histologic Healing of Membrane vs No-Membrane Approach to Buccal Overlay Grafting. Int. J. Periodontics Restor. Dent. 2019, 39, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Simion, M.; Baldoni, M.; Rossi, P.; Zaffe, D. A comparative study of the effectiveness of e-PTFE membranes with and without early exposure during the healing period. Int. J. Periodontics Restor. Dent. 1994, 14, 166–180. [Google Scholar]
- Ronda, M.; Rebaudi, A.; Torelli, L.; Stacchi, C. Expanded vs. dense polytetrafluoroethylene membranes in vertical ridge augmentation around dental implants: A prospective randomized controlled clinical trial. Clin. Oral Implant. Res. 2013, 25, 859–866. [Google Scholar] [CrossRef]
- Ling, L.-J.; Hung, S.-L.; Lee, C.-F.; Chen, Y.-T.; Wu, K.-M. The influence of membrane exposure on the outcomes of guided tissue regeneration: Clinical and microbiological aspects. J. Periodontal Res. 2003, 38, 57–63. [Google Scholar] [CrossRef]
- Naung, N.Y.; Shehata, E.; Van Sickels, J.E. Resorbable Versus Nonresorbable Membranes: When and Why? Dent. Clin. N. Am. 2019, 63, 419–431. [Google Scholar] [CrossRef]
- Soldatos, N.K.; Stylianou, P.; Koidou, V.P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guidedbone regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar]
- Laurito, D.; Lollobrigida, M.; Gianno, F.; Bosco, S.; Lamazza, L.; De Biase, A. Alveolar Ridge Preservation with nc-HA and d-PTFE Membrane: A Clinical, Histologic, and Histomorphometric Study. Int. J. Periodontics Restor. Dent. 2017, 37, 283–290. [Google Scholar] [CrossRef]
- Hoffmann, O.; Bartee, B.K.; Beaumont, C.; Kasaj, A.; Deli, G.; Zafiropoulos, G.-G. Alveolar Bone Preservation in Extraction Sockets Using Non-Resorbable dPTFE Membranes: A Retrospective Non-Randomized Study. J. Periodontol. 2008, 79, 1355–1369. [Google Scholar] [CrossRef]
- Laurito, D.; Cugnetto, R.; Lollobrigida, M.; Guerra, F.; Vestri, A.; Gianno, F.; Bosco, S.; Lamazza, L.; De Biase, A. Socket Preservation with d-PTFE Membrane: Histologic Analysis of the Newly Formed Matrix at Membrane Removal. Int. J. Periodontics Restor. Dent. 2016, 36, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.; Kernen, F.; Bidra, A.S. Clinical and histologic outcomes of socket grafting after flapless tooth extraction: A systematic review of randomized controlled clinical trials. J. Prosthet. Dent. 2015, 113, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; Rodriguez, J.C.; Rudek, I.; Benavides, E.; Rios, H.; Wang, H.-L. Effectiveness of three different alveolar ridge preservation techniques: A pilot randomized controlled trial. Int. J. Periodontics Restor. Dent. 2014, 34, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Mandarino, D.; Luz, D.; Moraschini, V.; Rodrigues, D.; Barboza, E. Alveolar ridge preservation using a non-resorbable membrane: Randomized clinical trial with biomolecular analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Barboza, E.P.; Stutz, B.; Mandarino, D.; Rodrigues, D.M.; Ferreira, V.F. Evaluation of a Dense Polytetrafluoroethylene Membrane to Increase Keratinized Tissue. Implant. Dent. 2014, 23, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Deli, G.; Petrone, V.; De Risi, V.; Tadic, D.; Zafiropoulos, G.-G. Longitudinal Implant Stability Measurements Based on Resonance Frequency Analysis After Placement in Healed or Regenerated Bone. J. Oral Implant. 2014, 40, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Malchiodi, L.; Balzani, L.; Cucchi, A.; Ghensi, P.; Nocini, P. Primary and Secondary Stability of Implants in Postextraction and Healed Sites: A Randomized Controlled Clinical Trial. Int. J. Oral Maxillofac. Implant. 2016, 31, 1435–1443. [Google Scholar] [CrossRef]
- Monje, A.; Ravidà, A.; Wang, H.-L.; A Helms, J.; Brunski, J.B. Relationship Between Primary/Mechanical and Secondary/Biological Implant Stability. Int. J. Oral Maxillofac. Implant. 2019, 34, s7–s23. [Google Scholar] [CrossRef]
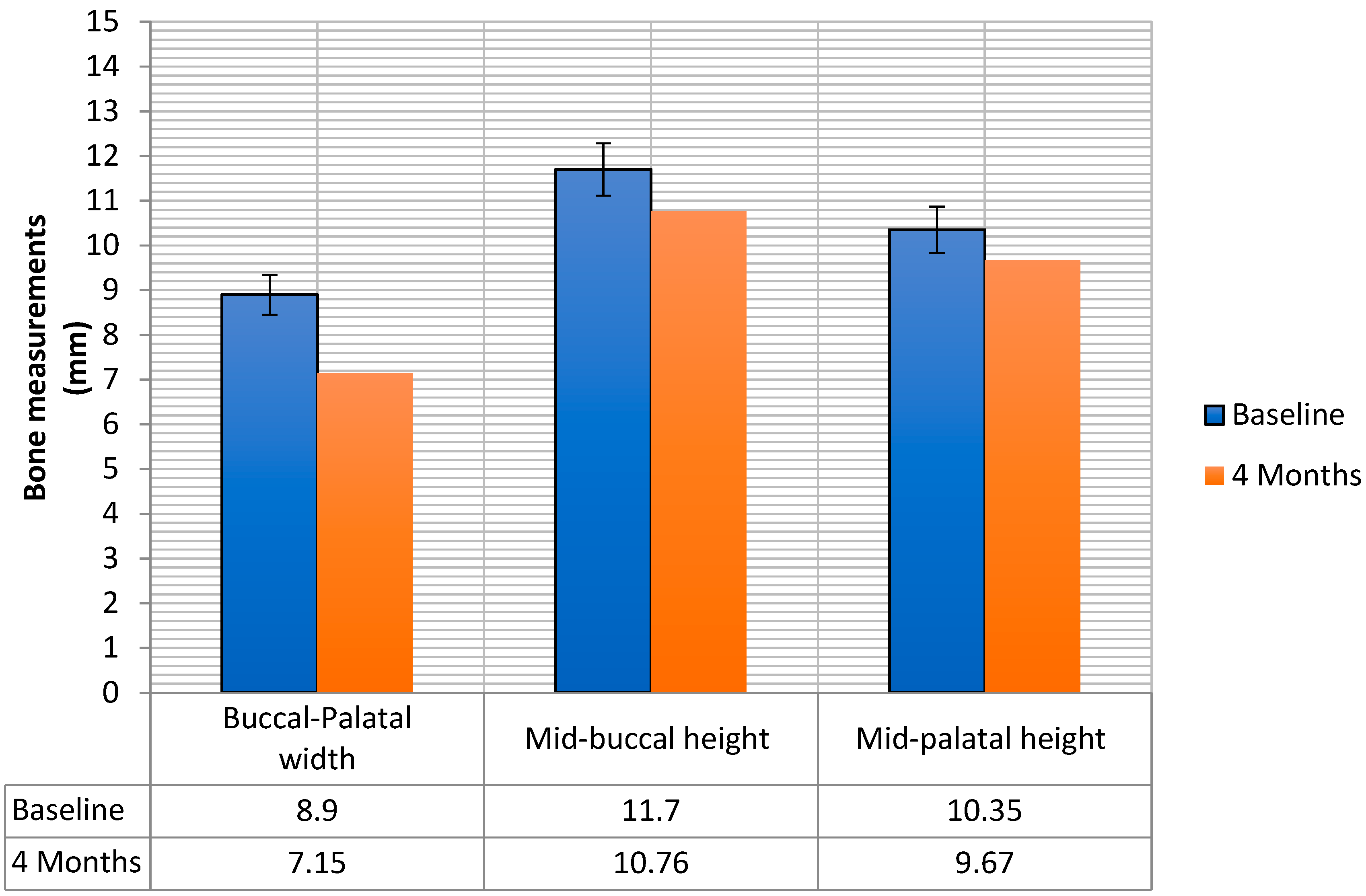

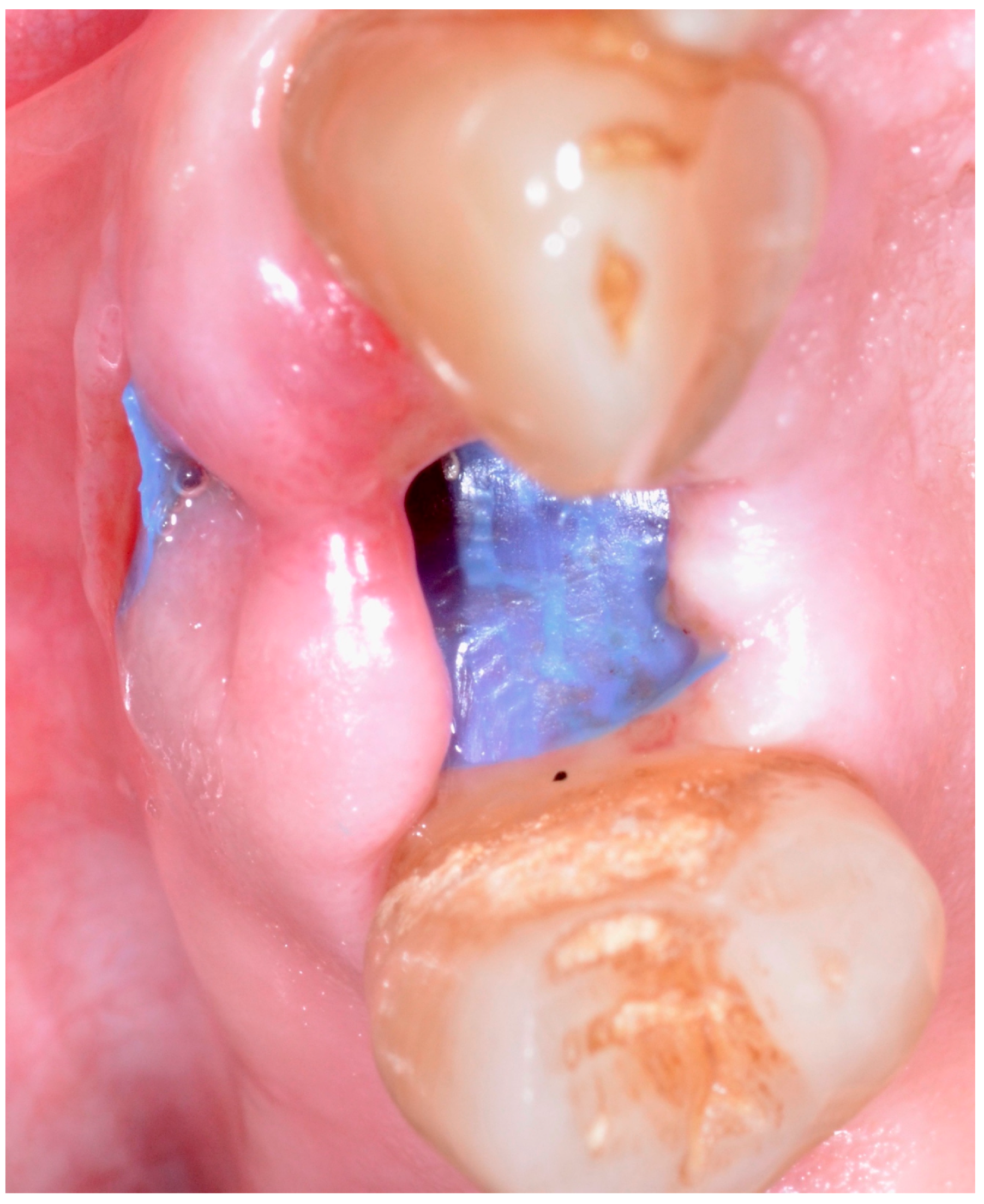
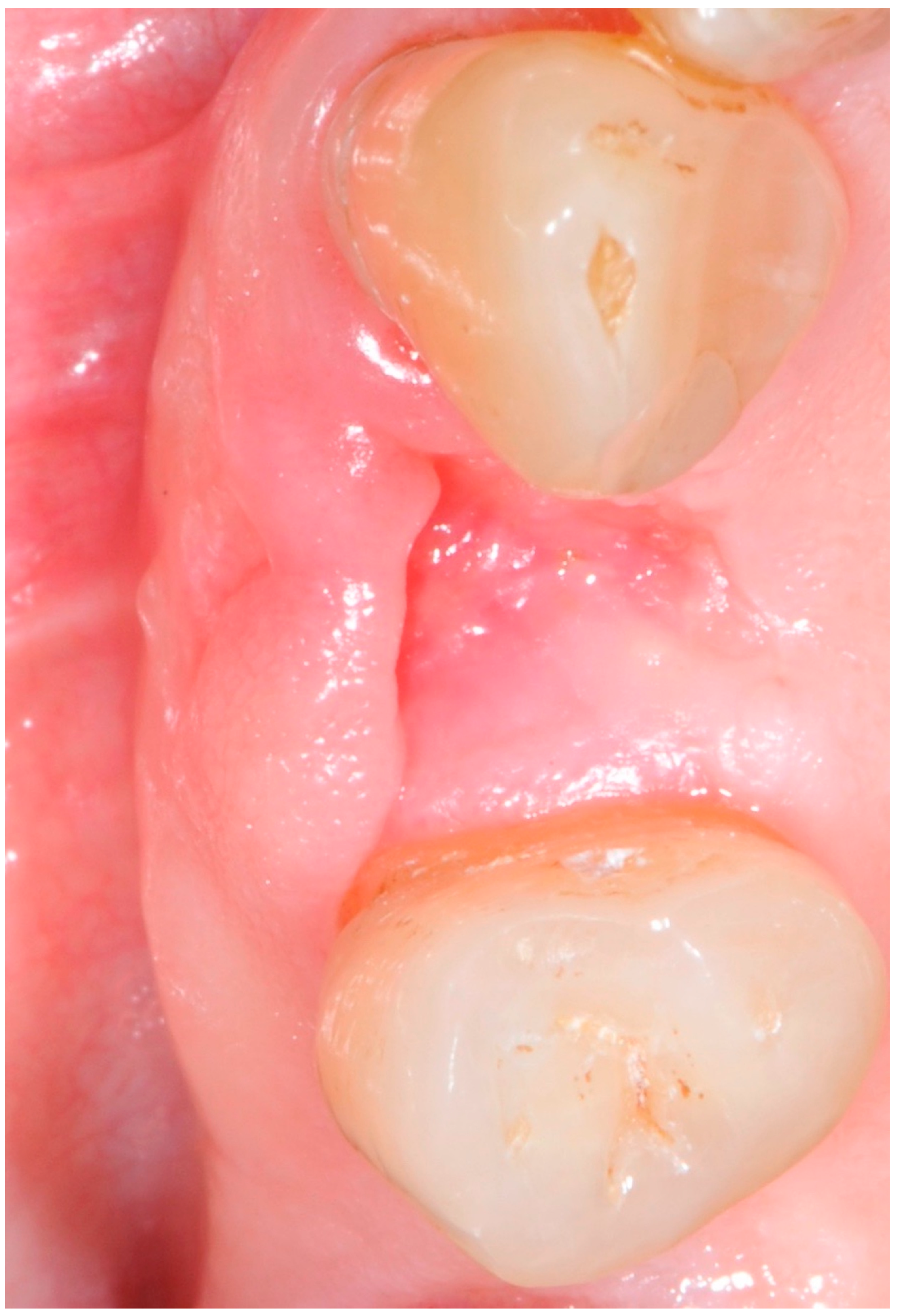



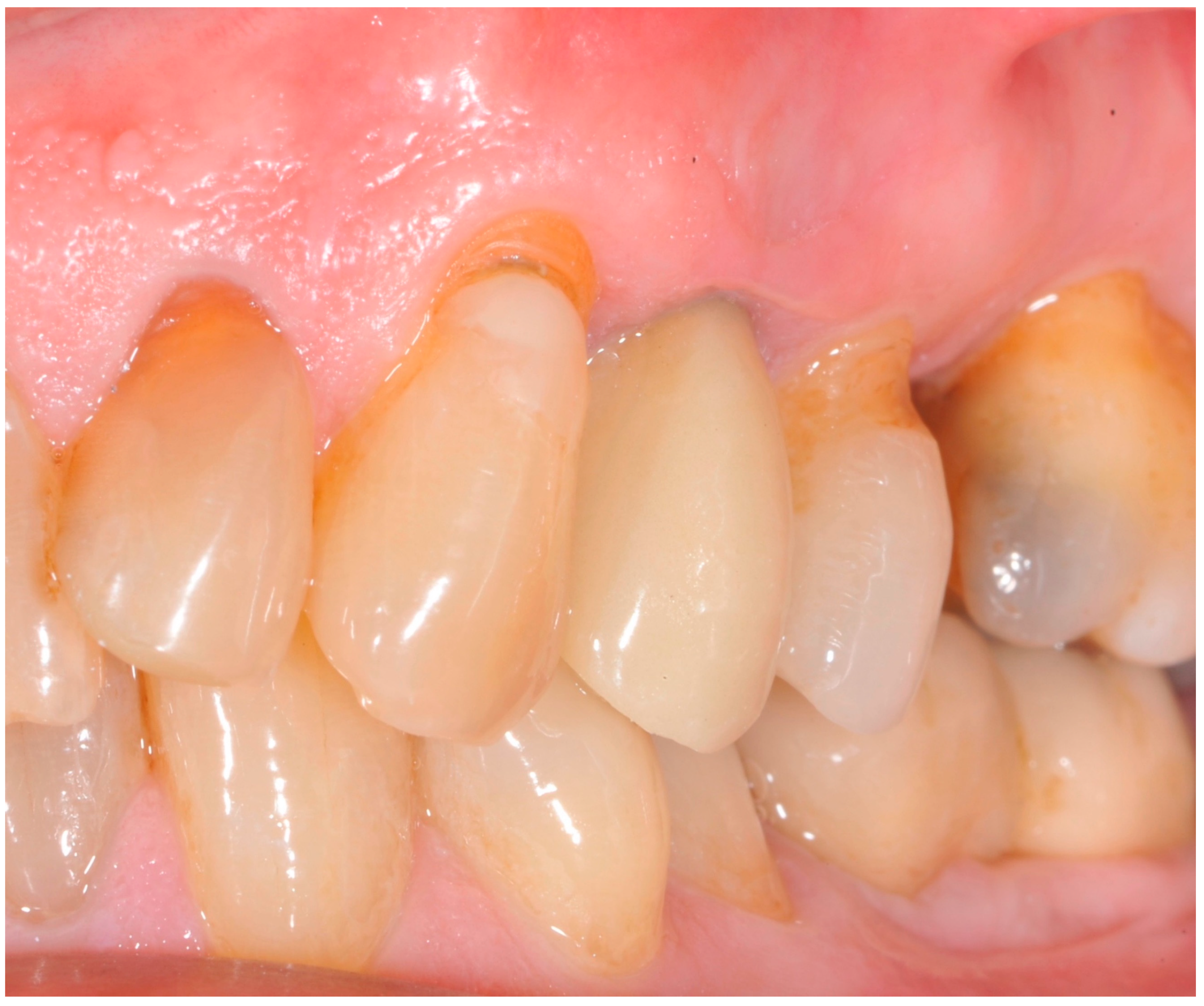
| Study Variable | Descriptive Statistics |
|---|---|
| Sample size (n) | 15 |
| Male | 9 |
| Female | 6 |
| Age (y) ± SD (range) | 49.4 ± 10.4 years (range: 35–67 years, median: 51 years) |
| Dental Implants BLX | |
| Diameter 4.5 mm | 9 |
| Diameter 3.75 mm | 6 |
| Length 10 mm | 10 |
| Length 12 mm | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papi, P.; Di Murro, B.; Tromba, M.; Passarelli, P.C.; D’Addona, A.; Pompa, G. The Use of a Non-Absorbable Membrane as an Occlusive Barrier for Alveolar Ridge Preservation: A One Year Follow-Up Prospective Cohort Study. Antibiotics 2020, 9, 110. https://doi.org/10.3390/antibiotics9030110
Papi P, Di Murro B, Tromba M, Passarelli PC, D’Addona A, Pompa G. The Use of a Non-Absorbable Membrane as an Occlusive Barrier for Alveolar Ridge Preservation: A One Year Follow-Up Prospective Cohort Study. Antibiotics. 2020; 9(3):110. https://doi.org/10.3390/antibiotics9030110
Chicago/Turabian StylePapi, Piero, Bianca Di Murro, Marco Tromba, Pier Carmine Passarelli, Antonio D’Addona, and Giorgio Pompa. 2020. "The Use of a Non-Absorbable Membrane as an Occlusive Barrier for Alveolar Ridge Preservation: A One Year Follow-Up Prospective Cohort Study" Antibiotics 9, no. 3: 110. https://doi.org/10.3390/antibiotics9030110
APA StylePapi, P., Di Murro, B., Tromba, M., Passarelli, P. C., D’Addona, A., & Pompa, G. (2020). The Use of a Non-Absorbable Membrane as an Occlusive Barrier for Alveolar Ridge Preservation: A One Year Follow-Up Prospective Cohort Study. Antibiotics, 9(3), 110. https://doi.org/10.3390/antibiotics9030110







