Successful High-Dosage Monotherapy of Tigecycline in a Multidrug-Resistant Klebsiella pneumoniae Pneumonia–Septicemia Model in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Genotypic Characterization of Bacterial Strains
2.3. Antibiotics and Anesthetics
2.4. Antimicrobial Susceptibility of K. pneumoniae Strains
2.5. Concentration- and Time-Dependent Bactericidal Activity of Antibiotics In Vitro
2.6. Animals
2.7. Ethics
2.8. Rat Model of Pneumonia–Septicemia
2.9. Characterization of Pneumonia–Septicemia Model
2.10. Antimicrobial Treatment of Pneumonia–Septicemia
2.11. Pharmacokinetics of Meropenem
2.12. Statistical Analysis
3. Results
3.1. Bacterial Strains
3.2. Antimicrobial Susceptibility of K. pneumoniae Strains
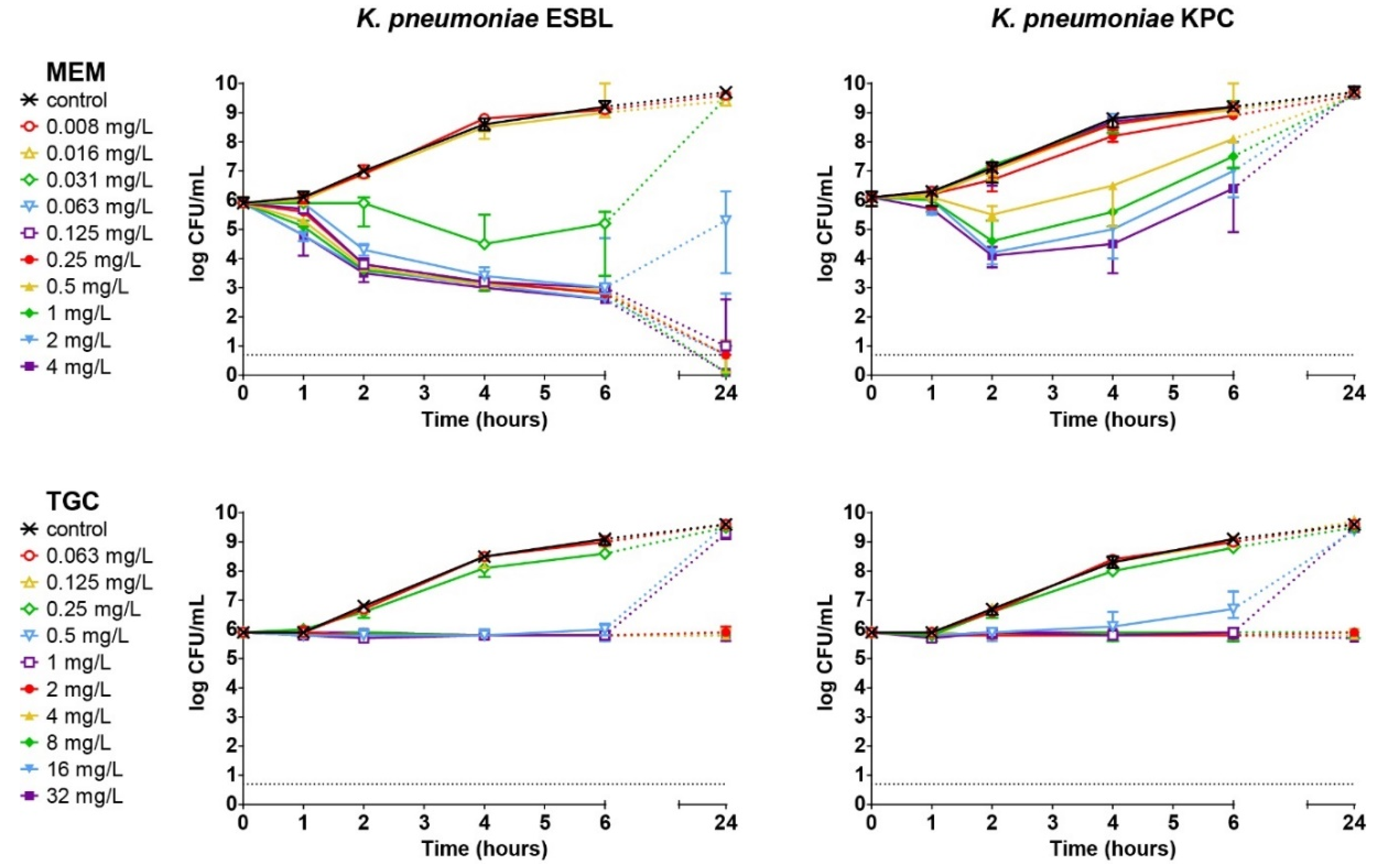
3.3. Concentration- and Time-Dependent Bactericidal Activity of Antibiotics In Vitro
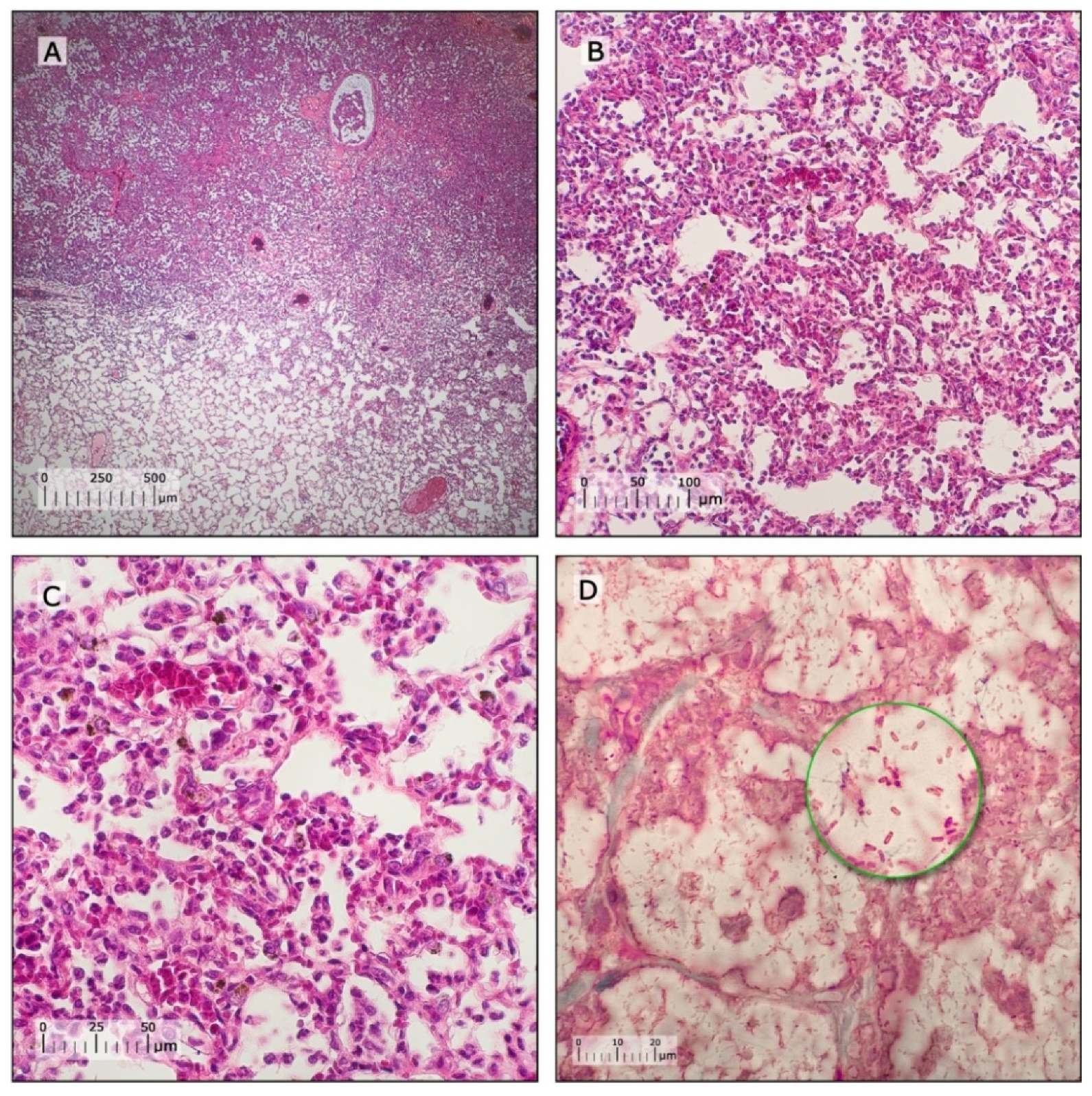
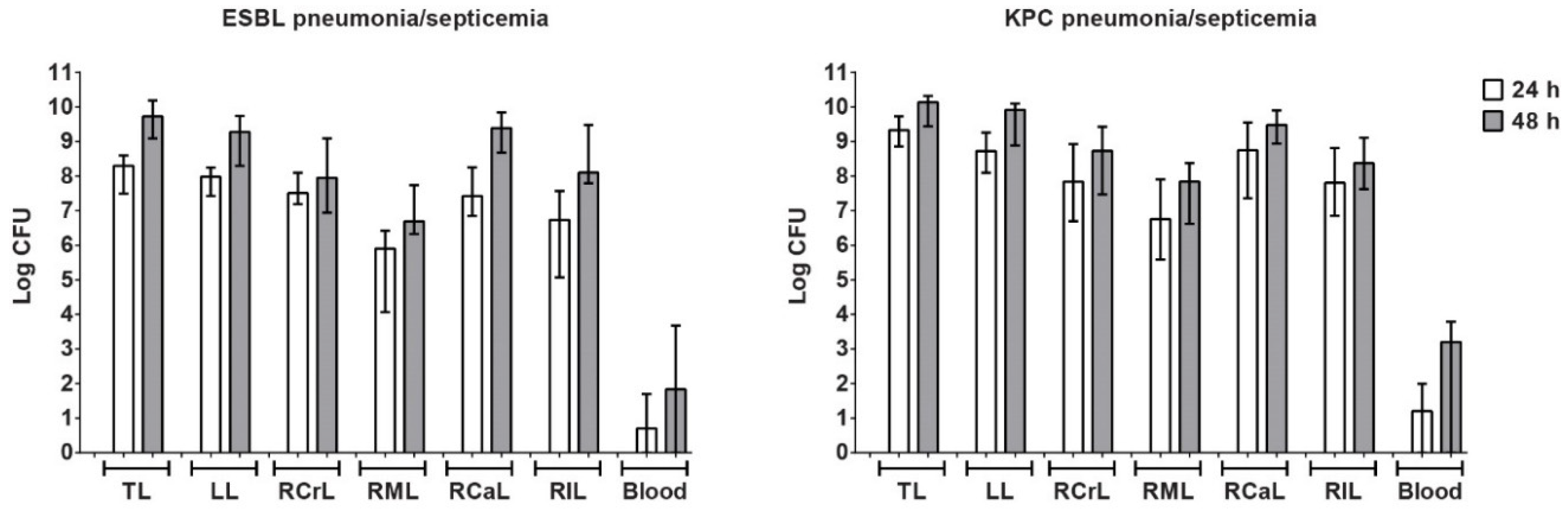
3.4. Characterization of Pneumonia–Septicemia Model
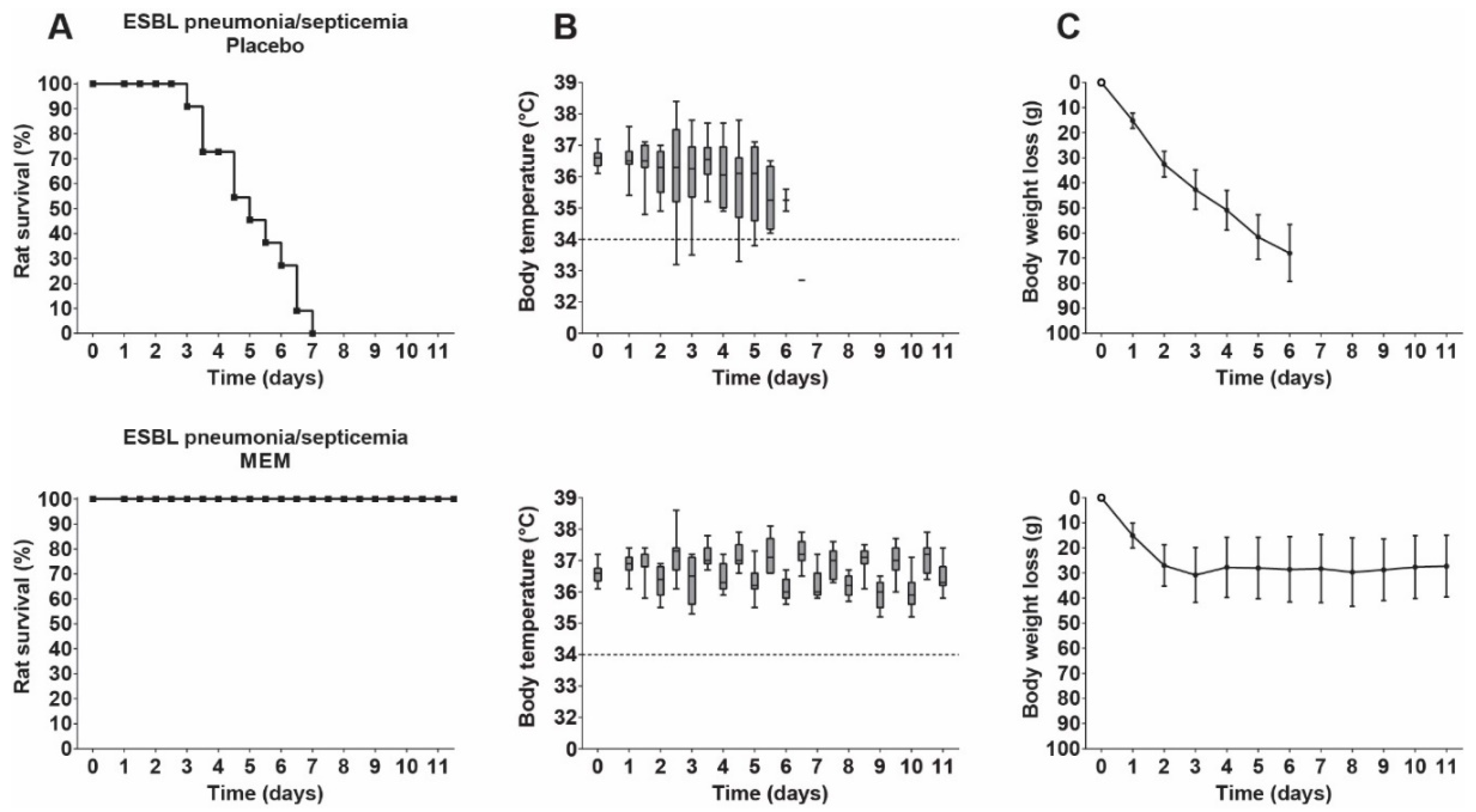
3.5. Therapeutic Efficacy of Meropenem in Rats with ESBL Pneumonia–Septicemia
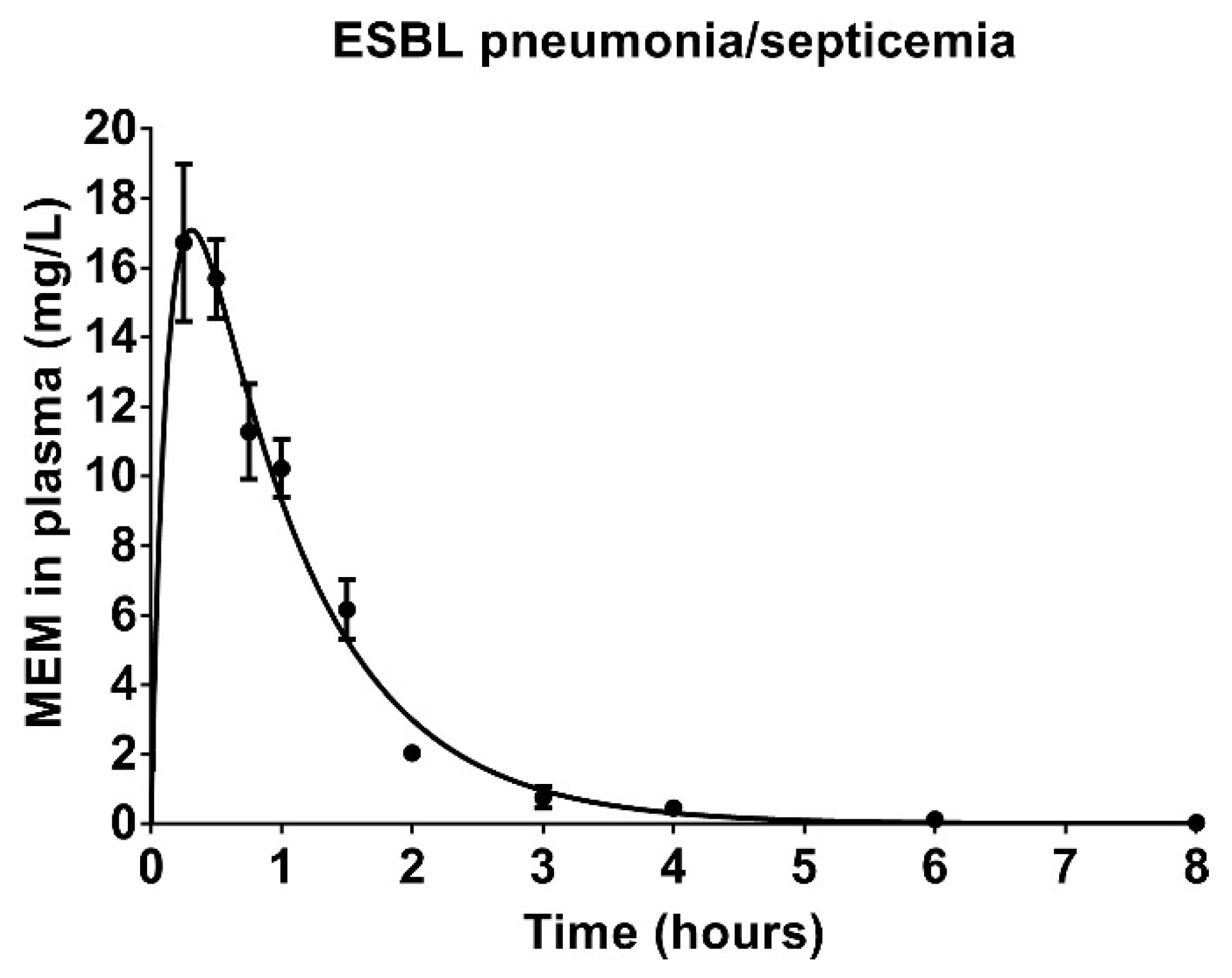
3.6. Pharmacokinetics of Meropenem in Rats with ESBL Pneumonia–Septicemia
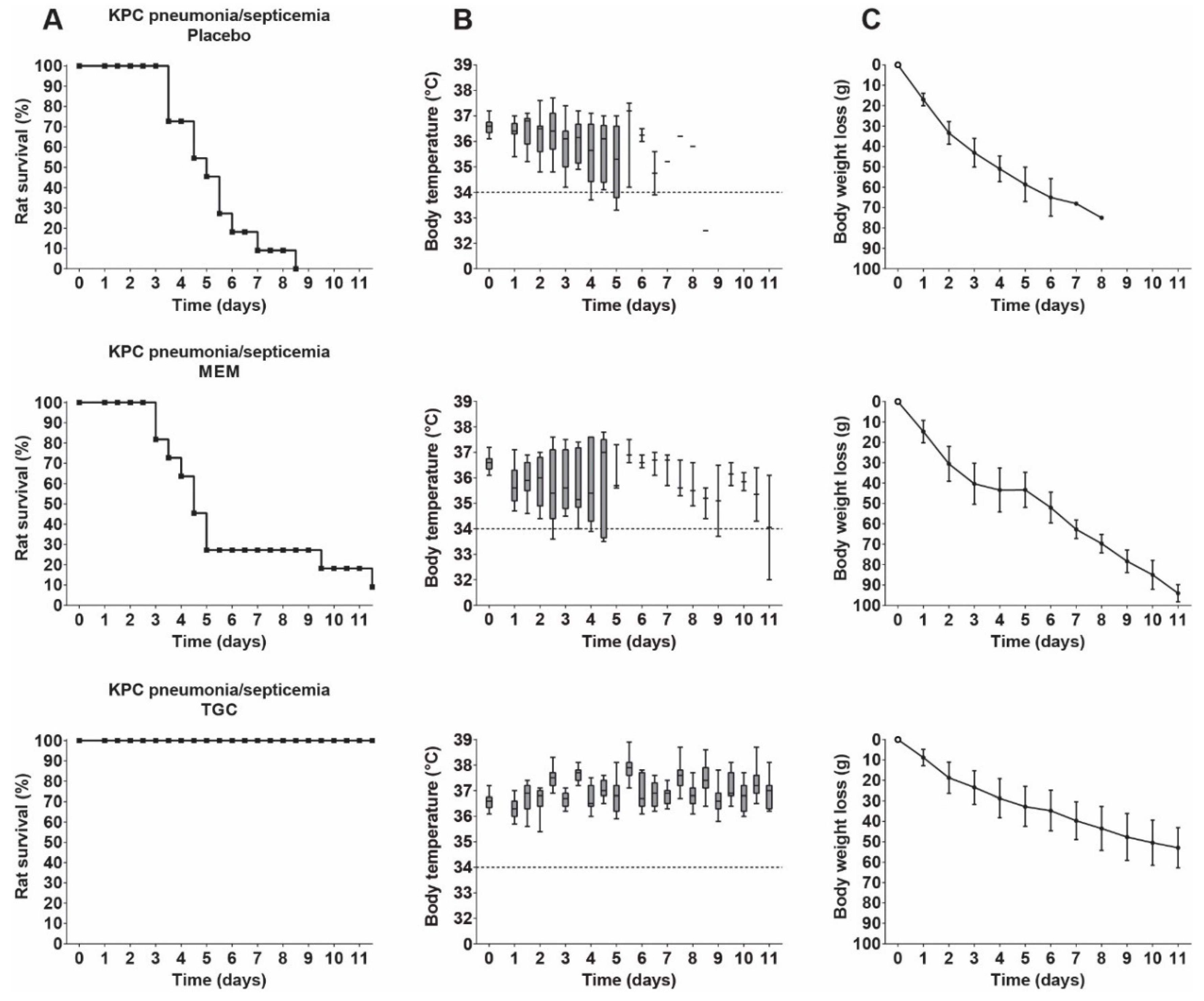
3.7. Therapeutic Efficacy of Meropenem and Tigecycline in Rats with KPC Pneumonia–Septicemia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Peleg, A.Y.; Hooper, D.C. Hospital-acquired infections due to gram-negative bacteria. N. Engl. J. Med. 2010, 362, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Girlich, D.; Poirel, L.; Nordmann, P. CTX-M expression and selection of ertapenem resistance in Klebsiella pneumoniae and Escherichia coli. Antimicrob. Agents Chemother. 2009, 53, 832–834. [Google Scholar] [CrossRef] [PubMed]
- Tzouvelekis, L.S.; Markogiannakis, A.; Psichogiou, M.; Tassios, P.T.; Daikos, G.L. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: An evolving crisis of global dimensions. Clin. Microbiol. Rev. 2012, 25, 682–707. [Google Scholar] [CrossRef] [PubMed]
- Duin, D.; Cober, E.D.; Richter, S.S.; Perez, F.; Cline, M.; Kaye, K.S.; Kalayjian, R.C.; Salata, R.A.; Evans, S.R.; Fowler, V.G. Tigecycline therapy for carbapenem-resistant Klebsiella pneumoniae (CRKP) bacteriuria leads to tigecycline resistance. Clin. Microbiol. Infect. 2014, 20, O1117–O1120. [Google Scholar] [CrossRef]
- Temkin, E.; Adler, A.; Lerner, A.; Carmeli, Y. Carbapenem-resistant Enterobacteriaceae: Biology, epidemiology, and management. Ann. N. Y. Acad. Sci. 2014, 1323, 22–42. [Google Scholar] [CrossRef]
- Burkhardt, O.; Rauch, K.; Kaever, V.; Hadem, J.; Kielstein, J.T.; Welte, T. Tigecycline possibly underdosed for the treatment of pneumonia: A pharmacokinetic viewpoint. Int. J. Antimicrob. Agents 2009, 34, 101–102. [Google Scholar] [CrossRef]
- Cunha, B.A.; Baron, J.; Cunha, C.B. Monotherapy with High-Dose Once-Daily Tigecycline is Highly Effective Against Acinetobacter baumanii and other Multidrug-Resistant (MDR) Gram-Negative Bacilli (GNB). Int. J. Antimicrob. Agents 2018, 52, 119. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Guidance Document on Tigecycline Dosing in association with Revision of Breakpoints for Enterobacterales and Other Species with an “Intermediate” Category (December 2018). Available online: http://www.eucast.org (accessed on 2 March 2020).
- Falagas, M.E.; Vardakas, K.Z.; Tsiveriotis, K.P.; Triarides, N.A.; Tansarli, G.S. Effectiveness and safety of high-dose tigecycline-containing regimens for the treatment of severe bacterial infections. Int. J. Antimicrob. Agents 2014, 44, 1–7. [Google Scholar] [CrossRef]
- Heizmann, W.; Löschmann, P.-A.; Eckmann, C.; Von Eiff, C.; Bodmann, K.-F.; Petrik, C. Clinical efficacy of tigecycline used as monotherapy or in combination regimens for complicated infections with documented involvement of multiresistant bacteria. Infection 2015, 43, 37–43. [Google Scholar] [CrossRef]
- Goessens, W.H.F.; Mouton, J.W.; Marian, T.; Sörgel, F.; Kinzig, M.; Bakker-Woudenberg, I.A.J.M. The therapeutic effect of tigecycline, unlike that of ceftazidime, is not influenced by whether the Klebsiella pneumoniae strain produces extended-spectrum β-lactamases in experimental pneumonia in rats. Antimicrob. Agents Chemother. 2013, 57, 643–646. [Google Scholar] [CrossRef][Green Version]
- Bassetti, M.; Welte, T.; Wunderink, R.G. Treatment of Gram-negative pneumonia in the critical care setting: Is the beta-lactam antibiotic backbone broken beyond repair? Crit. Care 2015, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Fasciana, T.; Gentile, B.; Aquilina, M.; Ciammaruconi, A.; Mascarella, C.; Anselmo, A.; Fortunato, A.; Fillo, S.; Petralito, G.; Lista, F. Co-existence of virulence factors and antibiotic resistance in new Klebsiella pneumoniae clones emerging in south of Italy. BMC Infect. Dis. 2019, 19, 928. [Google Scholar] [CrossRef] [PubMed]
- Hennequin, C.; Robin, F. Correlation between antimicrobial resistance and virulence in Klebsiella pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Woodford, N.; Fagan, E.J.; Ellington, M.J. Multiplex PCR for rapid detection of genes encoding CTX-M extended-spectrum β-lactamases. J. Antimicrob. Chemother. 2005, 57, 154–155. [Google Scholar] [CrossRef]
- Mabilat, C.; Goussard, S. PCR detection and identification of genes for extended-spectrum β-lactamases. In Diagnostic Molecular Microbiology: Principles and Applications; American Society for Microbiology: Washington, DC, USA, 1993; pp. 553–559. [Google Scholar]
- Nüesch-Inderbinen, M.T.; Hächler, H.; Kayser, F.H. Detection of genes coding for extended-spectrum SHV beta-lactamases in clinical isolates by a molecular genetic method, and comparison with the E test. Eur. J. Clin. Microbiol. Infect. Dis. 1996, 15, 398–402. [Google Scholar] [CrossRef]
- Dallenne, C.; Da Costa, A.; Decré, D.; Favier, C.; Arlet, G. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010, 65, 490–495. [Google Scholar] [CrossRef]
- Karisik, E.; Ellington, M.J.; Pike, R.; Warren, R.E.; Livermore, D.M.; Woodford, N. Molecular characterization of plasmids encoding CTX-M-15 β-lactamases from Escherichia coli strains in the United Kingdom. J. Antimicrob. Chemother. 2006, 58, 665–668. [Google Scholar] [CrossRef]
- Bradford, P.A.; Bratu, S.; Urban, C.; Visalli, M.; Mariano, N.; Landman, D.; Rahal, J.J.; Brooks, S.; Cebular, S.; Quale, J. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 β-lactamases in New York City. Clin. Infect. Dis. 2004, 39, 55–60. [Google Scholar] [CrossRef]
- Islam, M.A.; Talukdar, P.K.; Hoque, A.; Huq, M.; Nabi, A.; Ahmed, D.; Talukder, K.A.; Pietroni, M.A.C.; Hays, J.P.; Cravioto, A. Emergence of multidrug-resistant NDM-1-producing Gram-negative bacteria in Bangladesh. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2593–2600. [Google Scholar] [CrossRef]
- Boers, S.A.; Van der Reijden, W.A.; Jansen, R. High-throughput multilocus sequence typing: Bringing molecular typing to the next level. PLoS ONE 2012, 7, e39630. [Google Scholar] [CrossRef]
- Souverein, D.; Boers, S.A.; Veenendaal, D.; Euser, S.M.; Kluytmans, J.; Den Boer, J.W. Polyclonal Spread and Outbreaks with ESBL Positive Gentamicin Resistant Klebsiella spp. in the Region Kennemerland, The Netherlands. PLoS ONE 2014, 9, e101212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gruteke, P.; Goessens, W.; Van Gils, J.; Peerbooms, P.; Lemmens-den Toom, N.; Van Santen-Verheuvel, M.; Van Belkum, A.; Verbrugh, H. Patterns of resistance associated with integrons, the extended-spectrum β-lactamase SHV-5 gene, and a multidrug efflux pump of Klebsiella pneumoniae causing a nosocomial outbreak. J. Clin. Microbiol. 2003, 41, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Padilla, E.; Alonso, D.; Doménech-Sánchez, A.; Gomez, C.; Pérez, J.L.; Albertí, S.; Borrell, N. Effect of porins and plasmid-mediated AmpC β-lactamases on the efficacy of β-lactams in rat pneumonia caused by Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2006, 50, 2258–2260. [Google Scholar] [CrossRef][Green Version]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters, version 10.0. 2020. Available online: http://www.eucast.org (accessed on 2 March 2020).
- De Steenwinkel, J.E.M.; De Knegt, G.J.; Ten Kate, M.T.; Van Belkum, A.; Verbrugh, H.A.; Kremer, K.; Van Soolingen, D.; Bakker-Woudenberg, I.A.J.M. Time–kill kinetics of anti-tuberculosis drugs, and emergence of resistance, in relation to metabolic activity of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2010, 65, 2582–2589. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Antimicrobial Wild Type Distributions of Microorganisms, Version 5.26. 2019. Available online: http://mic.eucast.org/Eucast2/ (accessed on 2 March 2020).
- Balls, M.; Goldberg, A.M.; Fentem, J.H.; Broadhead, C.L.; Burch, R.L.; Festing, M.F.; Frazier, J.M.; Hendriksen, C.F.; Jennings, M.; Van der Kamp, M. The three Rs: The way forward: The report and recommendations of ECVAM Workshop 11. ATLA 1995, 23, 838. [Google Scholar]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, 100412. [Google Scholar] [CrossRef]
- Abdulla, A.; Bahmany, S.; Wijma, R.A.; Van der Nagel, B.C.H.; Koch, B.C.P. Simultaneous determination of nine β-lactam antibiotics in human plasma by an ultrafast hydrophilic-interaction chromatography–tandem mass spectrometry. J. Chromatogr. B 2017, 1060, 138–143. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- Hori, T.; Nakano, M.; Kimura, Y.; Murakami, K. Pharmacokinetics and tissue penetration of a new carbapenem, doripenem, intravenously administered to laboratory animals. In Vivo 2006, 20, 91–96. [Google Scholar]
- Bradford, P.A. Extended-spectrum β-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef]
- Freire, A.T.; Melnyk, V.; Kim, M.J.; Datsenko, O.; Dzyublik, O.; Glumcher, F.; Chuang, Y.-C.; Maroko, R.T.; Dukart, G.; Cooper, C.A. Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn. Microbiol. Infect. Dis. 2010, 68, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Duran, F.Y.; Yıldırım, H.; Şen, E.M. A Lesser Known Side Effect of Tigecycline: Hypofibrinogenemia. Turk. J. Hematol. 2018, 35, 83. [Google Scholar] [CrossRef] [PubMed]
- Pieringer, H.; Schmekal, B.; Biesenbach, G.; Pohanka, E. Severe coagulation disorder with hypofibrinogenemia associated with the use of tigecycline. Ann. Hematol. 2010, 89, 1063–1064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, S.; Zhou, J. Tigecycline treatment causes a decrease in fibrinogen levels. Antimicrob. Agents Chemother. 2015, 59, 1650–1655. [Google Scholar] [CrossRef]
- Routsi, C.; Kokkoris, S.; Douka, E.; Ekonomidou, F.; Karaiskos, I.; Giamarellou, H. High-dose tigecycline-associated alterations in coagulation parameters in critically ill patients with severe infections. Int. J. Antimicrob. Agents 2015, 45, 90–93. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, P.; Dong, L.; Zhang, X. A case report of patient with severe acute cholangitis with tigecycline treatment causing coagulopathy and hypofibrinogenemia. Medicine 2017, 96, e9124. [Google Scholar] [CrossRef]
- Gong, J.; Su, D.; Shang, J.; Yu, H.; Du, G.; Lin, Y.; Sun, Z.; Liu, G. Efficacy and safety of high-dose tigecycline for the treatment of infectious diseases: A meta-analysis. Medicine 2019, 98, e17091. [Google Scholar] [CrossRef]
- Cunha, B.A.; Baron, J.; Cunha, C.B. Once daily high dose tigecycline-pharmacokinetic/pharmacodynamic based dosing for optimal clinical effectiveness: Dosing matters, revisited. Expert Rev. Anti-Infect. Ther. 2017, 15, 257–267. [Google Scholar] [CrossRef]
- Baron, J.; Cai, S.; Klein, N.; Cunha, B. Once Daily High Dose Tigecycline Is Optimal: Tigecycline PK/PD Parameters Predict Clinical Effectiveness. J. Clin. Med. 2018, 7, 49. [Google Scholar] [CrossRef]
- De Pascale, G.; Montini, L.; Pennisi, M.A.; Bernini, V.; Maviglia, R.; Bello, G.; Spanu, T.; Tumbarello, M.; Antonelli, M. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit. Care 2014, 18, R90. [Google Scholar] [CrossRef]
- Geng, T.-T.; Xu, X.; Huang, M. High-dose tigecycline for the treatment of nosocomial carbapenem-resistant Klebsiella pneumoniae bloodstream infections: A retrospective cohort study. Medicine 2018, 97, e9961. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, X. Adverse events of high-dose tigecycline in the treatment of ventilator-associated pneumonia due to multidrug-resistant pathogens. Medicine 2018, 97, e12647. [Google Scholar] [CrossRef]
- Gao, H.; Yao, Z.; Li, Y.; Huang, S.; Liu, J.; Jing, Y. Safety and Effectiveness of High Dose Tigecycline for Treating Patients with Acute Leukemia after Ineffctiveness of Carbapenems Chemotherapy Combinating with Febrile Neutropenia: Retrospective study [Article in Chinese; spelling errors present in PubMed reference]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2018, 26, 684–690. [Google Scholar] [PubMed]
- Tsala, M.; Vourli, S.; Daikos, G.L.; Tsakris, A.; Zerva, L.; Mouton, J.W.; Meletiadis, J. Impact of bacterial load on pharmacodynamics and susceptibility breakpoints for tigecycline and Klebsiella pneumoniae. J. Antimicrob. Chemother. 2016, 72, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Li, G.; Zhao, J.; Cui, J.; Wang, R.; Gao, Z.; Liu, Y. Use of Monte Carlo simulation to evaluate the efficacy of tigecycline and minocycline for the treatment of pneumonia due to carbapenemase-producing Klebsiella pneumoniae. Infect. Dis. 2018, 50, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Jeng, Y.-Y.; Chen, T.-L.; Fung, C.-P. Bacteremic community-acquired pneumonia due to Klebsiella pneumoniae: Clinical and microbiological characteristics in Taiwan, 2001–2008. BMC Infect. Dis. 2010, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Wang, Y.-P.; Wang, F.-D.; Fung, C.-P. Community-onset Klebsiella pneumoniae pneumonia in Taiwan: Clinical features of the disease and associated microbiological characteristics of isolates from pneumonia and nasopharynx. Front. Microbiol. 2015, 6, 122. [Google Scholar] [CrossRef]
- Qureshi, Z.A.; Paterson, D.L.; Potoski, B.A.; Kilayko, M.C.; Sandovsky, G.; Sordillo, E.; Polsky, B.; Adams-Haduch, J.M.; Doi, Y. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: Superiority of combination antimicrobial regimens. Antimicrob. Agents Chemother. 2012. [Google Scholar] [CrossRef]
- Xie, J.; Roberts, J.A.; Alobaid, A.S.; Roger, C.; Wang, Y.; Yang, Q.; Sun, J.; Dong, H.; Wang, X.; Xing, J. Population pharmacokinetics of tigecycline in critically ill patients with severe infections. Antimicrob. Agents Chemother. 2017, 61, e00345-17. [Google Scholar] [CrossRef]
- Nicolau, D.P. Pharmacokinetic and pharmacodynamic properties of meropenem. Clin. Infect. Dis. 2008, 47, S32–S40. [Google Scholar] [CrossRef]
- Gilbert, D.N.; Chambers, H.F.; Eliopoulos, G.M.; Saag, M.S.; Pavia, A.T. The Sanford Guide to Antimicrobial Therapy, 47th ed.; Antimicrobial Theraphy, Inc.: Sperryville, VA, USA, 2017. [Google Scholar]
- Mouton, J.W.; Touw, D.J.; Horrevorts, A.M.; Vinks, A.A. Comparative pharmacokinetics of the carbapenems. Clin. Pharmacokinet. 2000, 39, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, G.; Micalizzi, M.; Speth, J.; Raible, D.; Troy, S. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob. Agents Chemother. 2005, 49, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, I.M.; Crandon, J.L.; Lesho, E.P.; McGann, P.; Nicolau, D.P. Efficacy of humanized high-dose meropenem, cefepime, and levofloxacin against Enterobacteriaceae isolates producing Verona integron-encoded metallo-β-lactamase (VIM) in a murine thigh infection model. Antimicrob. Agents Chemother. 2015, 59, 7145–7147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giannella, M.; Trecarichi, E.M.; Giacobbe, D.R.; De Rosa, F.G.; Bassetti, M.; Bartoloni, A.; Bartoletti, M.; Losito, A.R.; Del Bono, V.; Corcione, S. Effect of combination therapy containing a high-dose carbapenem on mortality in patients with carbapenem-resistant Klebsiella pneumoniae bloodstream infection. Int. J. Antimicrob. Agents 2018, 51, 244–248. [Google Scholar] [CrossRef]

| Strain | ATCC 43816 | EMC 2003 | EMC 2014 | ATCC 13883 | ATCC 700603 | ATCC BAA1705 | |
|---|---|---|---|---|---|---|---|
| Phenotype | WT Parent | ESBL | KPC | WT | ESBL | KPC | |
| MIC (mg/L) | CAZ | 0.5 S | 256 R | 256 R | 0.5 S | 64 R | 128 R |
| MEM | 0.063 S | 0.063 S | 16 R | 0.063 S | 0.063 S | 32 R | |
| AMK | 2 S | 2 S | 2 S | 1 S | 1 S | 32 R | |
| TOB | 0.5 S | ≥128 R | ≥128 R | 0.5 S | 8 R | 32 R | |
| CIP | 0.031 S | 0.125 S | 0.063 S | 0.125 S | 0.5 I | ≥64 R | |
| NOR | 0.125 S | 0.25 S | 0.25 S | 0.25 S | 2 R | 512 R | |
| SXT | 0.5 S | 1 S | 2 S | 0.5 S | 4 I | ≥128 R | |
| CST | 0.5 S | 1 S | 0.5 S | 2 S | 1 S | 0.5 S | |
| TGC | 1 R | 1 R | 2 R | 1 R | 16 R | 4 R | |
| Pharmacokinetic Parameter | V/F | CL/F | t1/2 | ƒCmax | ƒT > MIC | |||
|---|---|---|---|---|---|---|---|---|
| 0.063 mg/L | 16 mg/L | |||||||
| L/kg | L/kg/h | h | mg/L | h | % | h | % | |
| Estimate | 1.03 | 1.17 | 0.61 | 13.37 | 5.18 | 43.17 | 0.00 | 0.00 |
| SEM | 0.07 | 0.06 | 0.03 | 1.43 | 0.24 | 2.00 | 0.00 | 0.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van der Weide, H.; Ten Kate, M.T.; Vermeulen-de Jongh, D.M.C.; Van der Meijden, A.; Wijma, R.A.; Boers, S.A.; Van Westreenen, M.; Hays, J.P.; Goessens, W.H.F.; Bakker-Woudenberg, I.A.J.M. Successful High-Dosage Monotherapy of Tigecycline in a Multidrug-Resistant Klebsiella pneumoniae Pneumonia–Septicemia Model in Rats. Antibiotics 2020, 9, 109. https://doi.org/10.3390/antibiotics9030109
Van der Weide H, Ten Kate MT, Vermeulen-de Jongh DMC, Van der Meijden A, Wijma RA, Boers SA, Van Westreenen M, Hays JP, Goessens WHF, Bakker-Woudenberg IAJM. Successful High-Dosage Monotherapy of Tigecycline in a Multidrug-Resistant Klebsiella pneumoniae Pneumonia–Septicemia Model in Rats. Antibiotics. 2020; 9(3):109. https://doi.org/10.3390/antibiotics9030109
Chicago/Turabian StyleVan der Weide, Hessel, Marian T. Ten Kate, Denise M. C. Vermeulen-de Jongh, Aart Van der Meijden, Rixt A. Wijma, Stefan A. Boers, Mireille Van Westreenen, John P. Hays, Wil H. F. Goessens, and Irma A. J. M. Bakker-Woudenberg. 2020. "Successful High-Dosage Monotherapy of Tigecycline in a Multidrug-Resistant Klebsiella pneumoniae Pneumonia–Septicemia Model in Rats" Antibiotics 9, no. 3: 109. https://doi.org/10.3390/antibiotics9030109
APA StyleVan der Weide, H., Ten Kate, M. T., Vermeulen-de Jongh, D. M. C., Van der Meijden, A., Wijma, R. A., Boers, S. A., Van Westreenen, M., Hays, J. P., Goessens, W. H. F., & Bakker-Woudenberg, I. A. J. M. (2020). Successful High-Dosage Monotherapy of Tigecycline in a Multidrug-Resistant Klebsiella pneumoniae Pneumonia–Septicemia Model in Rats. Antibiotics, 9(3), 109. https://doi.org/10.3390/antibiotics9030109





