Brevinin-2GHk from Sylvirana guentheri and the Design of Truncated Analogs Exhibiting the Enhancement of Antimicrobial Activity
Abstract
1. Introduction
2. Results
2.1. Identification of Brevinin-2GHk from the Skin Secretion of Sylvirana guentheri
2.2. Chemical Synthesis of BR2GK and Design of Derivatives
2.3. Antimicrobial Activity of BR2GK and Derivatives
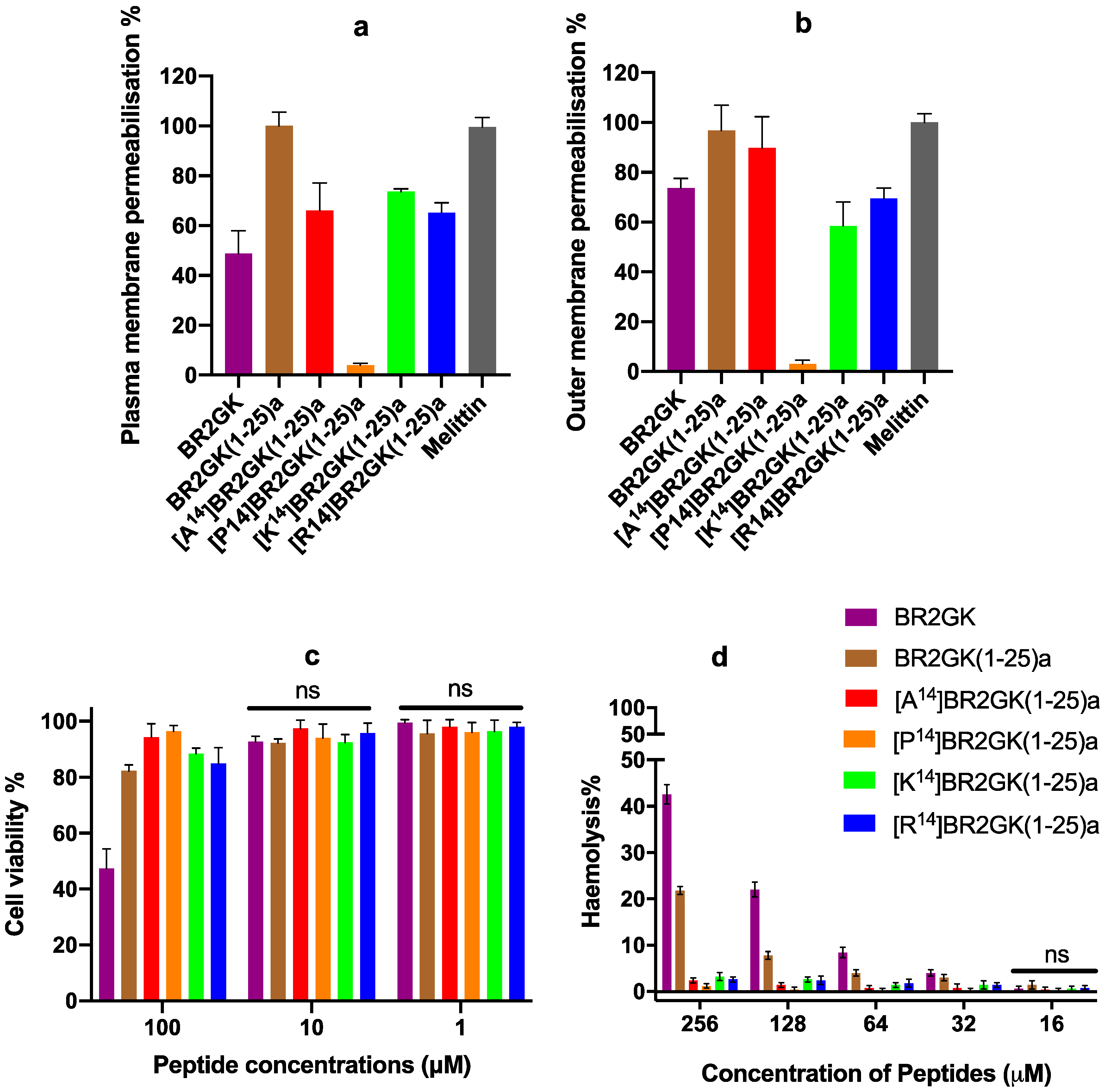
2.4. Cytotoxicity of BR2GK and Derivatives
3. Discussion
4. Materials and Methods
4.1. Acquisition of Skin Secretion of Sylvirana guentheri
4.2. Molecular Cloning of cDNA-Encoding Precursor from Skin Secretions
4.3. Fractionation of Skin Secretion and MS/MS Analysis
4.4. Solid Phase Peptide Synthesis
4.5. Secondary Structure Analysis
4.6. Antimicrobial Susceptibility Test
4.7. SYTOX Green Dye Uptake Assay
4.8. NPN Outer Membrane Assay
4.9. Evaluation of In Vitro Cytotoxicity
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Demori, I.; El Rashed, Z.; Corradino, V.; Catalano, A.; Rovegno, L.; Queirolo, L.; Salvidio, S.; Biggi, E.; Zanotti-Russo, M.; Canesi, L.; et al. Peptides for Skin Protection and Healing in Amphibians. Molecules 2019, 24, 347. [Google Scholar] [CrossRef] [PubMed]
- König, E.; Bininda-Emonds, O.R.P.; Shaw, C. The diversity and evolution of anuran skin peptides. Peptides 2015, 63, 96–117. [Google Scholar] [CrossRef] [PubMed]
- Ladram, A.; Nicolas, P. Antimicrobial peptides from frog skin: Biodiversity and therapeutic promises. Front. Biosci. Landmark 2016, 21, 1341–1371. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, N.; Hagiwara, K.; Nakajima, T. Brevinin-1 and-2, unique antimicrobial peptides from the skin of the frog, Rana brevipoda porsa. Biochem. Biophys. Res. Commun. 1992, 189, 184–190. [Google Scholar] [CrossRef]
- Savelyeva, A.; Ghavami, S.; Davoodpour, P.; Asoodeh, A.; Łos, M.J. An overview of brevinin superfamily: Structure, function and clinical perspectives. Adv. Exp. Med. Biol. 2014, 818, 197–212. [Google Scholar] [PubMed]
- Conlon, J.M.; Kolodziejek, J.; Nowotny, N. Antimicrobial peptides from ranid frogs: Taxonomic and phylogenetic markers and a potential source of new therapeutic agents. Biochim. Biophys. Acta Proteins Proteomics 2004, 1696, 1–14. [Google Scholar] [CrossRef]
- Conlon, J.M.; Ahmed, E.; Condamine, E. Antimicrobial properties of brevinin-2-related peptide and its analogs: Efficacy against multidrug-resistant acinetobacter baumannii. Chem. Biol. Drug Des. 2009, 74, 488–493. [Google Scholar] [CrossRef]
- Conlon, J.M.; Sonnevend, Á.; Patel, M.; Al-Dhaheri, K.; Nielsen, P.F.; Kolodziejek, J.; Nowotny, N.; Iwamuro, S.; Pál, T. A family of brevinin-2 peptides with potent activity against Pseudomonas aeruginosa from the skin of the Hokkaido frog, Rana pirica. Regul. Pept. 2004, 118, 135–141. [Google Scholar] [CrossRef]
- Conlon, J.M.; Kolodziejek, J.; Mechkarska, M.; Coquet, L.; Leprince, J.; Jouenne, T.; Vaudry, H.; Nielsen, P.F.; Nowotny, N.; King, J.D. Host defense peptides from Lithobates forreri, Hylarana luctuosa, and Hylarana signata (Ranidae): Phylogenetic relationships inferred from primary structures of ranatuerin-2 and brevinin-2 peptides. Comp. Biochem. Physiol. Part D Genomics Proteomics 2014, 9, 49–57. [Google Scholar] [CrossRef]
- Conlon, J.M.; Kolodziejek, J.; Nowotny, N.; Leprince, J.; Vaudry, H.; Coquet, L.; Jouenne, T.; Iwamuro, S. Cytolytic peptides belonging to the brevinin-1 and brevinin-2 families isolated from the skin of the Japanese brown frog, Rana dybowskii. Toxicon 2007, 50, 746–756. [Google Scholar] [CrossRef]
- Casciaro, B.; Cappiello, F.; Cacciafesta, M.; Mangoni, M.L. Promising approaches to optimize the biological properties of the antimicrobial peptide esculentin-1a(1-21)NH2: Amino acids substitution and conjugation to nanoparticles. Front. Chem. 2017, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Shukla, S.K.; Prakash, O.; Zhang, G. Structural determinants of host defense peptides for antimicrobial activity and target cell selectivity. Biochimie 2010, 92, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Seo, C.H.; Luchian, T.; Park, Y. Pse-T2, an Antimicrobial Peptide with High-Level, BroadSpectrum Antimicrobial Potency and Skin Biocompatibility against Multidrug-Resistant Pseudomonas aeruginosa Infection. Antimicrob. Agents Chemother. 2018, 62, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Won, H.S.; Kim, S.S.; Jung, S.J.; Son, W.S.; Lee, B.; Lee, B.J. Structure-activity relationships of antimicrobial peptides from the skin of Rana esculenta inhabiting in Korea. Mol. Cells 2004, 17, 469–476. [Google Scholar]
- Zhou, X.; Shi, D.; Zhong, R.; Ye, Z.; Ma, C.; Zhou, M.; Xi, X.; Wang, L.; Chen, T.; Kwok, H.F. Bioevaluation of ranatuerin-2Pb from the frog skin secretion of Rana pipiens and its truncated analogues. Biomolecules 2019, 9, 249. [Google Scholar] [CrossRef]
- Bao, K.; Yuan, W.; Ma, C.; Yu, X.; Wang, L.; Hong, M.; Xi, X.; Zhou, M.; Chen, T. Modification targeting the “Rana Box” motif of a novel nigrocin peptide from Hylarana latouchii enhances and broadens its potency against multiple bacteria. Front. Microbiol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Lee, J.K.; Gopal, R.; Park, S.C.; Ko, H.S.; Kim, Y.; Hahm, K.S.; Park, Y. A Proline-Hinge Alters the Characteristics of the Amphipathic α-helical AMPs. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Timmons, P.B.; O’Flynn, D.; Conlon, J.M.; Hewage, C.M. Structural and positional studies of the antimicrobial peptide brevinin-1BYa in membrane-mimetic environments. J. Pept. Sci. 2019, 25, e3208. [Google Scholar] [CrossRef]
- Park, S.; Park, S.H.; Ahn, H.C.; Kim, S.; Kim, S.S.; Lee, B.J.; Lee, B.J. Structural study of novel antimicrobial peptides, nigrocins, isolated from Rana nigromaculata. FEBS Lett. 2001, 507, 95–100. [Google Scholar] [CrossRef]
- Zhou, J.; McClean, S.; Thompson, A.; Zhang, Y.; Shaw, C.; Rao, P.; Bjourson, A.J. Purification and characterization of novel antimicrobial peptides from the skin secretion of Hylarana guentheri. Peptides 2006, 27, 3077–3084. [Google Scholar] [CrossRef]
- Parvin, A.; Anand, S.; Asha, R.; Reshmy, V.; Sanil, G.; Kumar, K.S. Structure-activity relationship and mode of action of a frog secreted antibacterial peptide B1CTcu5 using synthetically and modularly modified or deleted (SMMD) peptides. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Chen, Q.; Cheng, P.; Ma, C.; Xi, X.; Wang, L.; Zhou, M.; Bian, H.; Chen, T. Evaluating the Bioactivity of a Novel Broad-Spectrum Antimicrobial Peptide Brevinin-1GHa from the Frog Skin Secretion of Hylarana guentheri and Its Analogues. Toxins 2018, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Kolar, S.S.N.; Luca, V.; Baidouri, H.; Mannino, G.; McDermott, A.M.; Mangoni, M.L. Esculentin-1a(1-21)NH2: A frog skin-derived peptide for microbial keratitis. Cell. Mol. Life Sci. 2015, 72, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.Y.; Hong, S.Y.; Lee, K.H. Structure-activity analysis of brevinin 1E amide, an antimicrobial peptide from Rana esculenta. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1998, 1387, 239–248. [Google Scholar] [CrossRef]
- Haney, E.F.; Hunter, H.N.; Matsuzaki, K.; Vogel, H.J. Solution NMR studies of amphibian antimicrobial peptides: Linking structure to function? Biochim. Biophys. Acta Biomembr. 2009, 1788, 1639–1655. [Google Scholar] [CrossRef]
- Mourtada, R.; Herce, H.D.; Yin, D.J.; Moroco, J.A.; Wales, T.E.; Engen, J.R.; Walensky, L.D. Design of stapled antimicrobial peptides that are stable, nontoxic and kill antibiotic-resistant bacteria in mice. Nat. Biotechnol. 2019, 37, 1186–1197. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, S.S.; Lee, M.H.; Lee, B.J.; Ryu, P.D. Role of C-terminal heptapeptide in pore-forming activity of antimicrobial agent, gaegurin 4. J. Pept. Res. 2004, 64, 151–158. [Google Scholar] [CrossRef]
- Chi, S.W.; Kim, J.S.; Kim, D.H.; Lee, S.H.; Park, Y.H.; Han, K.H. Solution structure and membrane interaction mode of an antimicrobial peptide gaegurin 4. Biochem. Biophys. Res. Commun. 2007, 352, 592–597. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Guarnieri, M.T.; Vasil, A.I.; Vasil, M.L.; Mant, C.T.; Hodges, R.S. Role of peptide hydrophobicity in the mechanism of action of alpha-helical antimicrobial peptides. Antimicrob. Agents Chemother. 2007, 51, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kang, D.; Choi, J.; Huang, W.; Wadman, M.; Barron, A.E.; Seo, J. Effect of side chain hydrophobicity and cationic charge on antimicrobial activity and cytotoxicity of helical peptoids. Bioorg. Med. Chem. Lett. 2018, 28, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Spohn, R.; Daruka, L.; Lázár, V.; Martins, A.; Vidovics, F.; Grézal, G.; Méhi, O.; Kintses, B.; Számel, M.; Jangir, P.K.; et al. Integrated evolutionary analysis reveals antimicrobial peptides with limited resistance. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, D.; Wang, L.; Lin, C.; Ma, C.; Xi, X.; Zhou, M.; Duan, J.; Bininda-Emonds, O.R.P.; Chen, T. Targeted modification of a novel amphibian antimicrobial peptide from Phyllomedusa tarsius to enhance its activity against MRSA and microbial biofilm. Front. Microbiol. 2017, 8, 628. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zai, Y.; Xi, X.; Ma, C.; Wang, L.; Zhou, M.; Shaw, C.; Chen, T. BBA—General Subjects A novel membrane-disruptive antimicrobial peptide from frog skin secretion against cystic fi brosis isolates and evaluation of anti-MRSA e ff ect using Galleria mellonella model. BBA Gen. Subj. 2019, 1863, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Defraine, V.; Liebens, V.; Loos, E.; Swings, T.; Weytjens, B.; Fierro, C.; Marchal, K.; Sharkey, L.; O’Neill, A.J.; Corbau, R.; et al. 1-((2,4-Dichlorophenethyl)Amino)-3-phenoxypropan-2-ol kills Pseudomonas aeruginosa through extensive membrane damage. Front. Microbiol. 2018, 9, 129. [Google Scholar] [CrossRef]

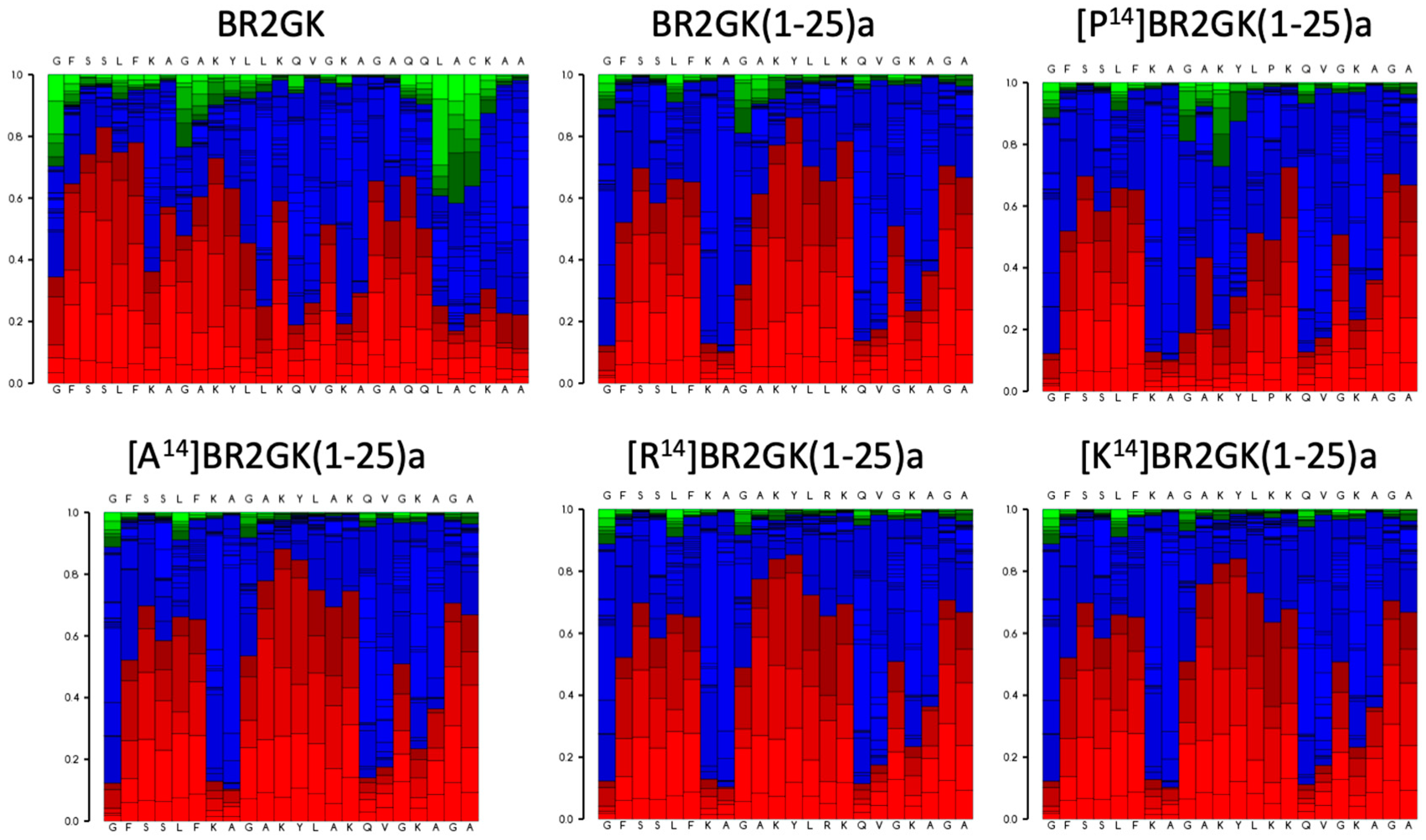

| Peptide | Sequence | Charge | Hydrophobicity | Helicity% a |
|---|---|---|---|---|
| BR2GK | GFSSLFKAGAKYLLKQVGKAGAQQLACKAANNC | +5 | 0.331 | 8.9/20.9 |
| BR2GK(1-25)a | GFSSLFKAGAKYLLKQVGKAGAQQL-NH2 | +5 | 0.364 | 14.1/20.1 |
| [P14]BR2GK(1-25)a | GFSSLFKAGAKYLPKQVGKAGAQQL-NH2 | +5 | 0.325 | 0.6/6.2 |
| [A14]BR2GK(1-25)a | GFSSLFKAGAKYLAKQVGKAGAQQL-NH2 | +5 | 0.308 | 7.2/16.0 |
| [K14]BR2GK(1-25)a | GFSSLFKAGAKYLKKQVGKAGAQQL-NH2 | +6 | 0.256 | 9.6/25.4 |
| [R14]BR2GK(1-25)a | GFSSLFKAGAKYLRKQVGKAGAQQL-NH2 | +6 | 0.256 | 11.0/25.7 |
| Peptide | MIC/MBC (µM) | ||||||
|---|---|---|---|---|---|---|---|
| S. aureus | E. faecalis | MRSA | E. coli | P. aeruginosa | K. pneumoniae | C. albicans | |
| BR2GK | 64/256 | 64/256 | >512/>512 | >512/>512 | >512/>512 | >512/>512 | 256/512 |
| BR2GK(1-25)a | 4/8 | 4/16 | 4/16 | 4/8 | 8/32 | 16/32 | 4/16 |
| [P14]BR2GK(1-25)a | 512/>512 | >512>512 | >512/>512 | >512/>512 | >512/>512 | >512/>512 | >512>512 |
| [A14]BR2GK(1-25)a | 8/16 | 8/16 | 16/64 | 8/32 | 32/128 | 128/256 | 16/32 |
| [K14]BR2GK(1-25)a | 16/32 | 16/64 | 64/128 | 8/32 | 16/64 | 128/256 | 64/128 |
| [R14]BR2GK(1-25)a | 16/32 | 16/32 | 64/128 | 8/16 | 16/32 | 128/256 | 64/128 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Miao, Y.; Ma, C.; Zhou, M.; Shi, Z.; Chen, X.; Burrows, J.F.; Xi, X.; Chen, T.; Wang, L. Brevinin-2GHk from Sylvirana guentheri and the Design of Truncated Analogs Exhibiting the Enhancement of Antimicrobial Activity. Antibiotics 2020, 9, 85. https://doi.org/10.3390/antibiotics9020085
Chen G, Miao Y, Ma C, Zhou M, Shi Z, Chen X, Burrows JF, Xi X, Chen T, Wang L. Brevinin-2GHk from Sylvirana guentheri and the Design of Truncated Analogs Exhibiting the Enhancement of Antimicrobial Activity. Antibiotics. 2020; 9(2):85. https://doi.org/10.3390/antibiotics9020085
Chicago/Turabian StyleChen, Guanzhu, Yuxi Miao, Chengbang Ma, Mei Zhou, Zhanzhong Shi, Xiaoling Chen, James F. Burrows, Xinping Xi, Tianbao Chen, and Lei Wang. 2020. "Brevinin-2GHk from Sylvirana guentheri and the Design of Truncated Analogs Exhibiting the Enhancement of Antimicrobial Activity" Antibiotics 9, no. 2: 85. https://doi.org/10.3390/antibiotics9020085
APA StyleChen, G., Miao, Y., Ma, C., Zhou, M., Shi, Z., Chen, X., Burrows, J. F., Xi, X., Chen, T., & Wang, L. (2020). Brevinin-2GHk from Sylvirana guentheri and the Design of Truncated Analogs Exhibiting the Enhancement of Antimicrobial Activity. Antibiotics, 9(2), 85. https://doi.org/10.3390/antibiotics9020085






