Clinical Experience with Ceftazidime-Avibactam for the Treatment of Infections due to Multidrug-Resistant Gram-Negative Bacteria Other than Carbapenem-Resistant Enterobacterales
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Microbiology
2.3. Characteristics of Ceftazidime-Avibactam Therapy
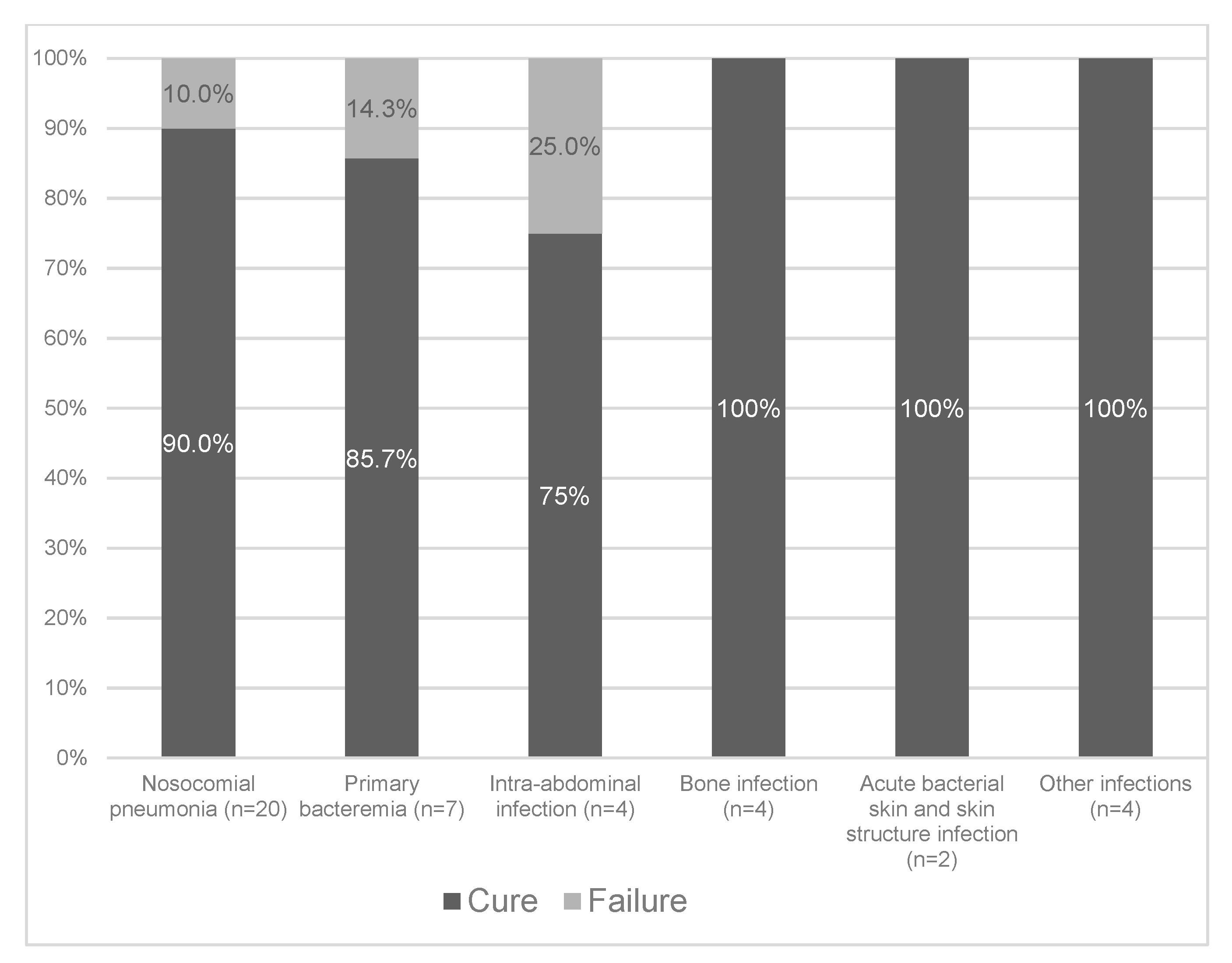
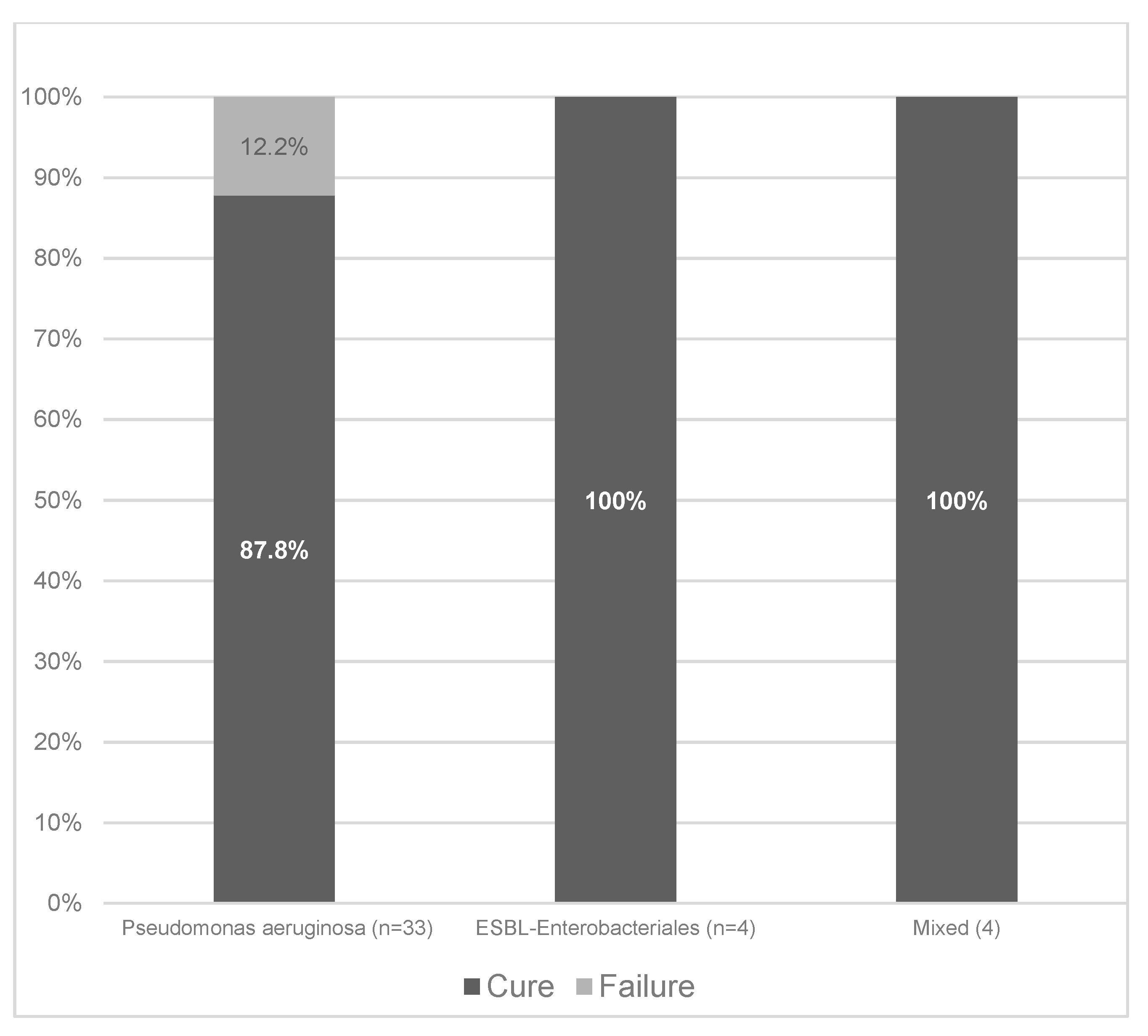
2.4. Clinical Cure
2.5. Risk factors for Clinical Failures
2.6. Adverse Events
3. Discussion
4. Materials and Methods
4.1. Definitions and Data Collection
4.2. Microbiological Methods
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tangden, T.; Giske, C.G. Global dissemination of extensively drug-resistant carbapenemase-producing Enterobacteriaceae: Clinical perspectives on detection, treatment and infection control. J. Intern. Med. 2015, 277, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Confronting the threat of multidrug-resistant Gram-negative bacteria in critically ill patients. J. Antimicrob. Chemother. 2013, 68, 490–491. [Google Scholar] [CrossRef] [PubMed]
- Karakonstantis, S.; Kritsotakis, E.I.; Gikas, A. Pandrug-resistant Gram-negative bacteria: A systematic review of current epidemiology, prognosis and treatment options. J. Antimicrob. Chemother. 2019. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Rello, J.; Marshall, J.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef]
- Qin, X.; Tran, B.G.; Kim, M.J.; Wang, L.; Nguyen, D.A.; Chen, Q.; Song, J.; Laud, P.J.; Stone, G.G.; Chow, J.W. A randomised, double-blind, phase 3 study comparing the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem for complicated intra-abdominal infections in hospitalised adults in Asia. Int. J. Antimicrob. Agents 2017, 49, 579–588. [Google Scholar] [CrossRef]
- Mazuski, J.E.; Gasink, L.B.; Armstrong, J.; Broadhurst, H.; Stone, G.G.; Rank, D.; Llorens, L.; Newell, P.; Pachl, J. Efficacy and Safety of Ceftazidime-Avibactam Plus Metronidazole Versus Meropenem in the Treatment of Complicated Intra-abdominal Infection: Results From a Randomized, Controlled, Double-Blind, Phase 3 Program. Clin. Infect. Dis. 2016, 62, 1380–1389. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.; Sobel, J.D.; Newell, P.; Armstrong, J.; Huang, X.; Stone, G.G.; Yates, K.; Gasink, L.B. Ceftazidime-avibactam Versus Doripenem for the Treatment of Complicated Urinary Tract Infections, Including Acute Pyelonephritis: RECAPTURE, a Phase 3 Randomized Trial Program. Clin. Infect. Dis. 2016, 63, 754–762. [Google Scholar] [CrossRef]
- Carmeli, Y.; Armstrong, J.; Laud, P.J.; Newell, P.; Stone, G.; Wardman, A.; Gasink, L.B. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): A randomised, pathogen-directed, phase 3 study. Lancet Infect. Dis. 2016, 16, 661–673. [Google Scholar] [CrossRef]
- De la Calle, C.; Rodriguez, O.; Morata, L.; Marco, F.; Cardozo, C.; Garcia-Vidal, C.; Rio, A.D.; Feher, C.; Pellice, M.; Puerta-Alcalde, P.; et al. Clinical characteristics and prognosis of infections caused by OXA-48 carbapenemase-producing Enterobacteriaceae in patients treated with ceftazidime-avibactam. Int. J. Antimicrob. Agents 2019, 53, 520–524. [Google Scholar] [CrossRef]
- Sousa, A.; Perez-Rodriguez, M.T.; Soto, A.; Rodriguez, L.; Perez-Landeiro, A.; Martinez-Lamas, L.; Nodar, A.; Crespo, M. Effectiveness of ceftazidime/avibactam as salvage therapy for treatment of infections due to OXA-48 carbapenemase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 3170–3175. [Google Scholar] [CrossRef]
- Temkin, E.; Torre-Cisneros, J.; Beovic, B.; Benito, N.; Giannella, M.; Gilarranz, R.; Jeremiah, C.; Loeches, B.; Machuca, I.; Jimenez-Martin, M.J.; et al. Ceftazidime-Avibactam as Salvage Therapy for Infections Caused by Carbapenem-Resistant Organisms. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Shields, R.K.; Nguyen, M.H.; Chen, L.; Press, E.G.; Kreiswirth, B.N.; Clancy, C.J. Pneumonia and Renal Replacement Therapy Are Risk Factors for Ceftazidime-Avibactam Treatment Failures and Resistance among Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Potoski, B.A.; Haidar, G.; Hao, B.; Doi, Y.; Chen, L.; Press, E.G.; Kreiswirth, B.N.; Clancy, C.J.; Nguyen, M.H. Clinical Outcomes, Drug Toxicity, and Emergence of Ceftazidime-Avibactam Resistance Among Patients Treated for Carbapenem-Resistant Enterobacteriaceae Infections. Clin. Infect. Dis. 2016, 63, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Van Duin, D.; Lok, J.J.; Earley, M.; Cober, E.; Richter, S.S.; Perez, F.; Salata, R.A.; Kalayjian, R.C.; Watkins, R.R.; Doi, Y.; et al. Colistin Versus Ceftazidime-Avibactam in the Treatment of Infections Due to Carbapenem-Resistant Enterobacteriaceae. Clin. Infect. Dis. 2018, 66, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Trecarichi, E.M.; Corona, A.; De Rosa, F.G.; Bassetti, M.; Mussini, C.; Menichetti, F.; Viscoli, C.; Campoli, C.; Venditti, M.; et al. Efficacy of Ceftazidime-Avibactam Salvage Therapy in Patients With Infections Caused by Klebsiella pneumoniae Carbapenemase-producing K. pneumoniae. Clin. Infect. Dis. 2019, 68, 355–364. [Google Scholar] [CrossRef]
- Rodriguez-Nunez, O.; Ripa, M.; Morata, L.; de la Calle, C.; Cardozo, C.; Feher, C.; Pellice, M.; Valcarcel, A.; Puerta-Alcalde, P.; Marco, F.; et al. Evaluation of ceftazidime/avibactam for serious infections due to multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa. J. Glob. Antimicrob. Resist. 2018, 15, 136–139. [Google Scholar] [CrossRef]
- Santevecchi, B.A.; Smith, T.T.; MacVane, S.H. Clinical experience with ceftazidime/avibactam for treatment of antibiotic-resistant organisms other than Klebsiella pneumoniae. Int. J. Antimicrob. Agents 2018, 51, 629–635. [Google Scholar] [CrossRef]
- Xipell, M.; Bodro, M.; Marco, F.; Losno, R.A.; Cardozo, C.; Soriano, A. Clinical experience with ceftazidime/avibactam in patients with severe infections, including meningitis and lung abscesses, caused by extensively drug-resistant Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2017, 49, 266–268. [Google Scholar] [CrossRef]
- Stone, G.G.; Newell, P.; Gasink, L.B.; Broadhurst, H.; Wardman, A.; Yates, K.; Chen, Z.; Song, J.; Chow, J.W. Clinical activity of ceftazidime/avibactam against MDR Enterobacteriaceae and Pseudomonas aeruginosa: Pooled data from the ceftazidime/avibactam Phase III clinical trial programme. J. Antimicrob. Chemother. 2018, 73, 2519–2523. [Google Scholar] [CrossRef]
- Magill, S.S.; O’Leary, E.; Janelle, S.J.; Thompson, D.L.; Dumyati, G.; Nadle, J.; Wilson, L.E.; Kainer, M.A.; Lynfield, R.; Greissman, S.; et al. Changes in Prevalence of Health Care-Associated Infections in U.S. Hospitals. N. Engl. J. Med. 2018, 379, 1732–1744. [Google Scholar] [CrossRef]
- Antimicrobial Resistance: Global Report on Surveillance 2014. Available online: http://www.who.int/drugresistance/documents/surveillancereport/en/ (accessed on 9 February 2014).
- Carbapenemase-Producing Bacteria in Europe. Available online: https://www.ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/antimicrobial-resistance-carbapenemase-producing-bacteria-europe.pdf (accessed on 9 February 2018).
- Vena, A.; Castaldo, N.; Bassetti, M. The role of new beta-lactamase inhibitors in gram-negative infections. Curr. Opin. Infect. Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Peghin, M.; Vena, A.; Giacobbe, D.R. Treatment of Infections Due to MDR Gram-Negative Bacteria. Front. Med. (Lausanne) 2019, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Rodriguez-Bano, J. The Use of Noncarbapenem beta-Lactams for the Treatment of Extended-Spectrum beta-Lactamase Infections. Clin. Infect. Dis. 2017, 64, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Bano, J.; Navarro, M.D.; Retamar, P.; Picon, E.; Pascual, A. Extended-Spectrum Beta-Lactamases-Red Espanola de Investigacion en Patologia Infecciosa/Grupo de Estudio de Infeccion Hospitalaria, G. beta-Lactam/beta-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli: A post hoc analysis of prospective cohorts. Clin. Infect. Dis. 2012, 54, 167–174. [Google Scholar] [CrossRef]
- Gavin, P.J.; Suseno, M.T.; Thomson, R.B., Jr.; Gaydos, J.M.; Pierson, C.L.; Halstead, D.C.; Aslanzadeh, J.; Brecher, S.; Rotstein, C.; Brossette, S.E.; et al. Clinical correlation of the CLSI susceptibility breakpoint for piperacillin- tazobactam against extended-spectrum-beta-lactamase-producing Escherichia coli and Klebsiella species. Antimicrob. Agents Chemother. 2006, 50, 2244–2247. [Google Scholar] [CrossRef]
- Harris, P.N.A.; Tambyah, P.A.; Lye, D.C.; Mo, Y.; Lee, T.H.; Yilmaz, M.; Alenazi, T.H.; Arabi, Y.; Falcone, M.; Bassetti, M.; et al. Effect of Piperacillin-Tazobactam vs Meropenem on 30-Day Mortality for Patients with E coli or Klebsiella pneumoniae Bloodstream Infection and Ceftriaxone Resistance: A Randomized Clinical Trial. JAMA 2018, 320, 984–994. [Google Scholar] [CrossRef]
- Ramalheira, E.; Stone, G.G. Longitudinal analysis of the in vitro activity of ceftazidime/avibactam versus Enterobacteriaceae, 2012–2016. J. Glob. Antimicrob. Resist. 2019, 19, 106–115. [Google Scholar] [CrossRef]
- Viaggi, V.; Pini, B.; Tonolo, S.; Luzzaro, F.; Principe, L. In vitro activity of ceftazidime/avibactam against clinical isolates of ESBL-producing Enterobacteriaceae in Italy. J. Chemother. 2019, 31, 195–201. [Google Scholar] [CrossRef]
- Lopez-Hernandez, I.; Alonso, N.; Fernandez-Martinez, M.; Zamorano, L.; Rivera, A.; Oliver, A.; Conejo, M.C.; Martinez-Martinez, L.; Navarro, F.; Pascual, A. Activity of ceftazidime-avibactam against multidrug-resistance Enterobacteriaceae expressing combined mechanisms of resistance. Enferm. Infecc. Microbiol. Clin. 2017, 35, 499–504. [Google Scholar] [CrossRef]
- Tsolaki, V.; Mantzarlis, K.; Mpakalis, A.; Malli, E.; Tsimpoukas, F.; Tsirogianni, A.; Papagiannitsis, K.; Zygoulis, P.; Papadonta, M.E.; Petinaki, E.; et al. Ceftazidime-avibactam to treat life-threatening infections from carbapenem resistant pathogens in critically ill mechanically ventilated patients. Antimicrob. Agents Chemother. 2019. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Garner, J.S.; Jarvis, W.R.; Emori, T.G.; Horan, T.C.; Hughes, J.M. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control. 1988, 16, 128–140. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 8.1, M.. 2018. Available online: http://www.eucast.org/clinical_breakpoints/ (accessed on 22 May 2019).
| Variables | n = 41 |
|---|---|
| Age (years), median (IQR) | 62 (41–70) |
| Sex, male, n (%) | 28 (68.3) |
| Ward, n (%) | |
| Medical | 17 (41.5) |
| Surgical | 7 (17.1) |
| Intensive care unit | 17 (41.5) |
| Underlying disease, n (%) | |
| Cardiovascular disease | 14 (34.1) |
| Chronic renal disease | 9 (22.0) |
| Diabetes mellitus | 8 (19.5) |
| Solid organ transplant | 8 (19.5) |
| Neurological disease | 7 (17.1) |
| Solid organ tumors | 7 (17.1) |
| Bronchiectasis | 6 (14.6) |
| Chronic obstructive pulmonary disease | 5 (12.2) |
| Gastrointestinal disease | 4 (9.8) |
| Hematological malignancy | 4 (9.8) |
| Charlson comorbidity index, mean (±SD) | 4 (2–6) |
| Other predisposing conditions #, n (%) | |
| Corticosteroids | 12 (29.3) |
| Chemotherapy | 7 (17.1) |
| Neutropenia (absolute neutrophil count <500 mm3) | 5 (12.2) |
| Invasive procedures/devices, n (%) | |
| Central venous catheter | 29 (70.7) |
| Urinary catheter | 26 (63.4) |
| Previous surgery # | 15 (36.6) |
| Mechanical ventilation | 14 (34.1) |
| Percutaneous endoscopic gastrostomy | 2 (4.9) |
| Severity of clinical presentation, n (%) | |
| No sepsis | 17 (41.5) |
| Sepsis | 17 (41.5) |
| Septic shock | 7 (17.1) |
| ICU admission due to the index infection n (%) | 10 (24.4) |
| Primary Site of Infection & | Overall | P. Aeruginosa | ESBL−Producing Enterobacterales | Polymicrobial |
|---|---|---|---|---|
| Nosocomial pneumonia | 20 (48.8) | 18 | 0 | 2 * |
| Primary bacteremia | 7 (17.1) | 5 | 1 | 1 ± |
| Intra-abdominal infection | 4 (9.8) | 2 | 1 | 1 # |
| Bone infection | 4 (9.8) | 3 | 1 | 0 |
| Acute bacterial skin and skin structure infection | 2 (4.9) | 2 | 0 | 0 |
| Other infections § | 4 (9.8) | 3 | 1 | 0 |
| Total | 41 | 33 | 4 | 4 |
| Non-Susceptible Isolates, n (%) | |||
|---|---|---|---|
| Antibiotic | Overall (n = 45) | P. Aeruginosa (n = 38) | ESBL-producing Enterobacterales (n = 7) |
| Amikacin | 25 (55.6) | 20 (52.6) | 5 (71.4) |
| Cefepime | 43 (95.6) | 36 (94.7) | 7 (100) |
| Ceftazidime | 40 (88.9) | 33 (86.8) | 7 (100) |
| Ceftolozane-tazobactam | 5 (11.1) | 4 (10.5) | 1 (14.8) |
| Ciprofloxacin | 41 (91.1) | 34 (89.4) | 7 (100) |
| Colistin | 12 (26.6) | 12 (31.5) | 0 |
| Gentamycin | 34 (75.6) | 29 (76.3) | 5 (71.4) |
| Imipenem | 35 (77.8) | 35 (92.1) | 0 |
| Meropenem | 33 (73.3) | 33 (86.8) | 0 |
| Piperacillin-tazobactam | 39 (86.7) | 34 (89.4) | 5 (71.4) |
| VARIABLE | n = 41 |
|---|---|
| Antibiotics before ceftazidime-avibactam for the current infection | |
| Received antibiotics before ceftazidime-avibactam, n (%) | 27 (65.9) |
| Number of antibiotics received, median (IQR) | 1 (1–2) |
| Days of antibiotic therapy, median (IQR) | 11 (4.5–13) |
| Main reason for ceftazidime-avibactam use | |
| Antimicrobial resistance to previous antibiotic | 25 (61.0) |
| Previous antibiotic failure | 14 (34.1) |
| Previous colonization with carbapenemase-producing microorganisms | 10 (24.4) |
| Ceftazidime-avibactam treatment | |
| Targeted therapy | 33 (80.5) |
| Empirical therapy | 8 (19.5) |
| Combination therapy | 33 (80.5) |
| Continuous renal replacement therapy | 5 (12.2) |
| Days of treatment, median (range) | 13 (3–49) |
| Intermittent infusion | 26 (63.4) |
| Continuous infusion | 2 (4.9) |
| Extended infusion | 13 (31.7) |
| Adequate source control of the infection, n (%) | 11/13 (84.6) |
| Clinical cure, n (%) | 37 (90.2) |
| Age/Sex | Underlying Condition | Type of Infection | Concomitant BSI | Clinical Presentation | Prior Therapy to C/A | Dose of C/A | CRRT | Other Interventions | Reason for Clinical Failure |
|---|---|---|---|---|---|---|---|---|---|
| 73/F | Wide intestinal resection and hemicolectomy for intestinal obstruction due to metastatic colon cancer; pulmonary embolism; CHF | PDR P. aeruginosa Intra−abdominal infection | No | Sepsis | No | 1.25 gr/8 h for 8 weeks | Yes. | Inadequate source control of the infection; concomitant colistin therapy | Lack of clinical response |
| 57/F | Systemic sclerosis; lung transplant; chronic renal failure | XDR P. aeruginosa Nosocomial pneumonia | No | No sepsis | No | 2.5 gr/8 h for 10 days | No | No concomitant antibiotics | Lack of clinical response |
| 41/M | Burn injury; acute kidney injury | XDR P. aeruginosa Primary bacteremia | Yes | Septic shock | No | 1.25 gr/8 h for 10 days | Yes | No concomitant antibiotic therapy | Recurrent infection |
| 76/M | Diabetes; CHF; urothelial carcinomas | XDR P. aeruginosa Nosocomial pneumonia | No | Sepsis requiring ICU admission | Meropenem and amikacin for 5 days | 1.25 gr/8 h for 4 days | Yes | Concomitant amikacin | Death |
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| VARIABLE | Successful Clinical Outcome (n = 37) | Clinical Failure (n = 4) | p-Value | OR (95% CI) | p-Value |
| Age (years), mean ± SD | 56.3 ± 18.4 | 61.7 ± 16.1 | 0.59 | 1.0 (0.88–1.13) | 0.96 |
| Sex, male, n (%) | 25 (67.6) | 3 (75.0) | 1 | − | |
| Charlson comorbidity index, mean (±SD) | 3.9 ± 3.0 | 6.2 ± 6.0 | 0.22 | − | |
| Underlying disease, n (%) | − | ||||
| Cardiovascular disease | 12 (32.4) | 2 (50.0) | 0.59 | − | |
| Chronic renal disease | 8 (21.6) | 1 (25.0) | 1 | − | |
| Diabetes mellitus | 7 (18.9) | 1 (25.0) | 1 | − | |
| Solid organ transplant | 7 (18.9) | 1 (25.0) | 1 | − | |
| Neurological disease | 7 (18.9) | 0 | 1 | − | |
| Solid organ tumors | 5 (13.5) | 2 (50.0) | 0.12 | 6.09 (0.30–123.61) | 0.42 |
| Bronchiectasis | 6 (16.2) | 0 | 1 | − | − |
| Chronic obstructive pulmonary disease | 5 (13.5) | 0 | 1 | − | − |
| Gastrointestinal disease | 4 (10.8) | 0 | 1 | − | − |
| Hematological malignancy | 4 (10.8) | 0 | 1 | − | − |
| Other predisposing conditions #, n (%) | − | − | |||
| Corticosteroids | 11 (29.7) | 1 (25.0) | 1 | − | − |
| Chemotherapy | 7 (18.9) | 0 | 1 | − | − |
| Neutropenia (absolute neutrophil count <500 mmc3) | 5 (13.5) | 0 | 1 | − | − |
| Invasive procedures, n (%) # | |||||
| Central venous catheter | 25 (67.6) | 4 (100) | 0.30 | − | − |
| Urinary catheter | 23 (62.2) | 3 (75.0) | 1 | − | − |
| Previous surgery | 12 (32.4) | 3 (75.0) | 0.13 | − | − |
| Mechanical ventilation | 11 (29.7) | 3 (75.0) | 0.10 | 3.74 (0.14–95.89) | 0.42 |
| Percutaneous endoscopic gastrostomy | 2 (5.4) | 0 | 1 | − | − |
| Severity of clinical presentation, n (%) | |||||
| No sepsis | 16 (43.2) | 1 (25.0) | 0.14 | − | − |
| Sepsis | 15 (40.5) | 2 (50.0) | 1 | − | − |
| Septic shock | 6 (16.2) | 1 (25.0) | 0.54 | − | − |
| Intensive care unit admission due to gram negative infection n (%) | 9 (24.3) | 1 (25.0) | 1 | − | − |
| Type of infection, n (%) | − | ||||
| Nosocomial pneumonia | 18 (48.6) | 2 (50.0) | 1 | − | |
| Primary bacteremia | 6 (16.2) | 1 (25.0) | 0.54 | − | − |
| Intra-abdominal infection | 3 (8.1) | 1 (25.0) | 0.34 | − | − |
| Bone infection | 4 (10.8) | 0 | 1 | − | − |
| Acute bacterial skin and soft tissue infection | 2 (5.4) | 0 | 1 | − | − |
| Other infections § | 4 (10.8) | 0 | 1 | − | − |
| Microorganisms | |||||
| P. aeruginosa | 342 (81.1) | 4 (100) | 1 | − | − |
| Enterobacteriaceae | 7 (18.9) | 0 | − | − | |
| C/T treatment | |||||
| Combination therapy | 31 (83.8) | 2 (50.0) | 0.16 | − | − |
| Empirical therapy | 7 (18.9) | 1 (25.0) | 1 | − | − |
| Intermittent hemodialysis | 2 (5.4) | 2 (25.0) | 0.27 | − | − |
| Continuous renal replacement therapy | 2 (5.4) | 3 (75.0) | 0.004 | 29.03 (1.69−498.35) | 0.02 |
| Intermittent Infusion | 23 (62.2) | 3 (75.0) | 1 | − | − |
| Continuous infusion | 2 (5.4) | 0 | 1 | − | − |
| Extended infusion | 12 (32.4) | 1 (25.0) | 1 | − | − |
| Adequate source control of the infection, n (%) | 9 (81.8) | 2 (100) | 1 | − | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vena, A.; Giacobbe, D.R.; Castaldo, N.; Cattelan, A.; Mussini, C.; Luzzati, R.; De Rosa, F.G.; Puente, F.D.; Mastroianni, C.M.; Cascio, A.; et al. Clinical Experience with Ceftazidime-Avibactam for the Treatment of Infections due to Multidrug-Resistant Gram-Negative Bacteria Other than Carbapenem-Resistant Enterobacterales. Antibiotics 2020, 9, 71. https://doi.org/10.3390/antibiotics9020071
Vena A, Giacobbe DR, Castaldo N, Cattelan A, Mussini C, Luzzati R, De Rosa FG, Puente FD, Mastroianni CM, Cascio A, et al. Clinical Experience with Ceftazidime-Avibactam for the Treatment of Infections due to Multidrug-Resistant Gram-Negative Bacteria Other than Carbapenem-Resistant Enterobacterales. Antibiotics. 2020; 9(2):71. https://doi.org/10.3390/antibiotics9020071
Chicago/Turabian StyleVena, Antonio, Daniele Roberto Giacobbe, Nadia Castaldo, Annamaria Cattelan, Cristina Mussini, Roberto Luzzati, Francesco Giuseppe De Rosa, Filippo Del Puente, Claudio Maria Mastroianni, Antonio Cascio, and et al. 2020. "Clinical Experience with Ceftazidime-Avibactam for the Treatment of Infections due to Multidrug-Resistant Gram-Negative Bacteria Other than Carbapenem-Resistant Enterobacterales" Antibiotics 9, no. 2: 71. https://doi.org/10.3390/antibiotics9020071
APA StyleVena, A., Giacobbe, D. R., Castaldo, N., Cattelan, A., Mussini, C., Luzzati, R., De Rosa, F. G., Puente, F. D., Mastroianni, C. M., Cascio, A., Carbonara, S., Capone, A., Boni, S., Sepulcri, C., Meschiari, M., Raumer, F., Oliva, A., Corcione, S., Bassetti, M., & for the Ceftabuse Study Group. (2020). Clinical Experience with Ceftazidime-Avibactam for the Treatment of Infections due to Multidrug-Resistant Gram-Negative Bacteria Other than Carbapenem-Resistant Enterobacterales. Antibiotics, 9(2), 71. https://doi.org/10.3390/antibiotics9020071









