Initial Bacterial Adhesion and Biofilm Formation on Aligner Materials
Abstract
1. Introduction
2. Results
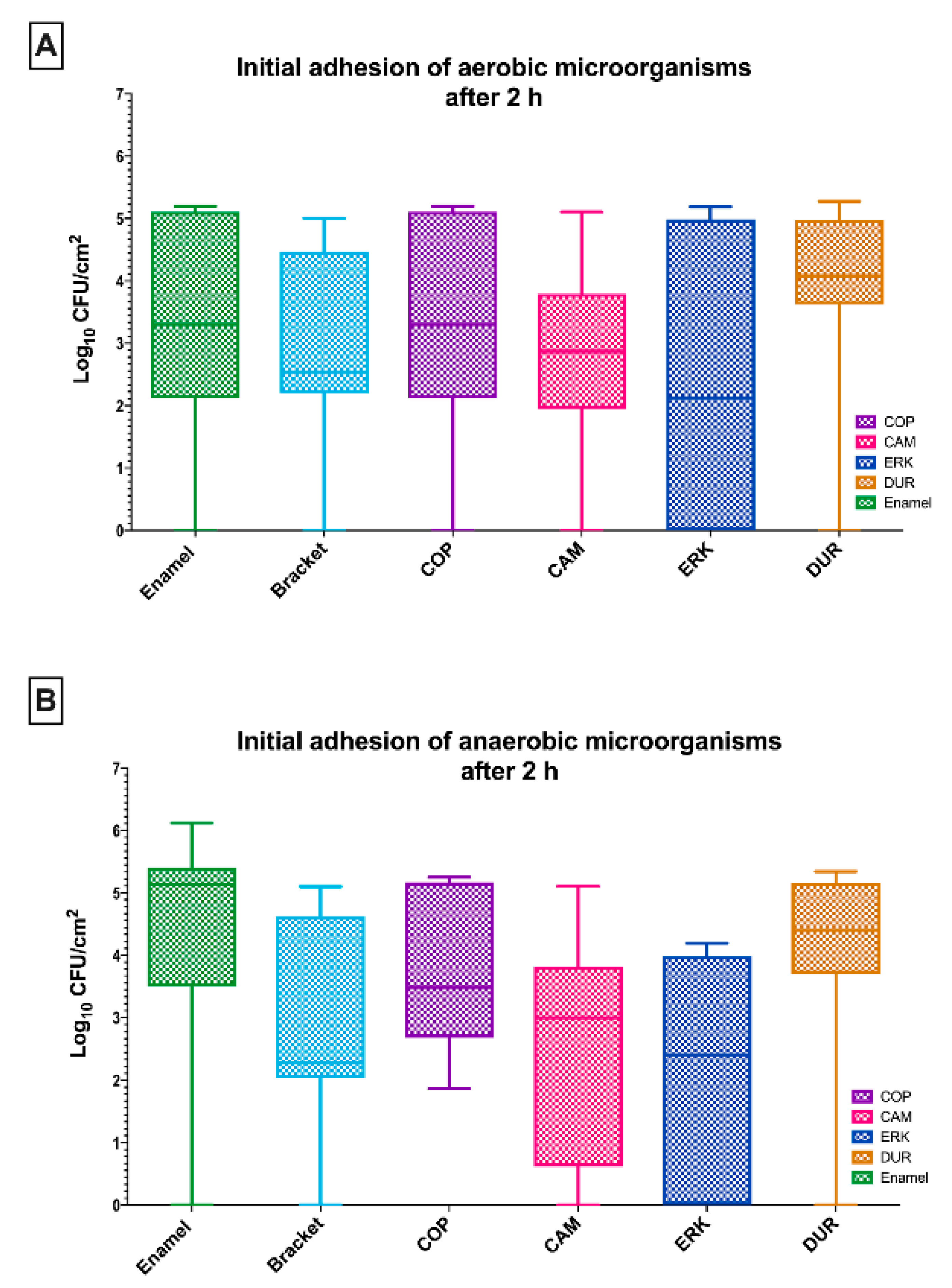
2.1. The Initial Adhesion of Aerobic and Anaerobic Microorganisms on Aligners Was Comparable to That on Enamel and Brackets
2.2. Aligner Materials and Controls Presented Similar Biofilm Formation
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Selection of Study Participants and Saliva Collection
4.3. Test Specimen Preparation
4.3.1. Aligner Samples
4.3.2. Enamel Specimens and Metal Brackets
4.4. Microbial Contamination of Aligner Materials and Controls
4.5. Quantification of the Adherent Oral Biofilm Microorganisms
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jeremiah, H.G.; Bister, D.; Newton, J.T. Social perceptions of adults wearing orthodontic appliances: A cross-sectional study. Eur. J. Orthod. 2011, 33, 476–482. [Google Scholar] [CrossRef]
- Rosvall, M.D.; Fields, H.W.; Ziuchkovski, J.; Rosenstiel, S.F.; Johnston, W.M. Attractiveness, acceptability, and value of orthodontic appliances. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 276.e1–276.e12. [Google Scholar] [CrossRef]
- Gracco, A.; Mazzoli, A.; Favoni, O.; Conti, C.; Ferraris, P.; Tosi, G.; Guarneri, M.P. Short-term chemical and physical changes in invisalign appliances. Aust. Orthod. J. 2009, 25, 34–40. [Google Scholar] [PubMed]
- Abbate, G.M.; Caria, M.P.; Montanari, P.; Mannu, C.; Orrù, G.; Caprioglio, A.; Levrini, L. Periodontal health in teenagers treated with removable aligners and fixed orthodontic appliances. J. Orofac. Orthop. 2015, 76, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Miethke, R.R.; Brauner, K. A Comparison of the periodontal health of patients during treatment with the Invisalign system and with fixed lingual appliances. J. Orofac. Orthop. 2007, 68, 223–231. [Google Scholar] [CrossRef]
- Ogaard, B.; Rølla, G.; Arends, J. Orthodontic appliances and enamel demineralization. Part 1. Lesion development. Am. J. Orthod. Dentofac. Orthop. 1988, 94, 68–73. [Google Scholar] [CrossRef]
- Marsh, P.D.; Bradshaw, D.J. Dental plaque as a biofilm. J. Ind. Microbiol. 1995, 15, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Dental plaque as a microbial biofilm. Caries Res. 2004, 38, 204–211. [Google Scholar] [CrossRef]
- Lindh, L.; Aroonsang, W.; Sotres, J.; Arnebrant, T. Salivary pellicles. Monogr. Oral Sci. 2014, 24, 30–39. [Google Scholar] [CrossRef]
- Thurnheer, T.; Bostanci, N.; Belibasakis, G.N. Microbial dynamics during conversion from supragingival to subgingival biofilms in an in vitro model. Mol. Oral Microbiol. 2016, 31, 125–135. [Google Scholar] [CrossRef]
- Eliades, T.; Eliades, G.; Brantley, W.A. Microbial attachment on orthodontic appliances: I. Wettability and early pellicle formation on bracket materials. Am. J. Orthod. Dentofac. Orthop. 1995, 108, 351–360. [Google Scholar] [CrossRef]
- Low, B.; Lee, W.; Seneviratne, C.J.; Samaranayake, L.P.; Hägg, U. Ultrastructure and morphology of biofilms on thermoplastic orthodontic appliances in ‘fast’ and ‘slow’ plaque formers. Eur. J. Orthod. 2011, 33, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Ireland, A.J.; Soro, V.; Sprague, S.V.; Harradine, N.W.; Day, C.; Al-Anezi, S.; Jenkinson, H.F.; Sherriff, M.; Dymock, D.; Sandy, J.R. The effects of different orthodontic appliances upon microbial communities. Orthod. Craniofac. Res. 2014, 17, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, C.M.; Brattström, V.; Malmberg, E.; Nord, C.E. Ligature wires and elastomeric rings: Two methods of ligation, and their association with microbial colonization of Streptococcus mutans and lactobacilli. Eur. J. Orthod. 1991, 13, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Yeo, I.S.; Lee, J.B.; Kim, S.H.; Kim, D.J.; Han, J.S. Initial in vitro bacterial adhesion on dental restorative materials. Int. J. Artif. Organs 2012, 35, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Lendenmann, U.; Grogan, J.; Oppenheim, F.G. Saliva and dental pellicle—A review. Adv. Dent. Res. 2000, 14, 22–28. [Google Scholar] [CrossRef]
- Gibbons, R.J. Bacterial adhesion to oral tissues: A model for infectious diseases. J. Dent. Res. 1989, 68, 750–760. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.M. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J. Clin. Periodontol. 1995, 22, 1–14. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm matrixome: Extracellular components in structured microbial communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Listgarten, M.A. Structure of the microbial flora associated with periodontal health and disease in man. A light and electron microscopic study. J. Periodontol. 1976, 47, 1–18. [Google Scholar] [CrossRef]
- Bradshaw, D.J.; Marsh, P.D. Use of continuous flow techniques in modeling dental plaque biofilms. Methods Enzymol. 1999, 310, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Maryan, C.; Verran, J. Retention of oral microorganisms on cobalt-chromium alloy and dental acrylic resin with different surface finishes. J. Prosthet. Dent. 1998, 80, 592–597. [Google Scholar] [CrossRef]
- Suter, F.; Zinelis, S.; Patcas, R.; Schätzle, M.; Eliades, G.; Eliades, T. Roughness and wettability of aligner materials. J. Orthod. 2020, 47, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Alexandropoulos, A.; Al Jabbari, Y.S.; Zinelis, S.; Eliades, T. Chemical and mechanical characteristics of contemporary thermoplastic orthodontic materials. Aust. Orthod. J. 2015, 31, 165–170. [Google Scholar]
- Crawford, R.J.; Webb, H.K.; Truong, V.K.; Hasan, J.; Ivanova, E.P. Surface topographical factors influencing bacterial attachment. Adv. Colloid Interface Sci. 2012, 179–182, 142–149. [Google Scholar] [CrossRef]
- Altmann, B.; Karygianni, L.; Al-Ahmad, A.; Butz, F.; Bächle, M.; Adolfsson, E.; Fürderer, T.; Courtois, N.; Palmero, P.; Follo, M.; et al. Assessment of novel long-lasting ceria-stabilized zirconia-based ceramics with different surface topographies as implant materials. Adv. Funct. Mater. 2017, 27, 1702512. [Google Scholar] [CrossRef]
- Jaggy, F.; Zinelis, S.; Polychronis, G.; Patcas, R.; Schätzle, M.; Eliades, G.; Eliades, T. ATR-FTIR analysis and one-week stress relaxation of four orthodontic aligner materials. Materials (Basel) 2020, 13, 1868. [Google Scholar] [CrossRef]
- Papadopoulou, A.K.; Cantele, A.; Polychronis, G.; Zinelis, S.; Eliades, T. Changes in Roughness and Mechanical Properties of Invisalign(®) Appliances after One- and Two-Weeks Use. Materials (Basel) 2019, 12, 2406. [Google Scholar] [CrossRef]
- Mondal, S.; Martin, D. Hydrolytic degradation of segmented polyurethane copolymers for biomedical applications. Polym. Degrad. Stab. 2012, 97, 1553–1561. [Google Scholar] [CrossRef]
- Marquis, R.E. Oxygen metabolism, oxidative stress and acid-base physiology of dental plaque biofilms. J. Ind. Microbiol. 1995, 15, 198–207. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Lee, S.J.; Lim, B.S.; Nahm, D.S. Quantitative determination of adhesion patterns of cariogenic streptococci to various orthodontic brackets. Am. J. Orthod. Dentofac. Orthop. 2007, 132, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Balenseifen, J.W.; Madonia, J.V. Study of dental plaque in orthodontic patients. J. Dent. Res. 1970, 49, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.A.; Roberts, W.E.; Eckert, G.J.; Kula, K.S.; González-Cabezas, C. Risk factors for incidence and severity of white spot lesions during treatment with fixed orthodontic appliances. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 188–194. [Google Scholar] [CrossRef]
- Mascarenhas, A.K. Oral hygiene as a risk indicator of enamel and dentin caries. Community Dent. Oral Epidemiol. 1998, 26, 331–339. [Google Scholar] [CrossRef]
- Gorelick, L.; Geiger, A.M.; Gwinnett, A.J. Incidence of white spot formation after bonding and banding. Am. J. Orthod. 1982, 81, 93–98. [Google Scholar] [CrossRef]
- Naranjo, A.A.; Triviño, M.L.; Jaramillo, A.; Betancourth, M.; Botero, J.E. Changes in the subgingival microbiota and periodontal parameters before and 3 months after bracket placement. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 275.e17–275.e22. [Google Scholar] [CrossRef]
- De Souza, R.A.; de Araújo Magnani, M.B.B.; Nouer, D.F.; da Silva, C.O.; Klein, M.I.; Sallum, E.A.; Gonçalves, R.B. Periodontal and microbiologic evaluation of 2 methods of archwire ligation: Ligature wires and elastomeric rings. Am. J. Orthod. Dentofac. Orthop. 2008, 134, 506–512. [Google Scholar] [CrossRef]
- Sifakakis, I.; Papaioannou, W.; Papadimitriou, A.; Kloukos, D.; Papageorgiou, S.N.; Eliades, T. Salivary levels of cariogenic bacterial species during orthodontic treatment with thermoplastic aligners or fixed appliances: A prospective cohort study. Prog. Orthod. 2018, 19, 25. [Google Scholar] [CrossRef]
- Rossini, G.; Parrini, S.; Castroflorio, T.; Deregibus, A.; Debernardi, C.L. Periodontal health during clear aligners treatment: A systematic review. Eur. J. Orthod. 2015, 37, 539–543. [Google Scholar] [CrossRef]
- Chhibber, A.; Agarwal, S.; Yadav, S.; Kuo, C.L.; Upadhyay, M. Which orthodontic appliance is best for oral hygiene? A randomized clinical trial. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Verrusio, C.; Iorio-Siciliano, V.; Blasi, A.; Leuci, S.; Adamo, D.; Nicolò, M. The effect of orthodontic treatment on periodontal tissue inflammation: A systematic review. Quintessence Int. 2018, 49, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Burchard, T.; Karygianni, L.; Hellwig, E.; Follo, M.; Wrbas, T.; Wittmer, A.; Vach, K.; Al-Ahmad, A. Inactivation of oral biofilms using visible light and water-filtered infrared A radiation and indocyanine green. Future Med. Chem. 2019, 11, 1721–1739. [Google Scholar] [CrossRef] [PubMed]

| Brand Name | Company | Code |
|---|---|---|
| CA-medium | Scheu-Dental, Iserlohn, Germany | CAM |
| Copolyester | Essix, Dentsply Raintree Essix Sarasota, FL, USA | COP |
| Duran | Great Lakes Dental Technologies, Tonawanda, NY, USA | DUR |
| Erkodur | Erkodent Erich Kopp, Pfalzgrafenweiler, Germany | ERK |
| Surface Parameters | |||||
|---|---|---|---|---|---|
| Group | Sa (nm) | Sz (nm) | Sq (nm) | Sdr (%) | Sv (nm3/nm2) |
| CAM | 8.1 (2.8) a | 54.8 (22.6) a | 13.1 (7.5) a | 0.002 (0.004) a | 1.0 (0.3) a |
| COP | 8.5 (0.6) a | 58.0 (3.5) a | 11.2 (1.2) a | 0.000 (0.000) a | 1.3 (0.2) a |
| DUR | 9.6 (2.5) a | 96.3 (24.1) a | 13.4 (4.8) a | 0.000 (0.000) a | 1.1 (0.2) a |
| ERK | 9.0 (2.0) a | 75.7 (26.4) a | 12.6 (3.2) a | 0.000 (0.000) a | 1.0 (0.2) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tektas, S.; Thurnheer, T.; Eliades, T.; Attin, T.; Karygianni, L. Initial Bacterial Adhesion and Biofilm Formation on Aligner Materials. Antibiotics 2020, 9, 908. https://doi.org/10.3390/antibiotics9120908
Tektas S, Thurnheer T, Eliades T, Attin T, Karygianni L. Initial Bacterial Adhesion and Biofilm Formation on Aligner Materials. Antibiotics. 2020; 9(12):908. https://doi.org/10.3390/antibiotics9120908
Chicago/Turabian StyleTektas, Sibel, Thomas Thurnheer, Theodore Eliades, Thomas Attin, and Lamprini Karygianni. 2020. "Initial Bacterial Adhesion and Biofilm Formation on Aligner Materials" Antibiotics 9, no. 12: 908. https://doi.org/10.3390/antibiotics9120908
APA StyleTektas, S., Thurnheer, T., Eliades, T., Attin, T., & Karygianni, L. (2020). Initial Bacterial Adhesion and Biofilm Formation on Aligner Materials. Antibiotics, 9(12), 908. https://doi.org/10.3390/antibiotics9120908






