Short-Term Lincomycin Exposure Depletion of Murine Microbiota Affects Short-Chain Fatty Acids and Intestinal Morphology and Immunity
Abstract
1. Introduction
2. Results
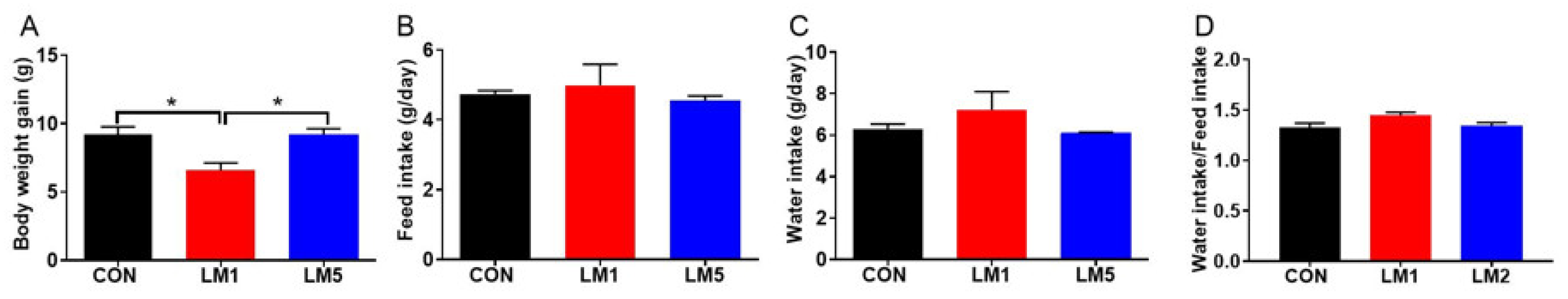
2.1. Antibiotic Exposure Can Decrease Body Weight Gain of Mice
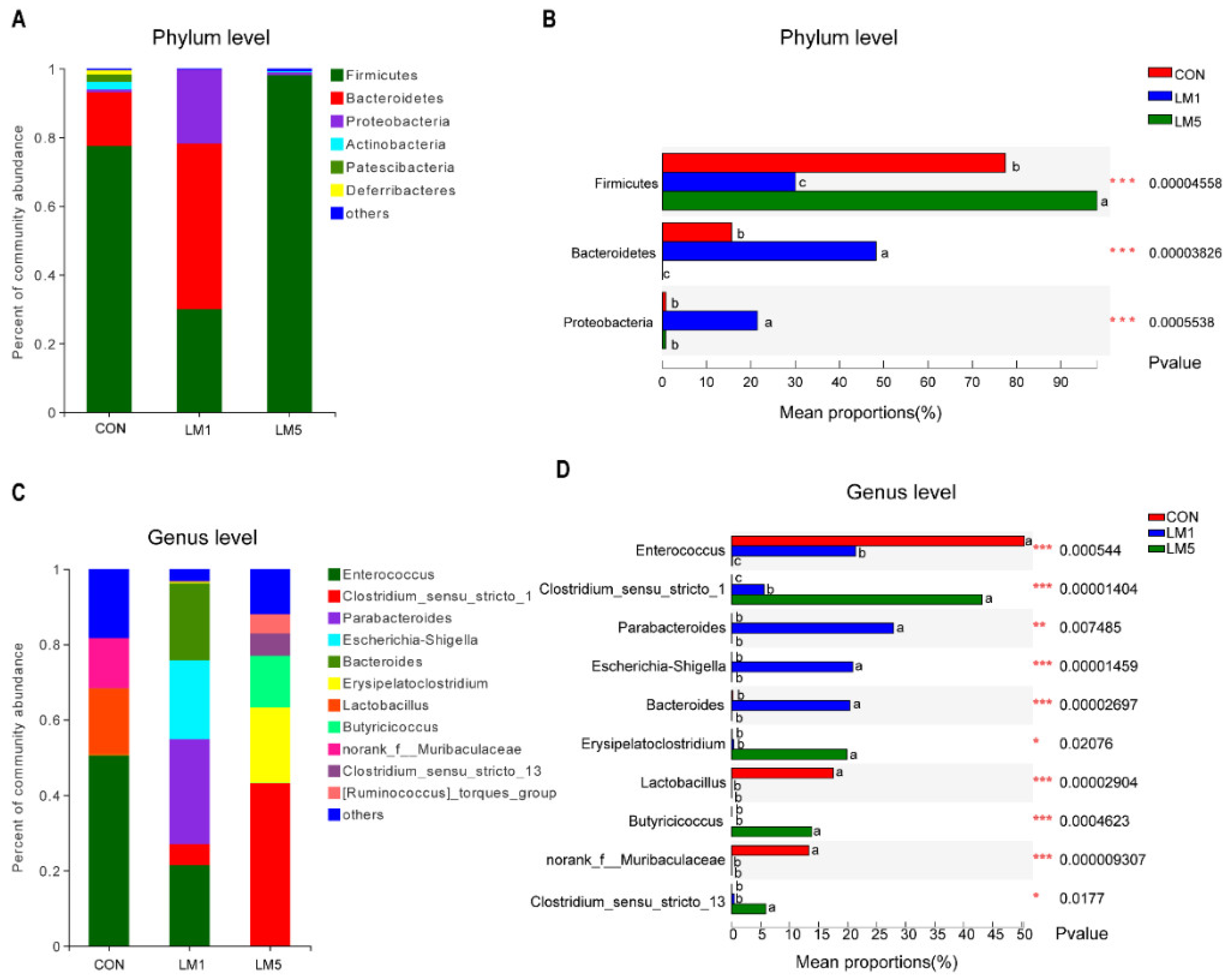
2.2. Antibiotic Treatment Reduces the Amount and Variability of the Gut Microbiota
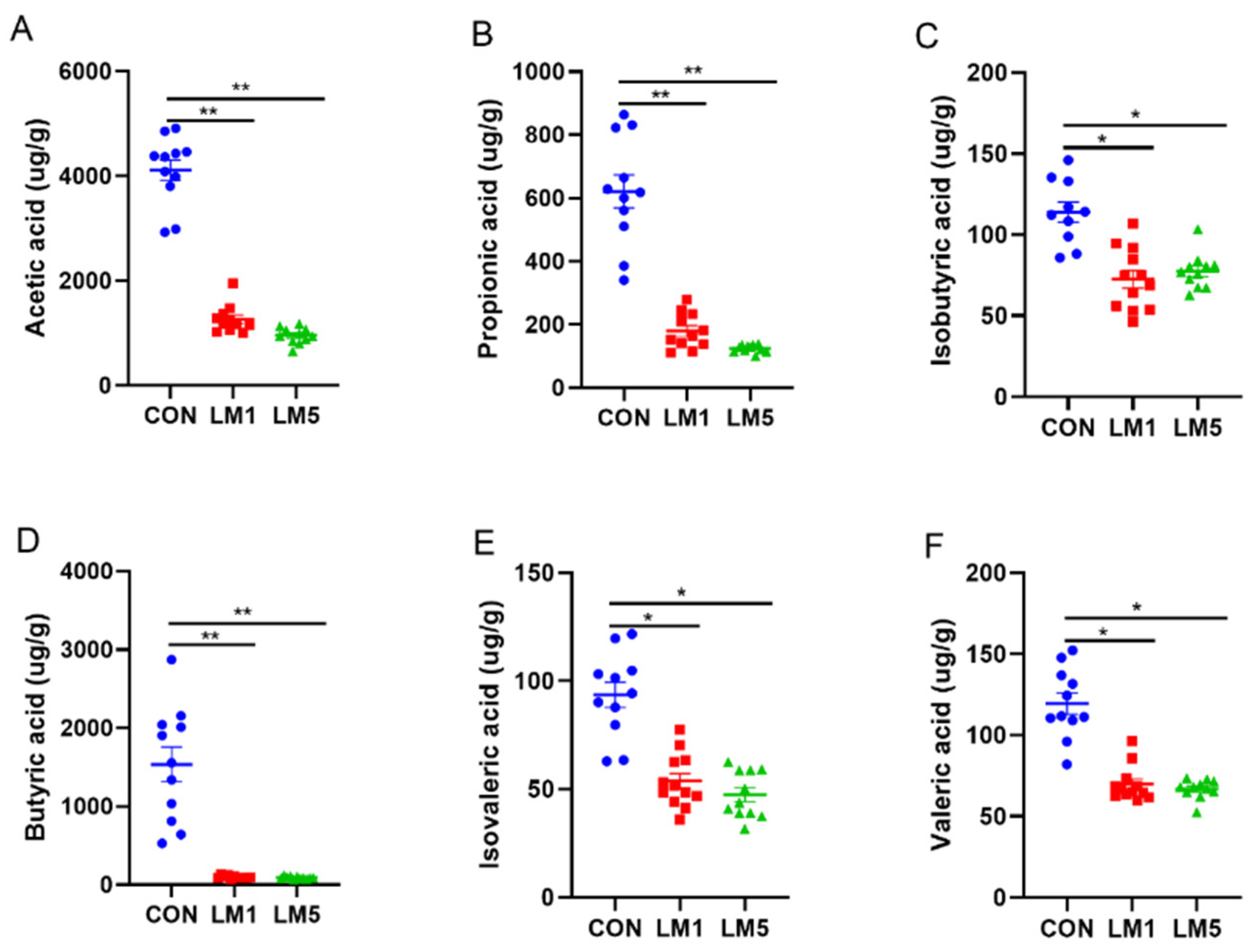
2.3. Effects of Antibiotic on SCFAs Production
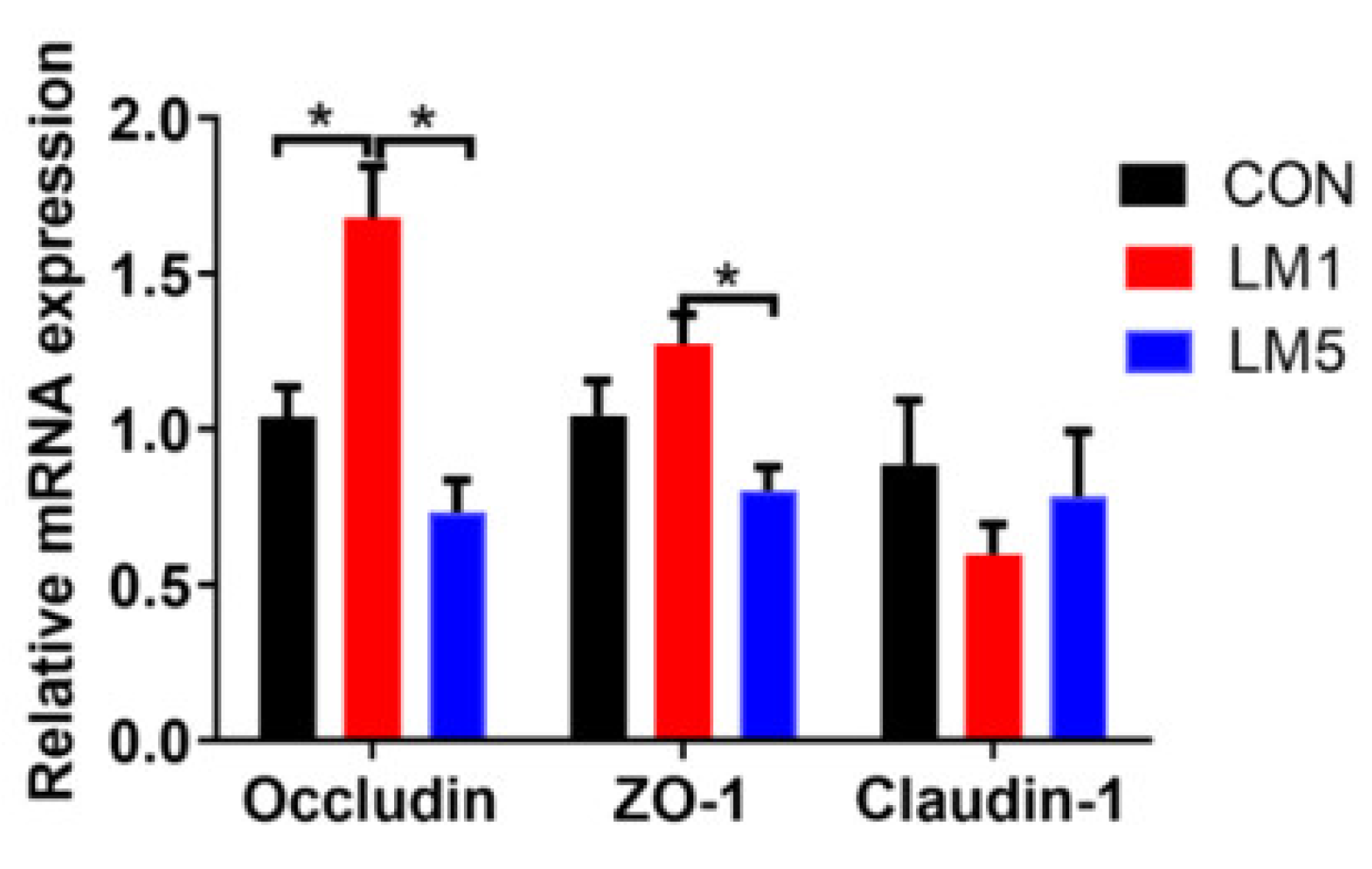
2.4. Antibiotic Exposure Influence Morphology of Jejunum and Ileum and Intestinal Barrier/gut integrity in Mice
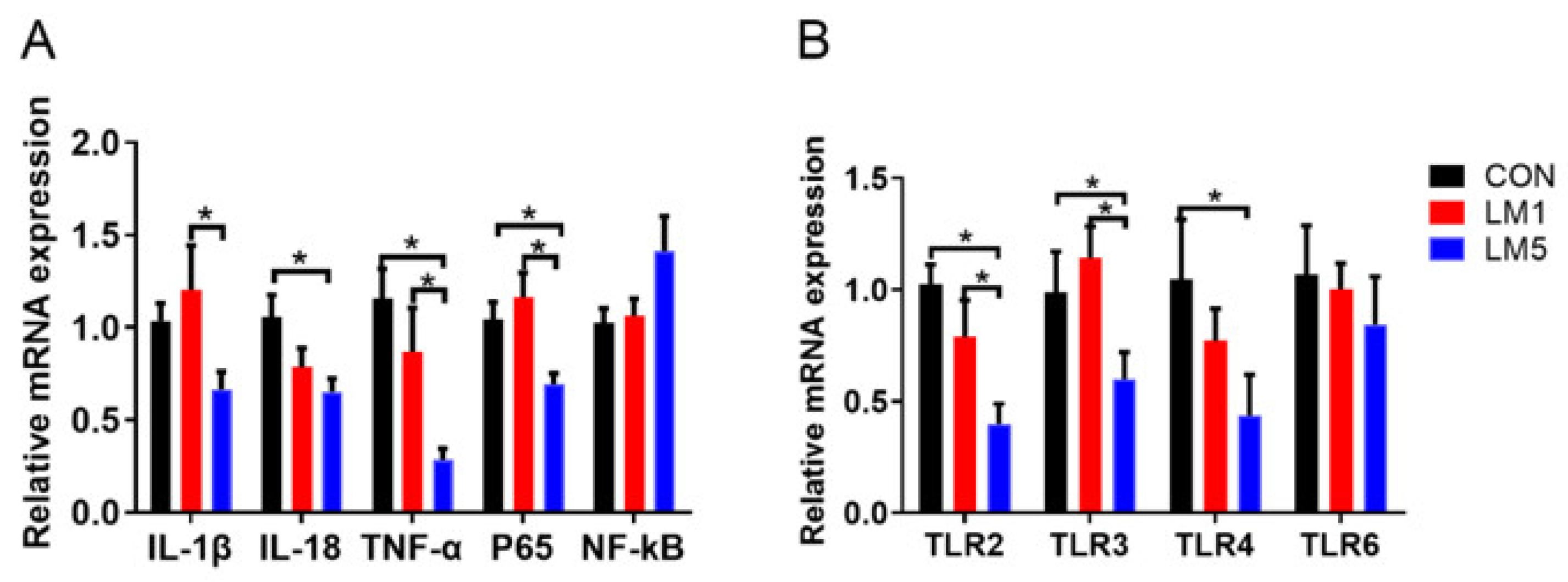
2.5. Effect of Antibiotic on TLRs Expression Patterns and Inflammation of Mice
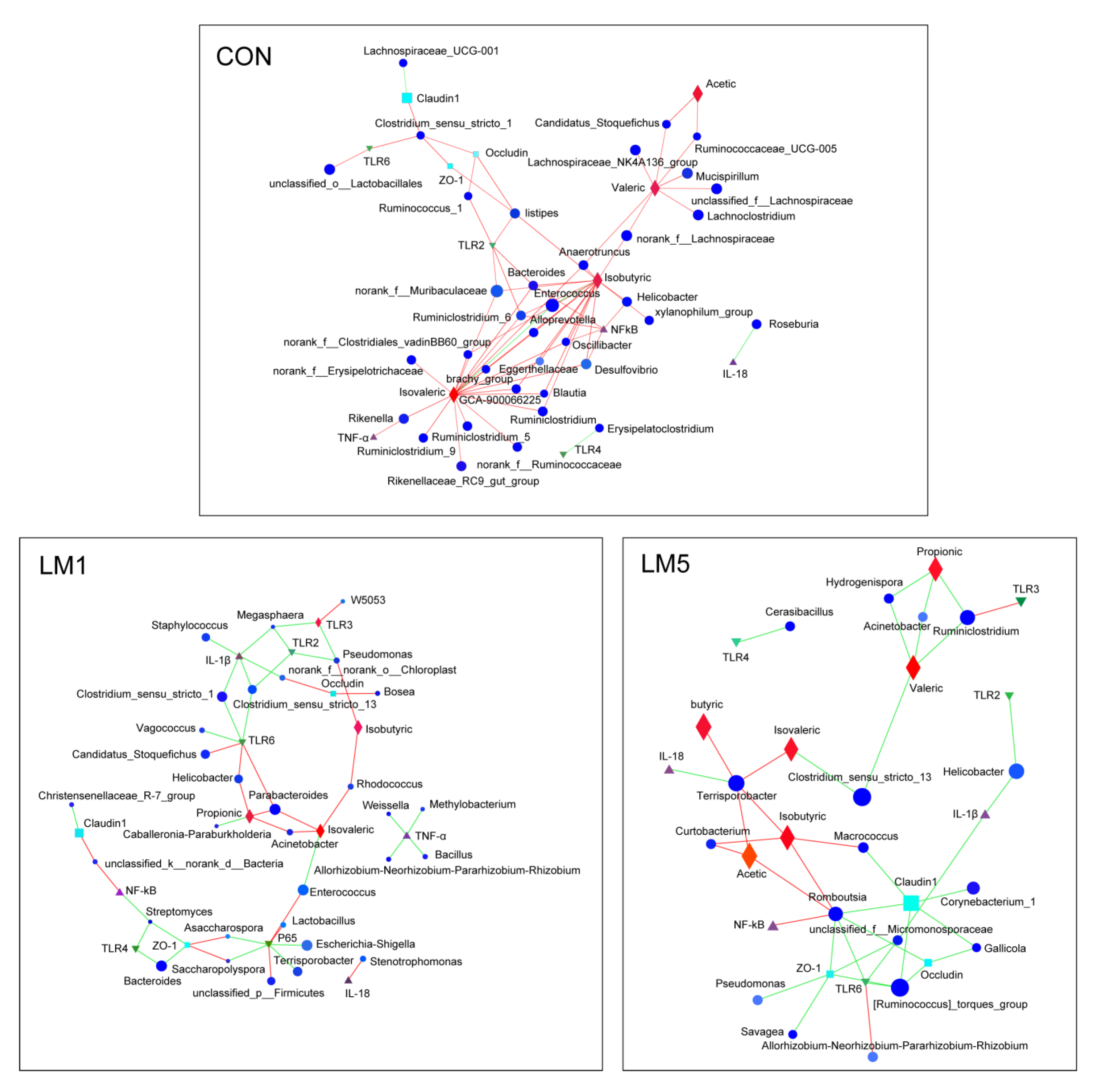
2.6. Antibiotic Modulates the Relevance Network between Relative Expression of Cytokine Contents, Bacterial Members, and Metabolites
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Mice and Antibiotics Treatment
4.3. Cytokine Genes Expression
4.4. DNA Extraction, 16S rRNA Gene Amplification and Sequencing
4.5. Sequence Processing and Analysis
4.6. The Relative Concentration of SCFAs
4.7. Tissue Sample and Intestinal Morphology
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Iwai, H.; Saito, M.; Yamauchi, C.; Nomura, T. Influence of intestinal microbes on digestion and absorption of nutrients in diet and nitrogen retention in germfree, gnotobiotic and conventional mice. 1. Protein and fat digestion and nitrogen retention in germfree and conventional mice. Jpn. J. Zootech. Sci. 1972, 43, 272–283. [Google Scholar] [CrossRef]
- Qayed, M.; Horan, J.T. The Role of Intestinal Microbiota in Graft versus Host Disease. Mini-Rev. Med. Chem. 2016, 16, 193–199. [Google Scholar] [CrossRef]
- Natarajan, N.; Pluznick, J.L. From microbe to man: The role of microbial short chain fatty acid metabolites in host cell biology. Am. J. Physiol. Cell Physiol. 2014, 307, C979–C985. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, X.; Zhang, Y.; Zheng, K.; Xiang, Q.; Chen, N.; Chen, Z.; Zhang, N.; Zhu, J.; He, Q. Antibiotic-Induced Disruption of Gut Microbiota Alters Local Metabolomes and Immune Responses. Front. Cell. Infect. Microbiol. 2019, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, S.H.; Hong, S.J. Antibiotics-Induced Dysbiosis of Intestinal Microbiota Aggravates Atopic Dermatitis in Mice by Altered Short-Chain Fatty Acids. Allergy Asthma Immunol. Res. 2020, 12, 137–148. [Google Scholar] [CrossRef]
- Manuzak, J.A.; Zevin, A.S.; Cheu, R.; Richardson, B.; Modesitt, J.; Hensley-McBain, T.; Miller, C.; Gustin, A.T.; Coronado, E.; Gott, T.; et al. Antibiotic-induced microbiome perturbations are associated with significant alterations to colonic mucosal immunity in rhesus macaques. Mucosal Immunol. 2020, 13, 471–480. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Zhao, Q.; Zhao, Y.; Fu, C.; Feng, X.; Wang, N.; Su, M.; Tang, C.; Jiang, F.; et al. Antibiotic Body Burden of Chinese School Children: A Multisite Biomonitoring-based Study. Environ. Sci. Technol. 2015, 49, 5070–5079. [Google Scholar] [CrossRef]
- Cheng, B.; Jiang, F.; Su, M.L.; Zhou, L.Q.; Zhang, H.; Cao, Z.G.; Liao, X.J.; Xiong, G.H.; Xiao, J.H.; Liu, F.S.; et al. Effects of lincomycin hydrochloride on the neurotoxicity of zebrafish. Ecotoxicol. Environ. Saf. 2020, 201, 110725. [Google Scholar] [CrossRef]
- Wang, Q.; Du, W.; Xu, J. Retrospective analysis of adverse reactions of lincomycin. Chin. J. New Drugs Clin. Remedies 2003, 22, 571–574. [Google Scholar]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Nogacka, A.M.; Salazar, N.; Arboleya, S.; Suarez, M.; Fernandez, N.; Solis, G.; de Los Reyes-Gavilan, C.G.; Gueimonde, M. Early microbiota, antibiotics and health. Cell. Mol. Life Sci. 2018, 75, 83–91. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, W.; Zhang, G. Effects of Enterococcus faecium on growth performance, intestinal Flora and immune function of weaner piglets. Chin. J. Anim. Nutr. 2013, 25, 1069–1076. [Google Scholar]
- Kierzkowska, M.; Majewska, A.; Szymanek-Majchrzak, K.; Sawicka-Grzelak, A.; Mlynarczyk, A.; Mlynarczyk, G. In vitro effect of clindamycin against Bacteroides and Parabacteroides isolates in Poland. J. Glob. Antimicrob. Resist. 2018, 13, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Khan, Z.; Malik, A.; Kalam, M.A.; Cash, P.; Ashraf, M.T.; Alshamsan, A. Colorectal cancer-inflammatory bowel disease nexus and felony of Escherichia coli. Life Sci. 2017, 180, 60–67. [Google Scholar] [CrossRef]
- Sears, C.L. Enterotoxigenic Bacteroides fragilis: A Rogue among Symbiotes. Clin. Microbiol. Rev. 2009, 22, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Guo, J.; Pu, F.; Wan, C.; Shi, L.; Li, H.; Yang, Y.; Huang, C.; Li, M.; He, F. Loading ceftriaxone, vancomycin, and Bifidobacteria bifidum TMC3115 to neonatal mice could differently and consequently affect intestinal microbiota and immunity in adulthood. Sci. Rep. 2019, 9, 3254. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology—Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Harata, G.; Kumar, H.; He, F.; Miyazawa, K.; Yoda, K.; Kawase, M.; Kubota, A.; Hiramatsu, M.; Rautava, S.; Salminen, S. Probiotics modulate gut microbiota and health status in Japanese cedar pollinosis patients during the pollen season. Eur. J. Nutr. 2017, 56, 2245–2253. [Google Scholar] [CrossRef]
- Yao, Y.; Yan, L.J.; Chen, H.; Wu, N.; Wang, W.B.; Wang, D.S. Cyclocarya paliurus polysaccharides alleviate type 2 diabetic symptoms by modulating gut microbiota and short-chain fatty acids. Phytomedicine 2020, 77, 153268. [Google Scholar] [CrossRef]
- Zhou, C.; Li, L.Z.; Li, T.M.; Sun, L.H.; Yin, J.H.; Guan, H.D.; Wang, L.C.; Zhu, H.B.; Xu, P.; Fan, X.; et al. SCFAs induce autophagy in intestinal epithelial cells and relieve colitis by stabilizing HIF-1 alpha. J. Mol. Med. JMM 2020, 98, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Romick-Rosendale, L.E.; Haslam, D.B.; Lane, A.; Denson, L.; Lake, K.; Wilkey, A.; Watanabe, M.; Bauer, S.; Litts, B.; Luebbering, N.; et al. Antibiotic Exposure and Reduced Short Chain Fatty Acid Production after Hematopoietic Stem Cell Transplant. Biol. Blood Marrow Transplant. 2018, 24, 2418–2424. [Google Scholar] [CrossRef] [PubMed]
- Rios-Covian, D.; Salazar, N.; Gueimonde, M.; de los Reyes-Gavilan, C.G. Shaping the Metabolism of Intestinal Bacteroides Population through Diet to Improve Human Health. Front. Microbiol. 2017, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, Y.; Mu, C.; Zhu, W. Ileum terminal antibiotic infusion affects jejunal and colonic specific microbial population and immune status in growing pigs. J. Anim. Sci. Biotechnol. 2018, 9, 51. [Google Scholar] [CrossRef]
- Ohland, C.L.; MacNaughton, W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef]
- Willing, B.P.; Russell, S.L.; Finlay, B.B. Shifting the balance: Antibiotic effects on host-microbiota mutualism. Nat. Rev. Microbiol. 2011, 9, 233–243. [Google Scholar] [CrossRef]
- Kaiko, G.E.; Stappenbeck, T.S. Host-microbe interactions shaping the gastrointestinal environment. Trends Immunol. 2014, 35, 538–548. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Carvalho, A.; Caso, J.; Sanz, Y.; Walder, K.; Maes, M. The Role of the Microbial Metabolites Including Tryptophan Catabolites and Short Chain Fatty Acids in the Pathophysiology of Immune-Inflammatory and Neuroimmune Disease. Mol. Neurobiol. 2017, 54, 4432–4451. [Google Scholar] [CrossRef]
- Ma, X.; Fan, P.X.; Li, L.S.; Qiao, S.Y.; Zhang, G.L.; Li, D.F. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J. Anim. Sci. 2012, 90, 266–268. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Yan, C.; Xie, R.; Guo, Z.; Wang, S.; Zhang, Y.; Li, Z.; Wang, B.; Cao, H. Diammonium Glycyrrhizinate Protects against Nonalcoholic Fatty Liver Disease in Mice through Modulation of Gut Microbiota and Restoration of Intestinal Barrier. Mol. Pharm. 2018, 15, 3860–3870. [Google Scholar] [CrossRef] [PubMed]
- Underhill, D.M.; Ozinsky, A. Toll-like receptors: Key mediators of microbe detection. Curr. Opin. Immunol. 2002, 14, 103–110. [Google Scholar] [CrossRef]
- Filippi, C.M. Toll-like receptor activation in immunity vs. tolerance. Front. Immunol. 2015, 6, 146. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, X.; Gao, X.-C.; Liu, J.; Ren, H.-Y. Effect of EPEC endotoxin and bifidobacteria on intestinal barrier function through modulation of toll-like receptor 2 and toll-like receptor 4 expression in intestinal epithelial cell-18. World J. Gastroenterol. 2017, 23, 4744–4751. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; Chang, W.J.; Chen, P.H.; Liu, W.C.; Hsu, C.M. TLR ligand decreases mesenteric ischemia and reperfusion injury-induced gut damage through TNF-alpha signaling. Shock 2008, 30, 563–570. [Google Scholar] [CrossRef]
- Ortega-Cava, C.F.; Ishihara, S.; Rumi, M.A.K.; Kawashima, K.; Ishimura, N.; Kazumori, H.; Udagawa, J.; Kadowaki, Y.; Kinoshita, Y. Strategic compartmentalization of toll-like receptor 4 in the mouse gut. J. Immunol. 2003, 170, 3977–3985. [Google Scholar] [CrossRef]
- Piras, V.; Selvarajoo, K. Beyonc MyD88 and TRIF pathways in Toll-like receptor signaling. Front. Immunol. 2014, 5, 70. [Google Scholar] [CrossRef]
- Wang, X.X.; Luo, B.J.; Lu, Y.Y.; Pang, D.G.; Zheng, J.Q.; Mo, J.L.; Huang, H.; Feng, J.F. The triggering receptor expressed by myeloid cells-1 activates TLR4-MyD88-NF-B-dependent signaling to aggravate ventilation-induced lung inflammation and injury in mice. Cell Tissue Res. 2018, 374, 137–148. [Google Scholar] [CrossRef]
- Xue, X.; Falcon, D.M. The Role of Immune Cells and Cytokines in Intestinal Wound Healing. Int. J. Mol. Sci. 2019, 20, 97. [Google Scholar] [CrossRef]
- Jena, P.K.; Sheng, L.; Liu, H.-X.; Kalanetra, K.M.; Mirsoian, A.; Murphy, W.J.; French, S.W.; Krishnan, V.V.; Mills, D.A.; Wan, Y.-J.Y. Western Diet-Induced Dysbiosis in Farnesoid X Receptor Knockout Mice Causes Persistent Hepatic Inflammation after Antibiotic Treatment. Am. J. Pathol. 2017, 187, 1800–1813. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Wick, E.C.; Sears, C.L. Bacteroides spp. and diarrhea. Curr. Opin. Infect. Dis. 2010, 23, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Nowak, E.C.; de Vries, V.C.; Wasiuk, A.; Ahonen, C.; Bennett, K.A.; Le Mercier, I.; Ha, D.-G.; Noelle, R.J. Tryptophan hydroxylase-1 regulates immune tolerance and inflammation. J. Exp. Med. 2012, 209, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Koliarakis, I.; Athanasakis, E.; Sgantzos, M.; Mariolis-Sapsakos, T.; Xynos, E.; Chrysos, E.; Souglakos, J.; Tsiaoussis, J. Intestinal Microbiota in Colorectal Cancer Surgery. Cancers 2020, 12, 3011. [Google Scholar] [CrossRef]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise Prevents Weight Gain and Alters the Gut Microbiota in a Mouse Model of High Fat Diet-Induced Obesity. PLoS ONE 2014, 9, e92193. [Google Scholar] [CrossRef]
- Shang, Q.S.; Song, G.R.; Zhang, M.F.; Shi, J.J.; Xu, C.Y.; Hao, J.J.; Li, G.Y.; Yu, G.L. Dietary fucoidan improves metabolic syndrome in association with increased Akkermansia population in the gut microbiota of high-fat diet-fed mice. J. Funct. Foods 2017, 28, 138–146. [Google Scholar] [CrossRef]
- Wu, W.; Xie, J.; Zhang, H. Dietary fibers influence the intestinal SCFAs and plasma metabolites profiling in growing pigs. Food Funct. 2016, 7, 4644–4654. [Google Scholar] [CrossRef]
- Zhong, G.; Shao, D.; Wang, Q.; Tong, H.; Shi, S. Effects of dietary supplemented of gamma-amino butyric acid on growth performance, blood biochemical indices and intestinal morphology of yellow-feathered broilers exposed to a high temperature environment. Ital. J. Anim. Sci. 2020, 19, 431–438. [Google Scholar] [CrossRef]

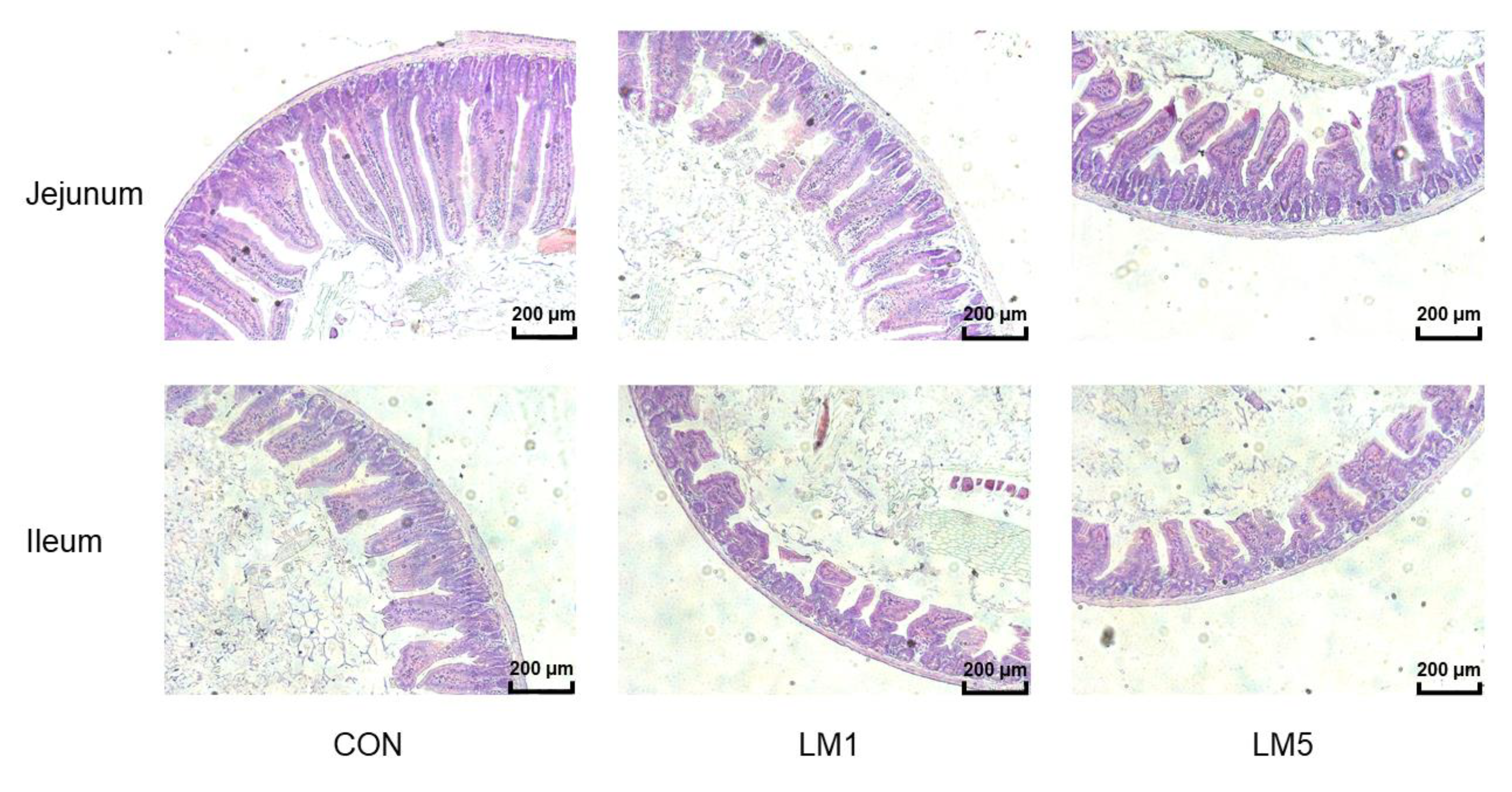
| Jejunum µm | Ileum µm | |||||
|---|---|---|---|---|---|---|
| Group | Villus Height | Crypt Depth | V/C | Villus Height | Crypt Depth | V/C |
| CON | 470.26 a | 111.02 a | 4.29 | 209.46 a | 79.16 a | 2.63 |
| LM1 | 405.51 ab | 95.24 ab | 4.23 | 161.12 ab | 65.47 ab | 2.45 |
| LM5 | 310.30 b | 78.58 b | 4.02 | 137.75 b | 55.68 b | 2.46 |
| SEM | 21.37 | 4.35 | 0.15 | 12.12 | 3.09 | 0.10 |
| p-value | 0.006 | 0.007 | 0.897 | 0.048 | 0.004 | 0.470 |
| Gene | Forward Nucleotide Sequence Primers (5′-3′) | Reverse Nucleotide Sequence Primers (5′-3′) | Product Size (bp) |
|---|---|---|---|
| GADPH | ACCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA | 172 |
| TLR2 | AGGTGCCCTGTGCCACCATT | CGGAACGAAGTCCCGCTTGT | 172 |
| TLR3 | GGTCCCCAGCCTTCAAAGAC | ACGAAGAGGGCGGAAAGGT | 85 |
| TLR4 | CTGGTGGCTGTGGAGACAAA | ATTCCCTGAAAGGCTTGGTC | 172 |
| TLR6 | TCATCTCAGCAAACACCGAGTATAGCG | CAACCTTATTGAATGTGACCCTCCAGC | 249 |
| IL-1β | TCGCAGCAGCACATCAACAAGAG | AGGTCCACGGGAAAGACACAGG | 97 |
| IL-18 | AGACCTGGAATCAGACAACTTT | TCAGTCATATCCTCGAACACAG | 117 |
| TNF-α | GGACTAGCCAGGAGGGAGAACAG | GCCAGTGAGTGAAAGGGACAGAAC | 103 |
| NF-kB | CAAAGACAAAGAGGAAGTGCAA | GATGGAATGTAATCCCACCGTA | 203 |
| P65 | TCGAGTCTCCATGCAGCTACGG | CGGTGGCGATCATCTGTGTCTG | 93 |
| ZO-1 | CTGGTGAAGTCTCGGAAAAATG | CATCTCTTGCTGCCAAACTATC | 97 |
| Occludin | TGCTTCATCGCTTCCTTAGTAA | GGGTTCACTCCCATTATGTACA | 155 |
| Claudin-1-1 | AGATACAGTGCAAAGTCTTCGA | CAGGATGCCAATTACCATCAAG | 86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Zhong, R.; Han, H.; Yi, B.; Yin, J.; Chen, L.; Zhang, H. Short-Term Lincomycin Exposure Depletion of Murine Microbiota Affects Short-Chain Fatty Acids and Intestinal Morphology and Immunity. Antibiotics 2020, 9, 907. https://doi.org/10.3390/antibiotics9120907
Zhang S, Zhong R, Han H, Yi B, Yin J, Chen L, Zhang H. Short-Term Lincomycin Exposure Depletion of Murine Microbiota Affects Short-Chain Fatty Acids and Intestinal Morphology and Immunity. Antibiotics. 2020; 9(12):907. https://doi.org/10.3390/antibiotics9120907
Chicago/Turabian StyleZhang, Shunfen, Ruqing Zhong, Hui Han, Bao Yi, Jie Yin, Liang Chen, and Hongfu Zhang. 2020. "Short-Term Lincomycin Exposure Depletion of Murine Microbiota Affects Short-Chain Fatty Acids and Intestinal Morphology and Immunity" Antibiotics 9, no. 12: 907. https://doi.org/10.3390/antibiotics9120907
APA StyleZhang, S., Zhong, R., Han, H., Yi, B., Yin, J., Chen, L., & Zhang, H. (2020). Short-Term Lincomycin Exposure Depletion of Murine Microbiota Affects Short-Chain Fatty Acids and Intestinal Morphology and Immunity. Antibiotics, 9(12), 907. https://doi.org/10.3390/antibiotics9120907








