Sequential Hypertonic-Hypotonic Treatment Enhances Efficacy of Antibiotic against Acinetobacter baumannii Biofilm Communities
Abstract
1. Introduction
2. Results
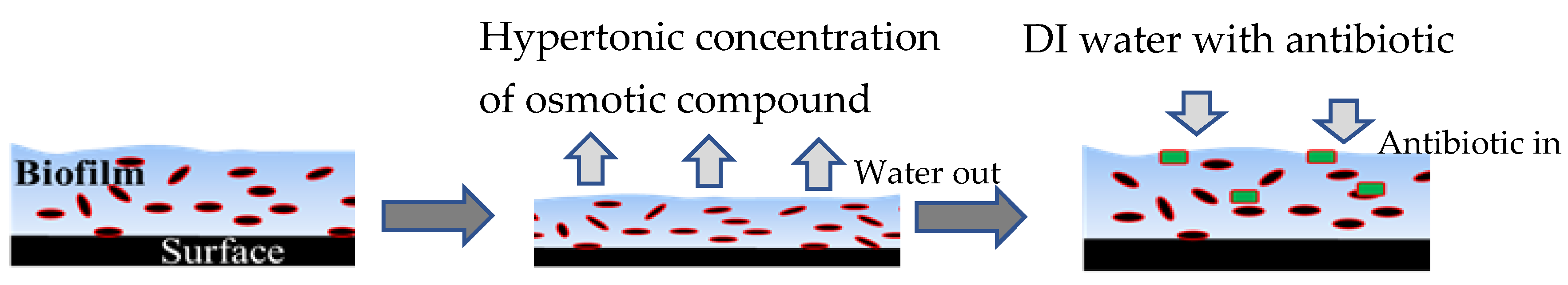
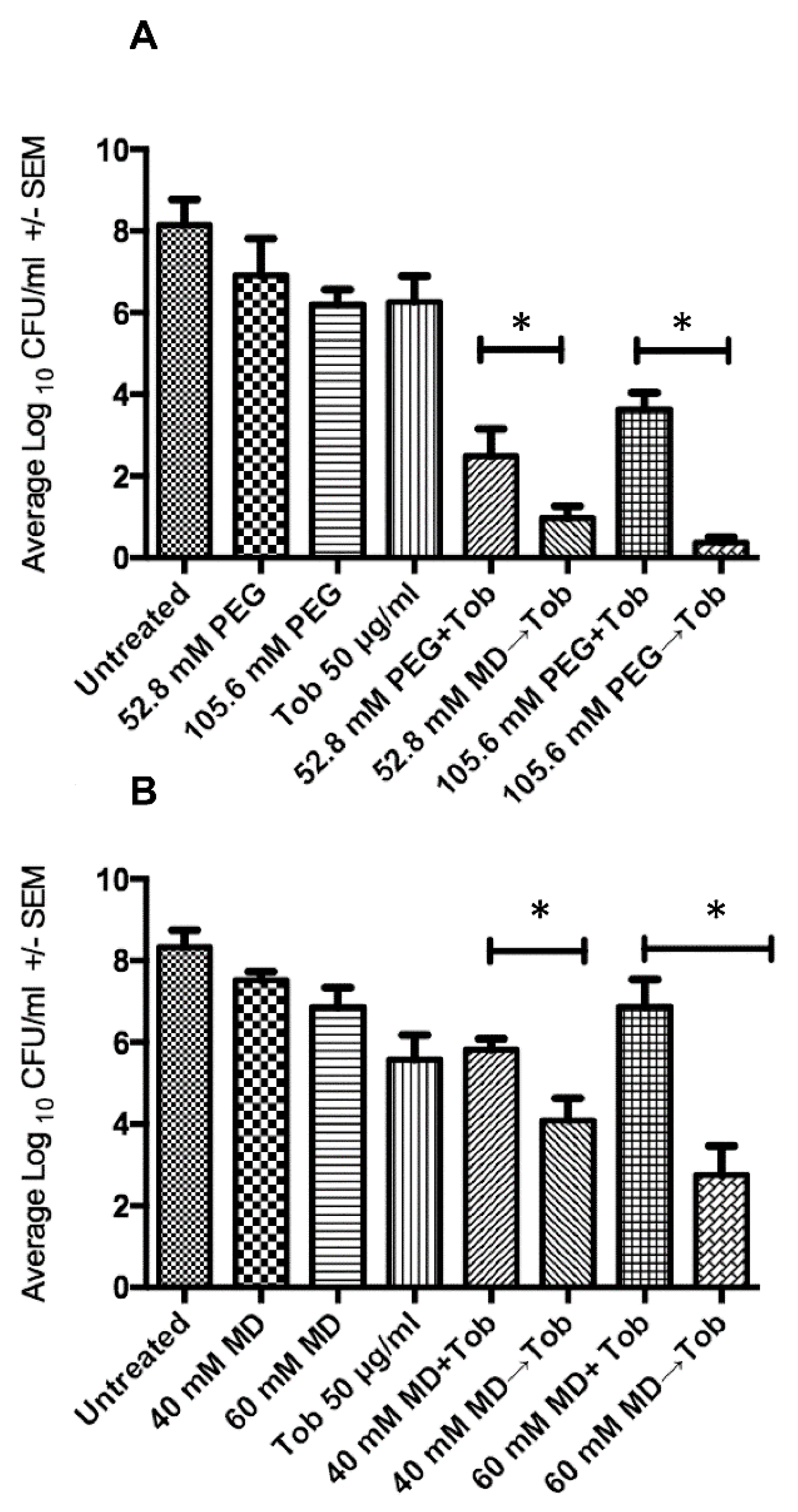
2.1. Sequential Treatment with Hypertonic and Hypotonic Conditions
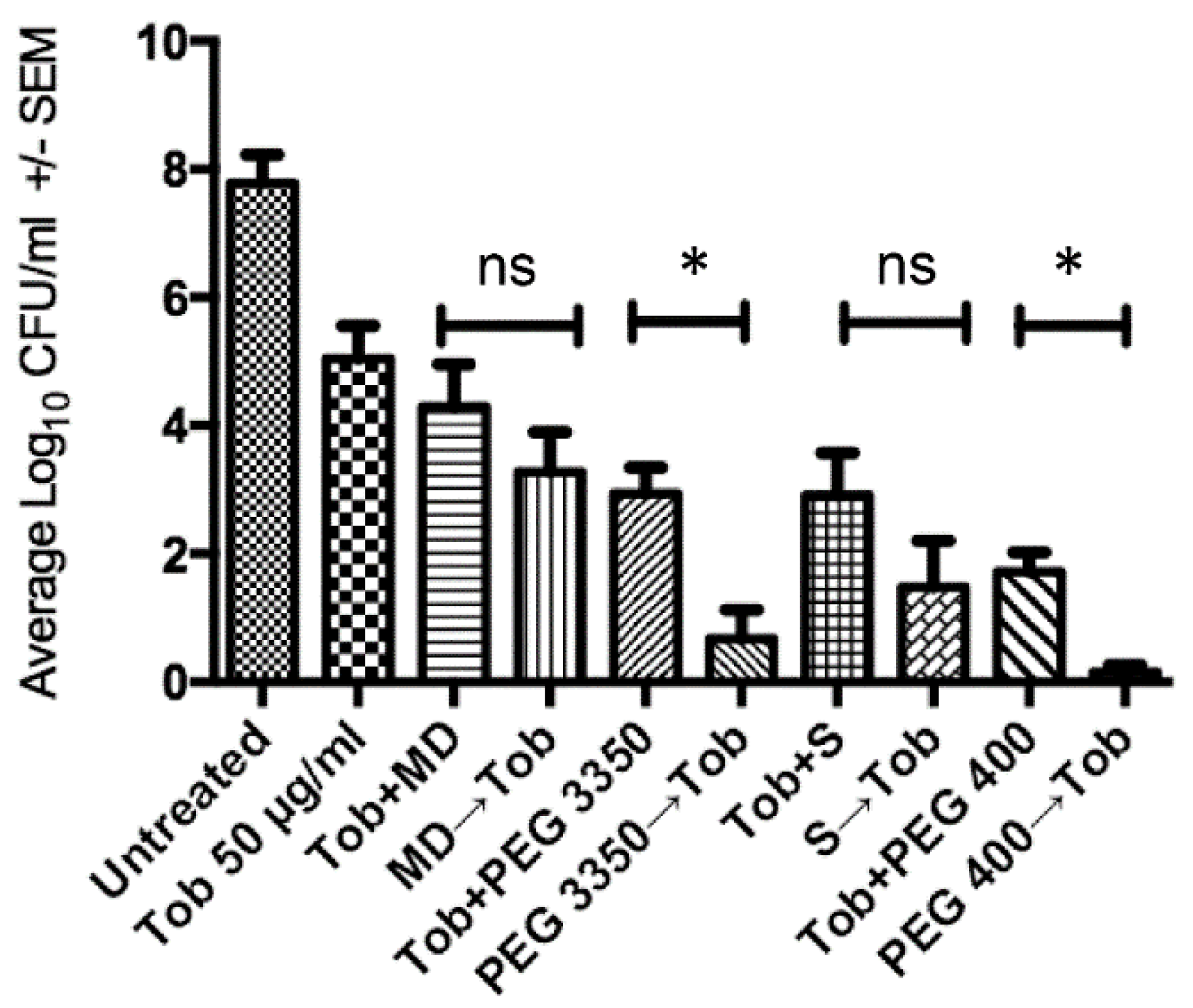
2.2. Penetration of Biofilms by Antibiotics is Concentration Dependent
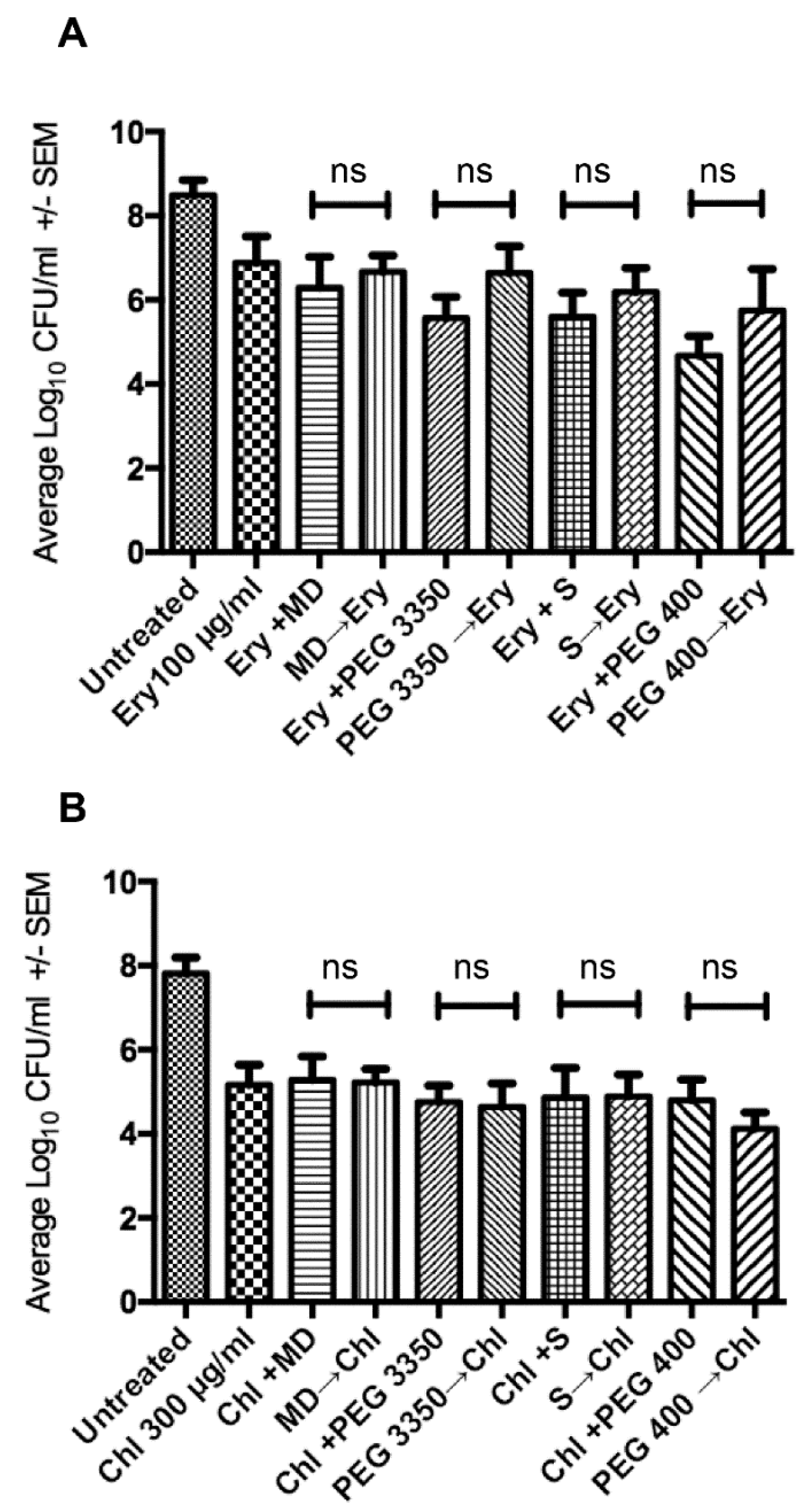
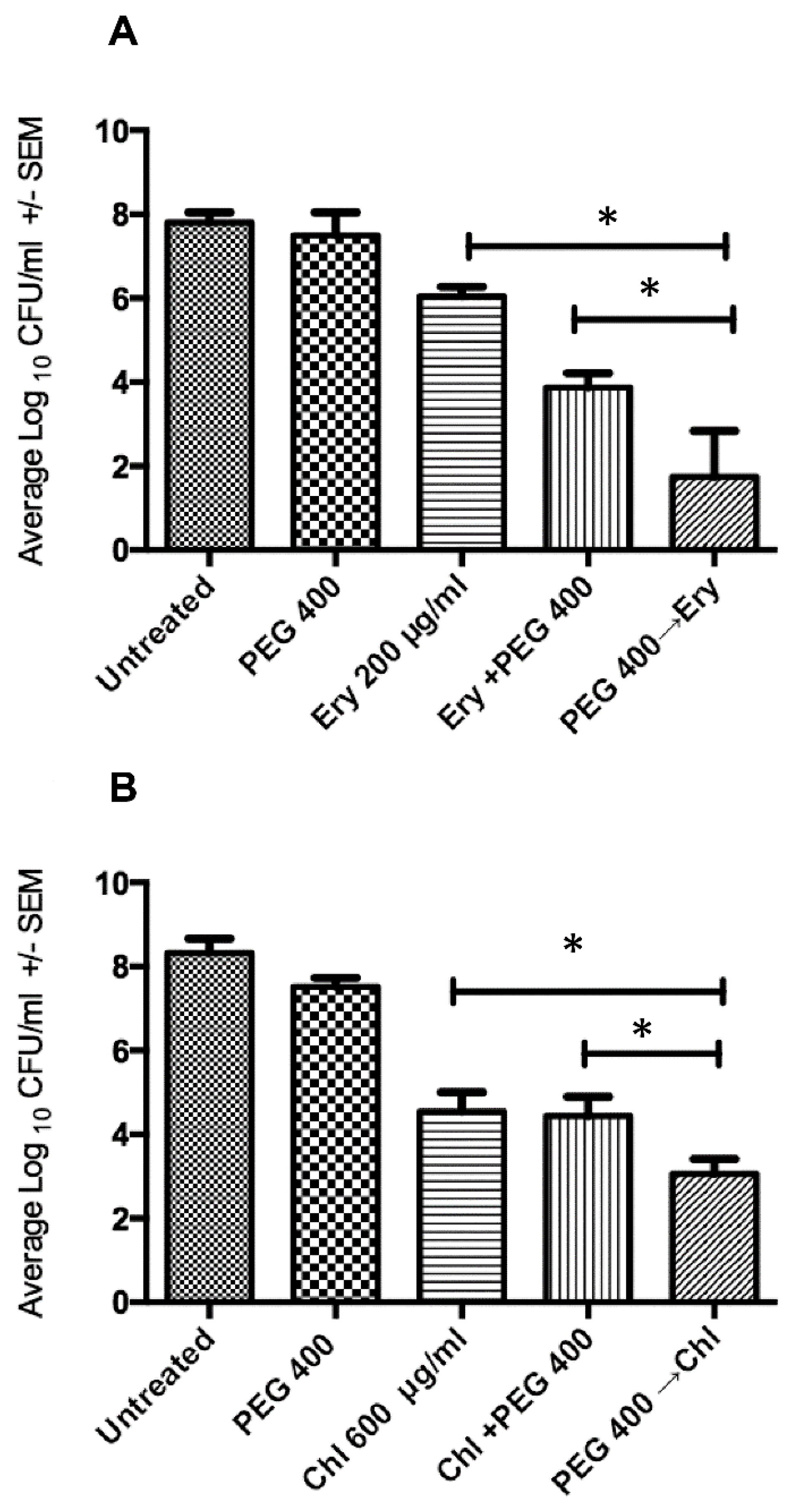
2.3. Sequential Treatment Negates the Limitations of Using Increased Concentration of Osmotic Compounds
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain and Reagents
4.2. Determining Minimum Inhibitory Concentration
4.3. Biofilm Preparation
4.4. Biofilm Treatment
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability: Samples of the compounds are not available from the authors, but compounds can be purchased from commercial vendors ....... |
References
- Lee, K.; Yong, D.; Jeong, S.H.; Chong, Y. Multidrug-resistant Acinetobacter Spp.: Increasingly problematic nosocomial pathogens. Yonsei Med. J. 2011, 52, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.; O’Donoghue, M.; Feeney, A.; Sleator, R.D. Acinetobacter Baumannii: An emerging opportunistic pathogen. Virulence 2012, 3, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter Baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.; Vuotto, C.; Donelli, G. Biofilm Formation in Acinetobacter Baumannii. New Microbiol. 2014, 37, 119–127. [Google Scholar]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter Baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef]
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. APMIS 2017, 125, 304–319. [Google Scholar] [CrossRef]
- Gordon, C.A.; Hodges, N.A.; Marriott, C. Antibiotic interaction and diffusion through alginate and exopolysaccharide of cystic fibrosis-derived Pseudomonas Aeruginosa. J. Antimicrob. Chemother. 1988, 22, 667–674. [Google Scholar] [CrossRef]
- Stewart, P.S. Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrob. Agents Chemother. 1996, 40, 2517–2522. [Google Scholar] [CrossRef]
- Van Acker, H.; Van Dijck, P.; Coenye, T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014, 22, 326–333. [Google Scholar] [CrossRef]
- Taff, H.T.; Mitchell, K.F.; Edward, J.A.; Andes, D.R. Mechanisms of candida biofilm drug resistance. Future Microbiol. 2013, 8, 1325–1337. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Davenport, E.K.; Call, D.R.; Beyenal, H. Differential protection from tobramycin by extracellular polymeric substances from Acinetobacter Baumannii and Staphylococcus Aureus biofilms. Antimicrob. Agents Chemother. 2014, 58, 4755–4761. [Google Scholar] [CrossRef] [PubMed]
- Kiamco, M.M.; Atci, E.; Khan, Q.F.; Mohamed, A.; Renslow, R.S.; Abu-Lail, N.; Fransson, B.A.; Call, D.R.; Beyenal, H. Vancomycin and maltodextrin affect structure and activity of Staphylococcus Aureus biofilms. Biotechnol. Bioeng. 2015, 112, 2562–2570. [Google Scholar] [CrossRef] [PubMed]
- Deliorman, M.; Gordesli Duatepe, F.P.; Davenport, E.K.; Fransson, B.A.; Call, D.R.; Beyenal, H.; Abu-Lail, N.I. Responses of Acinetobacter Baumannii bound and loose extracellular polymeric substances to hyperosmotic agents combined with or without tobramycin: An atomic force microscopy study. Langmuir 2019. [Google Scholar] [CrossRef]
- Falghoush, A.; Beyenal, H.; Besser, T.E.; Omsland, A.; Call, D.R. Osmotic compounds enhance antibiotic efficacy against Acinetobacter Baumannii biofilm communities. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef]
- Singh, R.; Sahore, S.; Kaur, P.; Rani, A.; Ray, P. Penetration barrier contributes to bacterial biofilm-associated resistance against only select antibiotics, and exhibits genus-, strain- and antibiotic-specific differences. Pathog. Dis. 2016, 74, ftw056. [Google Scholar] [CrossRef]
- Flemming, H.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Olsen, I. Biofilm-specific antibiotic tolerance and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 877–886. [Google Scholar] [CrossRef]
- Kiamco, M.M.; Mohamed, A.; Reardon, P.N.; Marean-Reardon, C.L.; Aframehr, W.M.; Call, D.R.; Beyenal, H.; Renslow, R.S. Structural and metabolic responses of Staphylococcus Aureus biofilms to hyperosmotic and antibiotic stress. Biotechnol. Bioeng. 2018, 115, 1594–1603. [Google Scholar] [CrossRef]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Jang, H.J.; Shin, C.Y.; Kim, K.B. Safety Evaluation of Polyethylene Glycol (PEG) compounds for cosmetic use. Toxicol. Res. 2015, 31, 105–136. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(Ethylene Glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Fruijtier-Pölloth, C. Safety assessment on Polyethylene Glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology. 2005, 214, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M.; Andrews, J.M. Determination of Minimum Inhibitory Concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 5–16. [Google Scholar] [CrossRef]
- Abdi-Ali, A.; Hendiani, S.; Mohammadi, P.; Gharavi, S. Assessment of biofilm formation and resistance to imipenem and ciprofloxacin among clinical isolates of Acinetobacter Baumannii in tehran. Jundishapur J. Microbiol. 2014, 7. [Google Scholar] [CrossRef]
- Pour, N.K.; Dusane, D.H.; Dhakephalkar, P.K.; Zamin, F.R.; Zinjarde, S.S.; Chopade, B.A. Biofilm formation by Acinetobacter Baumannii strains isolated from urinary tract infection and urinary catheters. FEMS Immunol. Med. Microbiol. 2011, 62, 328–338. [Google Scholar] [CrossRef]
- Chen, C.Y.; Nace, G.W.; Irwin, P.L. A 6x6 drop plate method for simultaneous colony counting and MPN enumeration of campylobacter jejuni, listeria monocytogenes, and escherichia coli. J. Microbiol. Methods 2003, 55, 475–479. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falghoush, A.; Beyenal, H.; Call, D.R. Sequential Hypertonic-Hypotonic Treatment Enhances Efficacy of Antibiotic against Acinetobacter baumannii Biofilm Communities. Antibiotics 2020, 9, 832. https://doi.org/10.3390/antibiotics9110832
Falghoush A, Beyenal H, Call DR. Sequential Hypertonic-Hypotonic Treatment Enhances Efficacy of Antibiotic against Acinetobacter baumannii Biofilm Communities. Antibiotics. 2020; 9(11):832. https://doi.org/10.3390/antibiotics9110832
Chicago/Turabian StyleFalghoush, Azeza, Haluk Beyenal, and Douglas R. Call. 2020. "Sequential Hypertonic-Hypotonic Treatment Enhances Efficacy of Antibiotic against Acinetobacter baumannii Biofilm Communities" Antibiotics 9, no. 11: 832. https://doi.org/10.3390/antibiotics9110832
APA StyleFalghoush, A., Beyenal, H., & Call, D. R. (2020). Sequential Hypertonic-Hypotonic Treatment Enhances Efficacy of Antibiotic against Acinetobacter baumannii Biofilm Communities. Antibiotics, 9(11), 832. https://doi.org/10.3390/antibiotics9110832




