African Plant-Based Natural Products with Antivirulence Activities to the Rescue of Antibiotics
Abstract
1. Introduction
2. Bacterial Virulence
2.1. Motilities and Virulence Factors Production
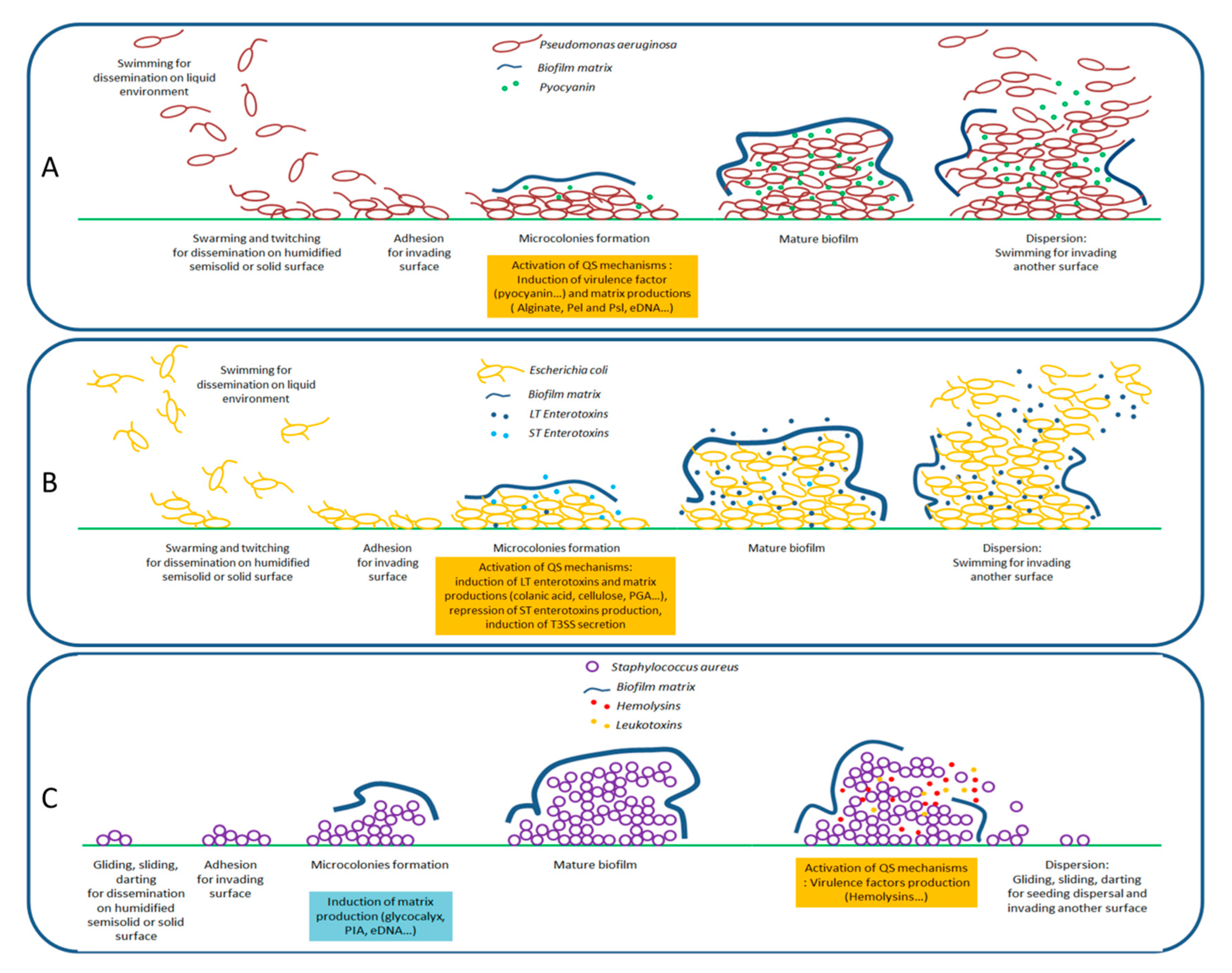
2.2. Formation of Biofilms
3. Quorum Sensing and Its Entanglement with the Expression of Virulence
4. African Plant Extracts with Antivirulence Activities
4.1. Activities on Gram-Negative Bacteria
4.2. Activities on Gram-Positive Bacteria
4.3. Activities on Gram-Indeterminate Bacteria
5. Compounds Isolated from African Plants with Antivirulence Activities
5.1. Activities on Gram-Negative Bacteria
5.2. Activities on Gram-Positive Bacteria
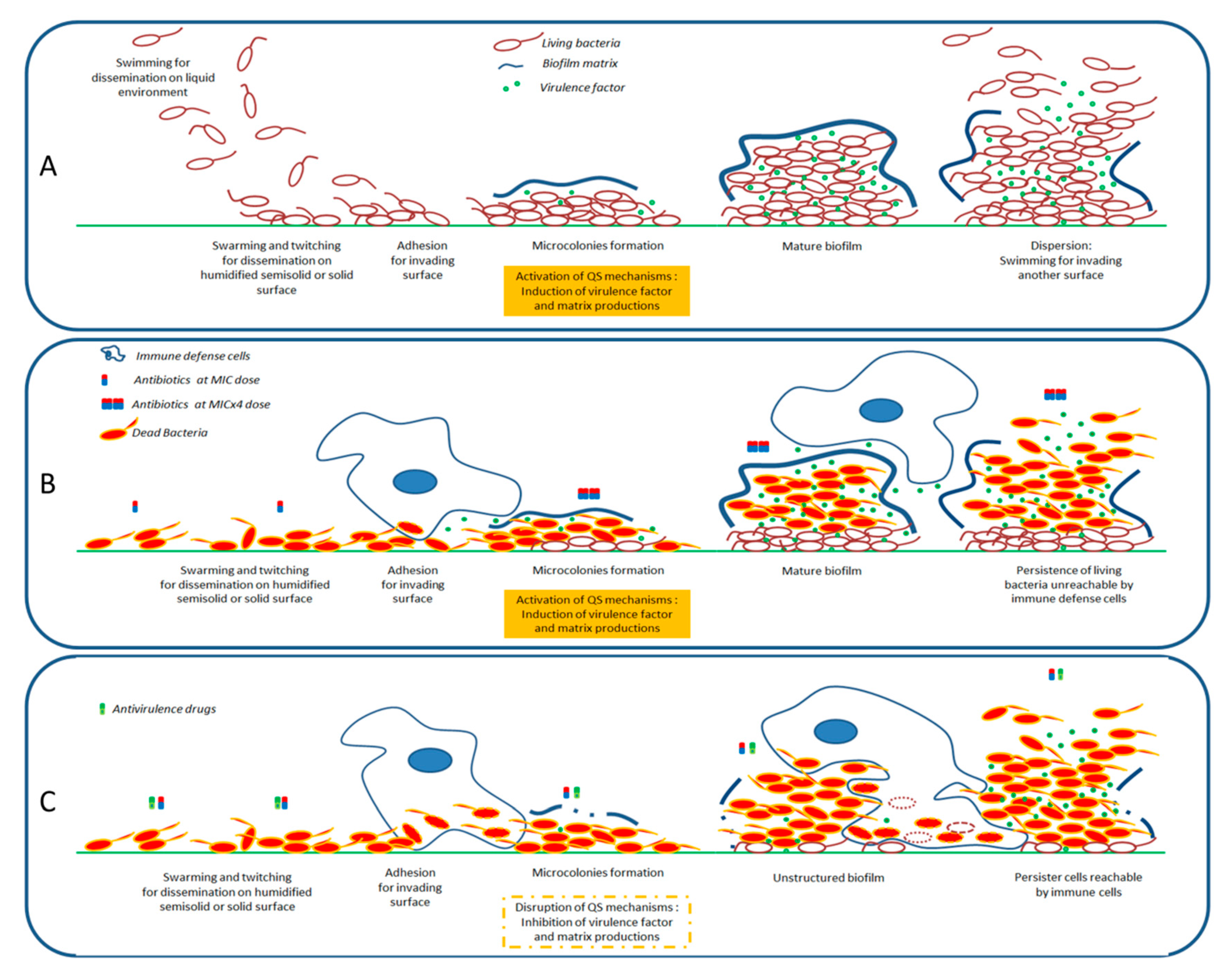
6. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation
| AHL | acyl homoserine lactone |
| AI | autoinducer |
| EPS | exopolysaccharide polymers |
| ETEC | enterotoxigenic E. coli |
| HSL | homoserine lactone |
| MIC | minimal inhibition concentration |
| quorum quenching | |
| QS | quorum sensing |
References
- WHO. World Health Organization Releases GLOBAL priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. J. Med. Soc. 2017, 32, 76–77. [Google Scholar]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Micol, V. Tackling Antibiotic Resistance with Compounds of Natural Origin: A Comprehensive Review. Biomedicines 2020, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I.; Hughes, D. Antibiotic Resistance and Its Cost: Is It Possible to Reverse Resistance? Nat. Rev. Microbiol. 2010, 8, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Talbot, G. Recommended Design Features of Future Clinical Trials of Antibacterial Agents for Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated Bacterial Pneumonia. Clin. Infect. Dis. 2010, 51, 150–170. [Google Scholar] [CrossRef] [PubMed]
- Rasamiravaka, T.; Vandeputte, O.M.; Pottier, L.; Huet, J.; Rabemanantsoa, C.; Kiendrebeogo, M.; Andriantsimavandy, A.; Rasamindrakotroka, A.; Stévigny, C.; Duez, P.; et al. Pseudomonas aeruginosa Biofilm Formation and Persistence, along with the Production of Quorum Sensing-Dependent Virulence Factors, Are Disrupted by a Triterpenoid Coumarate Ester Isolated from Dalbergia trichocarpa, a Tropical Legume. PLoS ONE 2015, 10, e0132791. [Google Scholar] [CrossRef] [PubMed]
- Carette, J.; Nachtergael, A.; Duez, P.; El Jaziri, M.; Rasamiravaka, T. Natural Compounds Inhibiting Pseudomonas aeruginosa Biofilm Formation by Targeting Quorum Sensing Circuitry. In Bacterial Biofilms; Intechopen: London, UK, 2020. [Google Scholar]
- Dickey, S.W.; Cheung, G.Y.C.; Otto, M. Different Drugs for Bad Bugs: Antivirulence Strategies in the Age of Antibiotic Resistance. Nat. Rev. Drug Discov. 2017, 16, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, H. Possible Drugs for the Treatment of Bacterial Infections in the Future: Anti-Virulence Drugs. J. Antibiot. 2020, 1–18. [Google Scholar] [CrossRef]
- Mion, S.; Rémy, B.; Plener, L.; Chabrière, É.; Daudé, D. Quorum Sensing and Quorum Quenching: How to Disrupt Bacterial Communication to Inhibit Virulence? Med. Sci. 2019, 35, 31–38. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Song, X.; Xia, Y.X.; He, Z.D.; Zhang, H.J. A Review of Natural Product with Anti-Biofilm Activity. Curr. Org. Chem. 2018, 22, 789–817. [Google Scholar] [CrossRef]
- Jakobsen, T.H.; van Gennip, M.; Phipps, R.K. Ajoene, a Sulfur-Rich Molecule from Garlic, Inhibits Genes Controlled by Quorum Sensing. Antimicrob. Agents Chemother. 2012, 56, 2314–2325. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Dong, B.; Wang, K.; Cai, S.; Liu, T.; Cheng, X.; Chen, Y. Baicalin Inhibits Biofilm Formation, Attenuates the Quorum Sensing-Controlled Virulence and Enhances Pseudomonas aeruginosa Clearance in a Mouse Peritoneal Implant Infection Model. PLoS ONE 2017, 12, e0176883. [Google Scholar] [CrossRef]
- Mahomoodally, M.F. Traditional Medicines in Africa: An Appraisal of Ten Potent African Medicinal Plants. Evid. Based Complement. Altern. Med. 2013, 2013, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kahumba, J.; Rasamiravaka, T.; Okusa, P.N.; Bakari, A.S.; Bizumukama, L.; Kiendrebeogo, M.; El Jaziri, M.; Williamson, E.M.; Duez, P. Traditional African Medicine: From Ancestral Know-How to Bright Future. Science 2015, 350, S61–S63. [Google Scholar]
- Ehrman, T.M.; Barlow, D.J.; Hylands, P.J. Phytochemical Databases of Chinese Herbal Constituents and Bioactive Plant Compounds with Known Target Specificities. J. Chem. Inf. Model. 2007, 47, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Wang, A.H.J.; Jennings, M.P. Discovery of Virulence Factors of Pathogenic Bacteria. Curr. Opin. Chem. Biol. 2008, 12, 93–101. [Google Scholar] [CrossRef]
- Rutherford, S.T.; Bassler, B.L. Bacterial Quorum Sensing: Its Role in Virulence and Possibilities for Its Control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Pollitt, E.J.G.; Diggle, S.P. Defining Motility in the Staphylococci. Cell. Mol. Life Sci. 2017, 74, 2943–2958. [Google Scholar] [CrossRef]
- Sharma, A.K.; Dhasmana, N.; Dubey, N.; Kumar, N.; Gangwal, A.; Gupta, M.; Singh, Y. Bacterial Virulence Factors: Secreted for Survival. Indian J. Microbiol. 2017, 57, 1–10. [Google Scholar] [CrossRef]
- Cress, B.F.; Englaender, J.A.; He, W.; Kasper, D.; Linhardt, R.J.; Koffas, M.A.G. Masquerading Microbial Pathogens: Capsular Polysaccharides Mimic Host-Tissue Molecules. FEMS Microbiol. Rev. 2014, 38, 660–697. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, Z.; Luo, Y.; Cox, E.; Devriendt, B. Heat-Stable Enterotoxins of Enterotoxigenic Escherichia coli and Their Impact on Host Immunity. Toxins 2019, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; McDermott, C.; Anoopkumar-Dukie, S.; McFarland, A.J.; Forbes, A.; Perkins, A.V.; Davey, A.K.; Chess-Williams, R.; Kiefel, M.J.; Arora, D.; et al. Cellular Effects of Pyocyanin, a Secreted Virulence Factor of Pseudomonas aeruginosa. Toxins 2016, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Neoh, H.M.; Nathan, S. Targeting Staphylococcus aureus Toxins: A Potential Form of Anti-Virulence Therapy. Toxins 2016, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Kristian, S.A.; Golda, T.; Ferracin, F.; Cramton, S.E.; Neumeister, B.; Peschel, A.; Götz, F.; Landmann, R. The Ability of Biofilm Formation Does Not Influence Virulence of Staphylococcus aureus and Host Response in a Mouse Tissue Cage Infection Model. Microb. Pathog. 2004, 36, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Archer, N.K.; Mazaitis, M.J.; Costerton, J.W.; Leid, J.G.; Powers, E.; Shirtliff, M.E. Staphylococcus aureus Biofilms Properties, Regulation and Roles in Human Disease. Virulence 2011, 2, 445–559. [Google Scholar] [CrossRef] [PubMed]
- Beloin, C.; Roux, A.; Ghigo, J. Escherichia coli Biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 249–289. [Google Scholar]
- Rasamiravaka, T.; Labtani, Q.; Duez, P.; El Jaziri, M. The Formation of Biofilms by Pseudomonas aeruginosa: A Review of the Natural and Synthetic Compounds Interfering with Control Mechanisms. Biomed. Res. Int. 2015, 2015, 1–17. [Google Scholar] [CrossRef]
- Franklin, M.J.; Nivens, D.E.; Weadge, J.T.; Lynne Howell, P. Biosynthesis of the Pseudomonas aeruginosa Extracellular Polysaccharides, Alginate, Pel, and Psl. Front. Microbiol. 2011, 2, 167. [Google Scholar] [CrossRef]
- Tseng, B.S.; Zhang, W.; Harrison, J.J.; Quach, T.P.; Song, J.L.; Penterman, J.; Singh, P.K.; Chopp, D.L.; Packman, A.I.; Parsek, M.R. The Extracellular Matrix Protects Pseudomonas aeruginosa Biofilms by Limiting the Penetration of Tobramycin. Environ. Microbiol. 2013, 15, 2865–2878. [Google Scholar]
- Miyaue, S.; Suzuki, E.; Komiyama, Y.; Kondo, Y.; Morikawa, M.; Maeda, S. Bacterial Memory of Persisters: Bacterial Persister Cells Can Retain Their Phenotype for Days or Weeks after Withdrawal from Colony-Biofilm Culture. Front. Microbiol. 2018, 9, 1396. [Google Scholar] [CrossRef]
- LaSarre, B.; Federle, M.J. Exploiting Quorum Sensing To Confuse Bacterial Pathogens. Microbiol. Mol. Biol. Rev. 2013, 77, 73–111. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Moustafa, D.; Smith, C.D.; Goldberg, J.B.; Bassler, B.L. The RhlR Quorum-Sensing Receptor Controls Pseudomonas aeruginosa Pathogenesis and Biofilm Development Independently of Its Canonical Homoserine Lactone Autoinducer. PLoS Pathog. 2017, 13, e1006504. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.M.; Bhangui, P.; Bhat, C. The First Report on Listeria monocytogenes Producing Siderophores and Responds Positively to N-Acyl Homoserine Lactone (AHL) Molecules by Enhanced Biofilm Formation. Arch. Microbiol. 2017, 199, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.; Sarode, S.; Chandrasekhar, K. Fundemantals of Bacterial Biofilm: Present State of Art; Springer Nature: Berlin, Germany, 2018; pp. 43–60. [Google Scholar]
- Haudecoeur, E.; Faure, D. A Fine Control of Quorum-Sensing Communication in Agrobacterium tumefaciens. Commun. Integr. Biol. 2010, 3, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.N.; Koch, G.; Thompson, J.A.; Xavier, K.B.; Cool, R.H.; Quax, W.J. The Multiple Signaling Systems Regulating Virulence in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 2012, 76, 46–65. [Google Scholar] [CrossRef] [PubMed]
- Bronner, S.; Monteil, H.; Prévost, G. Regulation of Virulence Determinants in Staphylococcus aureus: Complexity and Applications. FEMS Microbiol. Rev. 2004, 28, 183–200. [Google Scholar] [CrossRef]
- Garmyn, D.; Gal, L.; Lemaitre, J.P.; Hartmann, A.; Piveteau, P. Communication and Autoinduction in the Species Listeria monocytogenes: A Central Role for the Agr System. Commun. Integr. Biol. 2009, 2, 371–374. [Google Scholar] [CrossRef]
- Kim, C.S.; Gatsios, A.; Cuesta, S.; Lam, Y.C.; Wei, Z.; Chen, H.; Russell, M.R.; Shine, E.E.; Wang, R.; Wyche, T.P.; et al. Characterization of Autoinducer-3 Structure and Biosynthesis in E. coli. ACS Cent. Sci. 2020, 6, 197–206. [Google Scholar] [CrossRef]
- Roussel, C.; Cordonnier, C.; Livrelli, V.; Van de Wiele, T.; Blanquet-Diot, S. Enterotoxigenic and Enterohemorrhagic Escherichia coli: Survival and Modulation of Virulence in the Human Gastrointestinal Tract. In Escherichia coli—Recent Advances on Physiology, Pathogenesis and Biotechnological Applications; Intechopen: London, UK, 2017; pp. 3–24. [Google Scholar]
- Ujita, Y.; Sakata, M.; Yoshihara, A.; Hikichi, Y.; Kai, K. Signal Production and Response Specificity in the phc Quorum Sensing Systems of Ralstonia solanacearum Species Complex. ACS Chem. Biol. 2019, 14, 2243–2251. [Google Scholar] [CrossRef]
- Chenia, H.Y. Anti-Quorum Sensing Potential of Crude Kigelia africana Fruit Extracts. Sensors 2013, 13, 2802–2817. [Google Scholar] [CrossRef]
- Baloyi, I.T.; Cosa, S.; Combrinck, S.; Leonard, C.M.; Viljoen, A.M. Anti-Quorum Sensing and Antimicrobial Activities of South African Medicinal Plants against Uropathogens. S. Afr. J. Bot. 2019, 122, 484–491. [Google Scholar] [CrossRef]
- Bangré, Y.A.; Mètuor-dabiré, A.; Rouamba, A.; Kiendrebeogo, M.; Simporé, J. Antibacterial Activity of Methanol Bark Extract from Acacia dudgeoni craib. Ex Holl (Mimosaceae) on Growth of Cefotaxime Resistant Escherichia coli. Curr. Res. Microbiol. Biotechnol. 2018, 6, 1660–1664. [Google Scholar]
- Ouedraogo, V.; Compaoré, E.; Rouamba, A.; Compaoré, M.; Kiendrebeogo, M. Anti-Virulence Activity of Three Medicinal Plants: Cassia occidentalis L., Crossopteryx febrifuga (Afzel Ex G. Don) Benth. and Zanthoxylum zanthoxyloides (Lam) Zep. and Timl. Int. J. Microbiol. Res. 2019, 29, 1–7. [Google Scholar] [CrossRef]
- Ouedraogo, V.; Rouamba, A.; Compaore, M.; Sombié, P.A.E.D.; Kiendrebeogo, M. Acacia seyal Del Bark Extract Reduces Quorum Sensing—Controlled Virulence Factor Production and Biofilm Formation in Pseudomonas aeruginosa PAO1. Int. J. Curr. Res. Biosci. 2018, 5, 7–13. [Google Scholar] [CrossRef]
- Ouedraogo, V.; Rouamba, A.; Compaoré, E.; Compaoré, M.; Kiendrebeogo, M. Antioxidant, Antiquorum-Sensing and Antibiofilm Activities of Balanites aegyptiaca (L.) Del. (Balanitaceae) and Terminalia macroptera Guill. and Perr. (Combretaceae). Adv. Biochem. 2018, 6, 26–31. [Google Scholar] [CrossRef]
- Bacha, K.; Tariku, Y.; Gebreyesus, F.; Zerihun, S.; Mohammed, A.; Weiland-Bräuer, N.; Schmitz, R.A.; Mulat, M. Antimicrobial and Anti-Quorum Sensing Activities of Selected Medicinal Plants of Ethiopia: Implication for Development of Potent Antimicrobial Agents. BMC Microbiol. 2016, 16, 139. [Google Scholar] [CrossRef] [PubMed]
- Famuyide, I.M.; Aro, A.O.; Fasina, F.O.; Eloff, J.N.; McGaw, L.J. Antibacterial and Antibiofilm Activity of Acetone Leaf Extracts of Nine Under-Investigated South African Eugenia and Syzygium (Myrtaceae) Species and Their Selectivity Indices. BMC Complement. Altern. Med. 2019, 19, 141. [Google Scholar] [CrossRef]
- Omwenga, E.O.; Hensel, A.; Pereira, S.; Shitandi, A.A.; Goycoolea, F.M. Antiquorum Sensing, Antibiofilm Formation and Cytotoxicity Activity of Commonly Used Medicinal Plants by Inhabitants of Borabu Sub-County, Nyamira County, Kenya. PLoS ONE 2017, 12, e0185722. [Google Scholar] [CrossRef]
- Cosa, S.; Chenia, H. Investigating the Potential of Ten South African Indegenious Plants Extracts as Quorum Sensing Inhibitors (QSI) of Pseudomonas aeruginosa Virulence-Related Factors. J. Biotechnol. Biomater. 2014, 3, 112. [Google Scholar]
- Sarkar, R.; Chaudhary, S.K.; Sharma, A.; Yadav, K.K.; Nema, N.K.; Sekhoacha, M.; Karmakar, S.; Braga, F.C.; Matsabisa, G.; Mukherjee, P.K.; et al. Anti-Biofilm Activity of Marula—A Study with the Standardized Bark Extract. J. Ethnopharmacol. 2014, 154, 170–175. [Google Scholar] [CrossRef]
- Okusa, P.; Rasamiravaka, T.; Vandeputte, O.; Stvigny, C.; El Jaziri, M.; Duez, P. Extracts of Cordia gilletii De Wild (Boraginaceae) Quench the Quorum Sensing of Pseudomonas aeruginosa PAO1. J. Intercult. Ethnopharmacol. 2014, 3, 138. [Google Scholar] [CrossRef] [PubMed]
- Rasamiravaka, T.; Raveloson, P.A.; Rajaonarivelo, P.J.; Rabemanantsoa, C.; Andrianarisoa, B.; Duez, P.; El Jaziri, M. Malagasy Traditional Treatments of Infectious Plant Diseases Exert Anti-Virulence Activities Against Pseudomonas aeruginosa and Ralstonia solanacearum. J. Microbiol. Biotechnol. Food Sci. 2018, 7, 377–382. [Google Scholar] [CrossRef]
- Rasamiravaka, T.; Quentin, L.; Mol, A.; Megalizzi, V.; Rabemanantsoa, C.; Duez, P.; El Jaziri, M. An Active Fraction from Dalbergia trichocarpa Baker Disrupts the Formation and Maintenance of Biofilms in Pseudomonas aeruginosa PAO1. IARJSET 2016, 3, 124–133. [Google Scholar] [CrossRef]
- Ouedraogo, V.; Kiendrebeogo, M. Methanol Extract from Anogeissus leiocarpus (DC) Guill. et Perr. (Combretaceae) Stem Bark Quenches the Quorum Sensing of Pseudomonas aeruginosa PAO1. Medicines 2016, 3, 26. [Google Scholar] [CrossRef]
- Sandasi, M.; Leonard, C.M.; Viljoen, A.M. The In Vitro Antibiofilm Activity of Selected Culinary Herbs and Medicinal Plants against Listeria monocytogenes. Lett. Appl. Microbiol. 2010, 50, 30–35. [Google Scholar] [CrossRef]
- Akhalwaya, S.; van Vuuren, S.; Patel, M. An In Vitro Investigation of Indigenous South African Medicinal Plants Used to Treat Oral Infections. J. Ethnopharmacol. 2018, 210, 359–371. [Google Scholar] [CrossRef]
- Oosthuizen, C.B.; Gasa, N.; Hamilton, C.J.; Lall, N. Inhibition of Mycothione Disulphide Reductase and Mycobacterial Biofilm by Selected South African Plants. S. Afr. J. Bot. 2019, 120, 291–297. [Google Scholar] [CrossRef]
- Ouedraogo, V.; Karama, I.; Rouamba, A.; Compaoré, M.; Kiendrebeogo, M. Acacia dudgeoni Craib. Ex Holl (Mimosaceae): Potential Inhibitor of Biofilm Formation and Quorum Sensing in P. aeruginosa PAO1. Int. J. Biotechnol. 2019, 23, 1–6. [Google Scholar] [CrossRef]
- Sereme, A.; Millogo-Rasolodimby, J.; Guinko, S.; Nacro, M. Proprietes Therapeutiques des Plantes a Tanins Du Burkina Faso (Therapeutic Power of Tannins Producing Species of Burkina Faso). Afr. J. Tradit. Med. 2008, 15, 41–49. [Google Scholar]
- Alawi, S.M.A.; Hossain, M.A.; Abusham, A.A. Antimicrobial and Cytotoxic Comparative Study of Different Extracts of Omani and Sudanese Gum acacia. J. Basic Appl. Sci. 2018, 7, 22–26. [Google Scholar] [CrossRef]
- Rasamiravaka, T.; Jedrzejowski, A.; Kiendrebeogo, M.; Rajaonson, S.; Randriamampionona, D.; Rabemanantsoa, C.; Andriantsimavandy, A.; Rasamindrakotroka, A.; Duez, P.; El Jaziri, M.; et al. Endemic Malagasy Dalbergia species Inhibit Quorum Sensing in Pseudomonas aeruginosa PAO1. Microbiology 2013, 159, 924–938. [Google Scholar] [CrossRef]
- Rasamiravaka, T.; Rajaonarivelo, J.P.; Rabemanantsoa, C.; El Jaziri, M.; Andrianarisoa, B.; Duez, P. Malagasy Traditional Treatments for Food Crops: A Tool to Control Potato Bacterial Diseases? Crop. Prot. 2017, 102, 49–55. [Google Scholar] [CrossRef]
- Okusa, P.N.; Penge, O.; Devleeschouwer, M.; Duez, P. Direct and Indirect Antimicrobial Effects and Antioxidant Activity of Cordia gilletii De Wild (Boraginaceae). J. Ethnopharmacol. 2007, 112, 476–481. [Google Scholar] [CrossRef]
- Awolola, G.V.; Koorbanally, N.A.; Chenia, H.; Shode, F.O.; Baijnath, H. Antibacterial and Anti-Biofilm Activity of Flavonoids and Triterpenes Isolated. Afr. J. Tradit. Complement. Altern. Med. 2014, 3, 124–131. [Google Scholar] [CrossRef]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rajaonson, S.; Diallo, B.; Mol, A.; El Jaziri, M.; Baucher, M. Identification of Catechin as One of the Flavonoids from Combretum albiflorum Bark Extract That Reduces the Production of Quorum-Sensing-Controlled Virulence Factors in Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2010, 76, 243–253. [Google Scholar] [CrossRef]
- Ouedraogo, V.; Sombié, P.A.E.; Compaoré, M.; Kiendrébéogo, M. Anti-Quorum Quenching Activity of Methyl Gallate Isolated from Galls of Guiera senegalensis J. F. Gmel (Combretaceae). Afr. J. Microbiol. Res. 2019, 13, 290–297. [Google Scholar]
- Rasamiravaka, T.; Ngezahayo, J.; Pottier, L.; Ribeiro, S.O.; Souard, F.; Hari, L.; Stévigny, C.; El Jaziri, M.; Duez, P. Terpenoids from Platostoma rotundifolium (Briq.) A. J. Paton Alter the Expression of Quorum Sensing-Related Virulence Factors and the Formation of Biofilm in Pseudomonas aeruginosa PAO1. Int. J. Mol. Sci. 2017, 18, 1270. [Google Scholar] [CrossRef]
- Nyila, M.A.; Leonard, C.M.; Hussein, A.A.; Lall, N. Activity of South African Medicinal Plants against Listeria monocytogenes Biofilms, and Isolation of Active Compounds from Acacia karroo. S. Afr. J. Bot. 2012, 78, 220–227. [Google Scholar] [CrossRef]
- Rajaonson, S.; Vandeputte, O.M.; Vereecke, D.; Kiendrebeogo, M.; Ralambofetra, E.; Stévigny, C.; Duez, P.; Rabemanantsoa, C.; Mol, A.; Diallo, B.; et al. Virulence Quenching with a Prenylated Isoflavanone Renders the Malagasy Legume Dalbergia pervillei Resistant to Rhodococcus fascians. Environ. Microbiol. 2011, 13, 1236–1252. [Google Scholar] [CrossRef]
- Jongkind, C.C.H. Prodromus for a Revision of Combretum (Combretaceae) for Madagascar. Bull. Mus. Natl. Hist. Nat. 1995, 17, 191–200. [Google Scholar]
- Hossain, M.A.; Lee, S.J.; Park, N.H.; Mechesso, A.F.; Birhanu, B.T.; Kang, J.; Reza, M.A.; Suh, J.W.; Park, S.C. Impact of Phenolic Compounds in the Acyl Homoserine Lactone-Mediated Quorum Sensing Regulatory Pathways. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ngezahayo, J.; Havyarimana, F.; Hari, L.; Stévigny, C.; Duez, P. Medicinal Plants Used by Burundian Traditional Healers for the Treatment of Microbial Diseases. J. Ethnopharmacol. 2015, 173, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Babalola, I.T.; Shode, F.O. Ubiquitous Ursolic Acid: A Potential Pentacyclic Triterpene Natural Product. J. Pharmacogn. Phytochem. 2013, 2, 214–222. [Google Scholar]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on Plant Antimicrobials: A Mechanistic Viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 2–28. [Google Scholar] [CrossRef]
- Madureira, A.M.; Ramalhete, C.; Mulhovo, S.; Duarte, A.; Ferreira, M.J.U. Antibacterial Activity of Some African Medicinal Plants Used Traditionally Against Infectious Diseases. Pharm. Biol. 2012, 50, 481–489. [Google Scholar] [CrossRef]
- Van Vuuren, S.F. Antimicrobial Activity of South African Medicinal Plants. J. Ethnopharmacol. 2008, 119, 462–472. [Google Scholar] [CrossRef]
- Ngezahayo, J.; Pottier, L.; Ribeiro, S.O.; Delporte, C.; Fontaine, V.; Hari, L.; Stévigny, C.; Duez, P. Plastotoma rotundifolium Aerial Tissue Extract Has Antibacterial Activities. Ind. Crops Prod. 2016, 86, 301–310. [Google Scholar] [CrossRef]
- Xu, Q.; Bauer, R.; Hendry, B.M.; Fan, T.P.; Zhao, Z.; Duez, P.; Simmonds, M.S.J.; Witt, C.M.; Lu, A.; Robinson, N.; et al. The Quest for Modernisation of Traditional Chinese Medicine. BMC Complement. Altern. Med. 2013, 13, 132–143. [Google Scholar] [CrossRef]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New Technology for Rapid Determination of Antibiotic Susceptibilities of Bacterial Biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef]
- Strugeon, E.; Tilloy, V.; Ploy, M.-C.; Da Re, S. The Stringent Response Promotes Antibiotic Resistance Dissemination by Regulating Integron Integrase Expression in Biofilms. mBio 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Mellbye, B.; Schuster, M. The Sociomicrobiology of Antivirulence Drug Resistance: A Proof of Concept. mBio 2011, 2, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Schillaci, D.; Carnevale, I.; Giovannetti, E.; Diana, P.; Cirrincione, G.; Cascioferro, S. Synthetic Small Molecules as Anti-Biofilm Agents in the Struggle against Antibiotic Resistance. Eur. J. Med. Chem. 2019, 161, 154–178. [Google Scholar] [CrossRef]
- Theuretzbacher, U.; Outterson, K.; Engel, A.; Karlén, A. The Global Preclinical Antibacterial Pipeline. Nat. Rev. Microbiol. 2020, 18, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Rezzoagli, C.; Archetti, M.; Mignot, I.; Baumgartner, M.; Kümmerli, R. Combining Antibiotics with Antivirulence Compounds Can Have Synergistic Effects and Reverse Selection for Antibiotic Resistance in Pseudomonas aeruginosa. PLoS Biol. 2020, 18, e3000805. [Google Scholar] [CrossRef] [PubMed]
| Bacterial Model Strains | Plant Family 1 | Plant Species 1 | Plant Part and Traditional Usage | Active Extracts (or Fraction) | Bactericidal Effect MIC (µg/mL) | Impact on Bacterial Virulence 2 | Synergy with Antibiotics | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Motility | Virulence Factors Production | Biofilm Formation | QS | |||||||||
| Group of Gram-negative bacteria | A. tumefaciens | Bignoniaceae | Kigelia africana (Lam.) Benth | Fruit; dysentery, toothaches, malaria, diabetes | Hexane | NC | NC | ↘ | NC | ↘ | NC | [43] |
| C. violaceum | Asteraceae | Baccharoides adoensis (Sch. Bip. ex Walp.) H. Rob. [synonym of Vernonia adoensis Schi.Bip. ex Walp.] | Bark; urinary tract infections | Distilled water | 4000 | NC | ↔ | ↘ | NC | NC | [44] | |
| C. violaceum ATCC 12472 | Aristolochiaceae | Hydnora africana Thumb. | Bark; urinary tract infections | Methanol | 1000 | NC | ↘ | ↘ | NC | NC | [44] | |
| Aspagaceae | Eucomis autumnalis (Mill.) Chitt | Bulb; urinary tract infections | Methanol | 2000–4000 | ||||||||
| Bignoniaceae | Kigelia africana (Lam.) Benth | Fruit; dysentery, toothaches, malaria, diabetes | Hexane | NC | NC | ↘ | NC | ↘ | NC | [43] | ||
| Hypoxidaceae | Hypoxis hemerocallidea Fish., C.A.Mey. & Avé-Lall. | Corm; urinary tract infections | Dichloromethane | 2000 | NC | ↘ | ↘ | NC | NC | [44] | ||
| Lauraceae | Cryptocarya latifolia Sond. | Bark; urinary tract infections | Methanol | 2000–4000 | ↔ | |||||||
| Poacae | Cenchrus ciliaris L. | Leaves; urinary tract infections | Methanol | ↘ | ||||||||
| Vitaceae | Rhoicissus tridentata (L.f.) Wild & R.B.Drumm | Roots; urinary tract infections | Methanol | ↔ | ||||||||
| C. violaceum CV026 | Combretaceae | Terminalia leiocarpa (DC.) Baill. [synonym of Anogeissus leiocarpus (DC) Guill. et Perr.] | Stem bark; treat infected burn wounds | Methanol | 1250 | NC | ↘ | ↔ | NC | NC | [45] | |
| Terminalia macroptera Guill. and Perr. | Bark; respiratory tract diseases, skin diseases, and wound | NC | ↘ | ↘ | ↘ | NC | [46] | |||||
| Fabaceae | Vachellia seyal (Delile) P.J.H.Hurter [synonym of Acacia seyal Delile] | Bark; toothache, dysentery, burns | Methanol | NC | NC | ↘ | NC | NC | NC | [47] | ||
| Mimosaceae | Acacia dudgeoni Craib. ex Holland | Bark; diarrhea, childhood dysentery | Methanol | NC | NC | ↘ | NC | ↘ | NC | [48] | ||
| Zygophyllaceae | Balanites aegyptiaca (L.) Delille. | Galls and stem bark; respiratory tract diseases, skin diseases and wound | Methanol | 1250 | NC | ↘ | ↘ | ↔ | NC | [46] | ||
| E. coli | Acanthaceae | Justicia schimperiana (Hochst. ex Nees) T. Anderson | Whole plant; malaria, cough, stomach, asthma | Petroleum ether | NC | NC | NC | NC | ↘ | NC | [49] | |
| Fabaceae | Albizia Schimperiana Oliv. | Leaves; antifungal | Methanol | NC | NC | NC | NC | ↘ | NC | [49] | ||
| E. coli ATCC 25922 | Myrtaceae | Eugenia erythrophylla Strey | Leaves; diarrhea, diabetes, reproductive problems, and respiratory conditions | Acetone | 80–310 | NC | NC | ↘ | NC | NC | [50] | |
| Eugenia umtamvunensis A.E.van Wyk | ||||||||||||
| Eugenia capensis subsp. zeyheri (Harv.) F.White [synonym of Eugenia zeyheri (Harv.) Harv.] | ||||||||||||
| Syzygium legatii Burtt Davy & Greenway | ||||||||||||
| Syzygium masukuense (Baker) R.E.Fr. | ||||||||||||
| Syzygium sp. | ||||||||||||
| E. coli pBCA9145-jtk2828:: sfGFP | Celastraceae | Elaeodendron buchananii (Loes). Loes. | Roots; gastrointestinal tract, urinary tract, skin, and oral cavity | Ethanol | 500 | NC | ↔ | ↔ | ↘ | NC | [51] | |
| Fabaceae | Vachellia gerrardii (Benth.) P.J.H.Hurter [synonym of Acacia gerrardii Benth] | |||||||||||
| P. aeruginosa ATCC 27853 | Myrtaceae | Eugenia erythrophylla Strey | Leaves; diarrhea, diabetes, reproductive problems, and respiratory conditions | Acetone | 80–310 | NC | NC | ↘ | NC | NC | [50] | |
| Eugenia umtamvunensis A.E.van Wyk | ||||||||||||
| Eugenia capensis subsp. zeyheri (Harv.) F.White [synonym of Eugenia zeyheri (Harv.) Harv.] | ||||||||||||
| Syzygium legatii Burtt Davy & Greenway | ||||||||||||
| P. aeruginosa ATCC 35032 | Fabaceae | Lessertia frutescens (L.) Goldblatt & J.C.Manning [synonym of Sutherlandia frutescens (L) R. Br.] | Leaves; cancers, fever, diabetes | Ethanol | NC | NC | ↘ | ↘ | NC | NC | [52] | |
| P. aeruginosa MTCC 2453 | Anacardiaceae | Sclerocarya birrea (A.Rich.) Hoch | Stem bark; dysentery, diarrhea | Methanol | NC | ↘ | ↘ | ↘ | NC | NC | [53] | |
| P. aeruginosa PAO1 | Baroginaceae | Cordia gilletii De Wild | Root barks; malaria, diarrhea, wounds, and skin diseases | Dichloromethane | NC | NC | ↘ | ↘ | ↘ | NC | [54] | |
| Buddlejaceae | Buddleja madagascariensis Lam. | Leaves; potato wilt diseases | Methanol | 4000 | NC | NC | ↘ | ↘ | NC | [55] | ||
| Combretaceae | Terminalia leiocarpa (DC.) Baill. [synonym of Anogeissus leiocarpus (DC) Guill. et Perr.] | Stem bark; treat infected burn wounds | Methanol | 1250 | NC | ↘ | ↔ | NC | NC | [45] | ||
| Fabaceae | Vachellia seyal (Delile) P.J.H.Hurter [synonym of Acacia seyal Delile] | Bark; toothache, dysentery, burns | Methanol | NC | NC | ↘ | ↘ | NC | NC | [47] | ||
| Dalbergia trichocarpa Baker | Bark; laryngitis, diarrhea | Hexane (F1 Fraction) | 4000 | ↘ | ↘ | ↘ | ↔ | yes | [56] | |||
| Tephrosia purpurea (L.) Pers. | Leaves; potato wilt diseases | Methanol | 4000 | NC | NC | ↘ | ↔ | NC | [55] | |||
| Mimosaceae | Acacia dudgeoni Craib. ex Holland | Bark; diarrhea, childhood dysentery | Methanol | NC | NC | ↘ | ↘ | NC | NC | [48] | ||
| Rubiaceae | Crossopteryx febrifuga (Afzel ex G. Don) Benth | Leaves and stem; typhoid fever, respiratory infections, infected wounds, dental diseases | Methanol | NC | NC | ↘ | NC | NC | NC | [57] | ||
| Rutaceae | Zanthoxylum zanthoxyloides (Lam) Zepern. and Timler | Stem bark; typhoid fever, respiratory infections, infected wounds, dental diseases | ||||||||||
| Zygophyllaceae | Balanites aegyptiaca (L.) Delille | Galls and stem bark; respiratory tract diseases, skin diseases and wound | Methanol | 625 | NC | ↘ | ↘ | ↔ | NC | [46] | ||
| R. solanacearum | Fabaceae | Tephrosia purpurea (L.) Pers. | Leaves; potato wilt diseases | Methanol | 4000 | NC | NC | ↘ | ↔ | NC | [55] | |
| Scrophulariaceae | Buddleja madagascariensis Lam | Leaves; potato wilt diseases | Methanol | 4000 | NC | NC | ↘ | ↘ | NC | |||
| Salmonella ser. Typhimurium ATCC 39183 | Myrtaceae | Eugenia erythrophylla Strey | Leaves; diarrhea, diabetes, reproductive problems, and respiratory conditions | Acetone | 40–310 | NC | NC | ↘ | NC | NC | [50] | |
| Eugenia umtamvunensis A.E.van Wyk | ||||||||||||
| Eugenia capensis subsp. zeyheri (Harv.) F.White [synonym of Eugenia zeyheri (Harv.) Harv.] | ||||||||||||
| Syzygium legatii Burtt Davy & Greenway | ||||||||||||
| Syzygium masukuense (Baker) R.E.Fr. | ||||||||||||
| Syzygium sp. | ||||||||||||
| Group of Gram-positive bacteria | B. cereus ATCC 21366 | Myrtaceae | Eugenia erythrophylla Strey | Leaves; diarrhea, diabetes, reproductive problems, and respiratory conditions | Acetone | 20–160 | NC | NC | ↘ | NC | NC | [50] |
| Eugenia umtamvunensis A.E.van Wyk | ||||||||||||
| Eugenia capensis subsp. zeyheri (Harv.) F.White [synonym of Eugenia zeyheri (Harv.) Harv.] | ||||||||||||
| Syzygium legatii Burtt Davy & Greenway | ||||||||||||
| Syzygium sp. | ||||||||||||
| Syzygium gerrardii (Harv. ex Hook.f.) Burtt Davy | ||||||||||||
| E. faecalis ATCC 29212 | Myrtaceae | Eugenia umtamvunensis A.E.van Wyk | Leaves; diarrhea, diabetes, reproductive problems, and respiratory conditions | Acetone | 40–160 | NC | NC | ↘ | NC | NC | [50] | |
| Syzygium masukuense (Baker) R.E.Fr. | ||||||||||||
| Syzygium legatii Burtt Davy & Greenway | ||||||||||||
| Syzygium gerrardii (Harv. ex Hook.f.) Burtt Davy | ||||||||||||
| L. monocytogenes ATCC 19111 | Fabaceae | Aspalathus linearis (Burn.f.) R. Dahlgren | Leaves; antioxidant and antifungal activities | Dichloromethane/Methanol | NC | NC | NC | ↘ | NC | NC | [58] | |
| Rutaceae | Agathosma betulina (P.J. Bergius) Pillans | Dried plant material; urinary tract infectious | ||||||||||
| S. aureus ATCC 25923 | Aristolochiaceae | Hydnora africana Thumb. | Bark; urinary tract infections | Methanol | 500–4000 | NC | ↘ | ↘ | NC | NC | [44] | |
| Asparagaceae | Eucomis autumnalis (Mill.) Chitt | Bulb; urinary tract infections | ||||||||||
| Asteraceae | Baccharoides adoensis (Sch.Bip. ex Walp.) H.Rob. [synonym of Vernonia adoensis Sch.Bip. ex Walp.] | Bark; urinary tract infections | Distilled water | ↔ | ↘ | |||||||
| Fabaceae | Bauhinia bowkeri Harv. | Roots; urinary tract infections | Water | ↘ | ↔ | |||||||
| Hypoxidaceae | Hypoxis hemerocallidea Fisch., C.A.Mey. & Avé-Lall. | Corm; urinary tract infections | Dichloromethane | ↘ | ↘ | |||||||
| Lauraceae | Cryptocarya latifolia Sond. | Bark; urinary tract infections | Methanol | ↘ | ↔ | |||||||
| S. aureus ATCC 29213 | Myrtaceae | Eugenia erythrophylla Strey | Leaves; diarrhea, diabetes, reproductive problems, and respiratory conditions | Acetone | 40–160 | NC | NC | ↘ | NC | NC | [50] | |
| Eugenia umtamvunensis A.E.van Wyk | ||||||||||||
| Eugenia capensis subsp. zeyheri (Harv.) F.White [synonym of Eugenia zeyherii (Harv.) Harv.] | ||||||||||||
| Syzygium legatii Burtt Davy & Greenway | ||||||||||||
| Syzygium masukuense (Baker) R.E.Fr. | ||||||||||||
| Syzygium gerrardii (Harv. ex Hook.f.) Burtt Davy | ||||||||||||
| S. mutans ATCC 25175 | Asteraceae | Tarchonanthus camphoratus L. | Leaves; toothache | Dichloromethane/methanol | 500–1000 | NC | NC | ↘ | NC | NC | [59] | |
| Bignoniaceae | Tecoma capensis (Thumb.) Lindl., | Leaves; rubbed onto bleeding | Dichloromethane/methanol | |||||||||
| Euphorbiaceae | Spyrostachys africana Sond. | Leaves; toothache remedy | Dichloromethane/methanol | |||||||||
| Fabaceae | Vachellia karroo (Hayne) Banfi & Galasso [synonym of Acacia karroo Hayne] | Leaves; oral thrush | Dichloromethane/methanol | |||||||||
| Fabaceae | Erythrina lysistemon Hutch. | Bark; toothache | Dichloromethane/methanol | |||||||||
| Group of Gram-indeterminate bacteria | Mycobacterium smegmatis MC155 M.tuberculosis H37Rv ATCC 27264 | Asphodelaceae | Kumara plicatilis (L.) G.D.Rowley [synonym of Aloe plicatilis (L.) Mill.] | Roots; diarrhea | Ethanol | 31–1000 | NC | NC | ↘ | NC | NC | [60] |
| Asparagaceae | Dracaena aletriformis (Haw.) Bos | Leaves; chest pains | ||||||||||
| Dracaena draco (L.) L. | Leaves; fever, respiratory ailments | |||||||||||
| Eucomis vandermerwei Verd. | Leaves; anti-inflammatory | |||||||||||
| Merwilla plumbea (Lindl.) Speta | Leaves; chest pains and lung infections | |||||||||||
| Euphorbiaceae | Euphorbia tirrucalli L. | Stems; cough | ||||||||||
| Icacinaceae | Cassinopsis ilicifolia (Hochst.) Sleumer | Leaves and stem; stomach ailments | ||||||||||
| Lamiaceae | Leonotis leonurus (L) R.Br. | Leaves and stem; fever, headache, and cough | ||||||||||
| Salvia aurea L. [synonym of Salvia africana lutea L.] | Leaves and stem; cough, colds, and bronchitis | |||||||||||
| Malpighiaceae | Sphedamnocarpus pruriens (A. Juss.) Szyszył | Seeds and roots; snake bites | ||||||||||
| Orobanchaceae | Alectra sessiliflora (Vahl) Kuntze | Roots; diarrhea | ||||||||||
| Proteaceae | Faurea saligna Harv. | Leaves; diarrhea | ||||||||||
| Solanaceae | Withania somnifera (L) Dunal | Leaves and stem; fever and anti-inflammatory | ||||||||||
| Typhaceae | Typha capensis (Rohrb.) N.E.Br. | Leaves and roots; diarrhea | ||||||||||
| Burkinabe Medicinal Plant | Plant Part | Tested Extract and Concentration | Production of Violacein in C. violaceum CV026 | Production of Pyocyanin in P. aeruginosa PAO 1 | Production of Elastase in P. aeruginosa PAO1 | Production of Biofilm in P. aeruginosa PAO 1 | References |
|---|---|---|---|---|---|---|---|
| Acacia dudgeoni Craib. ex Holl. | Stem bark | Methanol 50–400 µg/mL 2,3 | −25% to −69% | −33% to −66% | NC | −25% to −59% | [61] |
| Balanites aegyptiaca (L.) Delille. | Leafy galls 1 | Methanol 100 µg/mL 2 | −10% | −15% | NC | −33% | [48] |
| Stem bark | Methanol 100 µg/mL 2 | −15% | −20% | NC | −20% | ||
| Crossopteryx febrifuga (Afzel ex G. Don) Benth | Leave and stem | Methanol 100 µg/mL 2 | NC | −52% | −48% | NC | [46] |
| Terminalia leiocarpa (DC.) Baill. [synonym of Anogeissus leiocarpus (DC) Guill. et Perr.] | Stem bark | Methanol 100 µg/mL 2 | −50% | −66% | NC | NC | [57] |
| Terminalia macroptera Guill. and Perr. | Stem bark | Methanol 100 µg/mL 2 | −35% | −50% | NC | −30% | [48] |
| Vachellia seyal (Delile) P.J.H.Hurter [synonym of Acacia seyal Delile] | Bark | Methanol 50–800 µg/mL 2,3 | −25% to −97% | −22% to −86% | −8% to −56% | At 800 µg/mL: −69% | [47] |
| Zanthoxylum zanthoxyloides (Lam) Zepern. and Timler | Stem bark | Methanol 100 µg/mL 2 | NC | −28% | −15% | NC | [46] |
| Bacterial Model Strains | Plant Family 1 | Plant Species 1 | Compound | Chemical Structure | Plant Part and Traditional Usage | Bactericidal Effect MIC (µg/mL) | Impact on Bacterial Virulence 2 | Synergy with Antibiotics | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Motility | Virulence Factors Production | Biofilm Formation | QS | ||||||||||
| Group of Gram-negative bacteria | E. coli ATCC 25922 | Moraceae | Ficus sansibarica Warb. | Epicatechin | 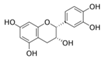 | Leaves and fruits; wound, dysentery, diarrhea, tuberculosis | 8000 | NC | NC | ↘ | NC | NC | [67] |
| C. violaceum CV026 | Combretaceae | Combretum albiflorum (Tul.) Jongkind | Catechin |  | Bark; bacterial infection, fever, pneumonia | 2000 | NC | ↘ | NC | ↘ | NC | [68] | |
| Combretaceae | Guiera senegalensis J. F. Gmel | Methyl Gallate | 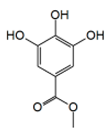 | Bark; cough, dysentery, malaria | 64 | NC | ↘ | NC | ↘ | NC | [69] | ||
| P. aeruginosa PAO1 | 512 | ↘ | ↘ | ↘ | ↘ | NC | |||||||
| Combretaceae | Combretum albiflorum (Tul.) Jongkind | Catechin | 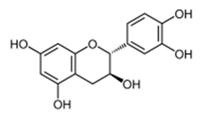 | Bark; bacterial infection, fever, pneumonia | 2000 | NC | ↘ | ↘ | ↘ | NC | [68] | ||
| Fabaceae | Dalbergia trichocarpa Baker | Oleanolic aldehyde coumarate |  | Bark; laryngitis, diarrhea | 4000 | ↘ | ↘ | ↘ | ↘ | yes | [5] | ||
| Lamiaceae | Platostoma rotundifolium (Briq.) A. J. Paton | β-sitosterol |  | Aerial part; bacterial diseases | 4000 | ↗ | ↘ | ↘ | ↘ | yes | [70] | ||
| Cassipourol | 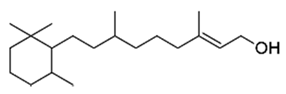 | 4000 | ↗ | ↘ | ↘ | ↘ | yes | ||||||
| α-amyrin | 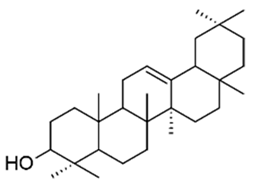 | 4000 | ↗ | ↘ | ↘ | ↔ | yes | ||||||
| Group of Gram-positive bacteria | L. monocytogenes LMG 21263 | Fabaceae | Vachellia karroo (Hyane) Banfi & Galasso [synonym of Acacia karroo Hayne] | Epigallocatechin |  | Leaves; dysentery, diarrhea, | 31 | NC | NC | ↘ | NC | NC | [71] |
| β-sitosterol | 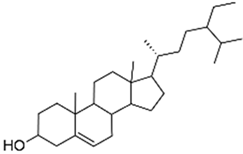 | 62 | NC | NC | ↘ | NC | NC | ||||||
| R. fascians D188 | Dalbergia pervillei Vatke | Perbergin | 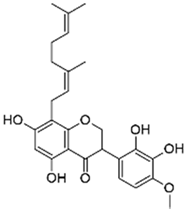 | Bark; unknown | 5 | NC | ↘ | NC | NC | NC | [72] | ||
| S. aureus ATCC 29213 | Moraceae | Ficus sansibarica Warb. | Isovitexin (apigenin 6C glycoside) | 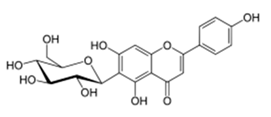 | Leaves and fruits; dysentery, diarrhea, wound, tuberculosis | 1600 | NC | NC | ↘ | NC | NC | [68] | |
| Epicatechin | 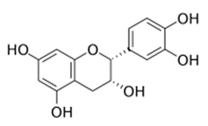 | 8000 | NC | NC | ↘ | NC | NC | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahavy, C.E.; Duez, P.; ElJaziri, M.; Rasamiravaka, T. African Plant-Based Natural Products with Antivirulence Activities to the Rescue of Antibiotics. Antibiotics 2020, 9, 830. https://doi.org/10.3390/antibiotics9110830
Mahavy CE, Duez P, ElJaziri M, Rasamiravaka T. African Plant-Based Natural Products with Antivirulence Activities to the Rescue of Antibiotics. Antibiotics. 2020; 9(11):830. https://doi.org/10.3390/antibiotics9110830
Chicago/Turabian StyleMahavy, Christian Emmanuel, Pierre Duez, Mondher ElJaziri, and Tsiry Rasamiravaka. 2020. "African Plant-Based Natural Products with Antivirulence Activities to the Rescue of Antibiotics" Antibiotics 9, no. 11: 830. https://doi.org/10.3390/antibiotics9110830
APA StyleMahavy, C. E., Duez, P., ElJaziri, M., & Rasamiravaka, T. (2020). African Plant-Based Natural Products with Antivirulence Activities to the Rescue of Antibiotics. Antibiotics, 9(11), 830. https://doi.org/10.3390/antibiotics9110830






