Abstract
The present study assessed the proportion of intensive care unit (ICU) patients who had a vancomycin serum concentration between 20 and 25 mg/L after 24–48 h of intravenous vancomycin administration. From 2016 to 2018, adult ICU patients with vancomycin continuous infusion (CI) for any indication were included. The primary outcome was the proportion of patients with a first-available vancomycin serum concentration between 20–25 mg/L at 24 h (D2) or 48 h (D3). Of 3894 admitted ICU patients, 179 were included. A median loading dose of 15.6 (interquartile range (IQR) = (12.5–20.8) mg/kg) was given in 151/179 patients (84%). The median daily doses of vancomycin infusion for D1 and D2 were 2000 [(IQR (1600–2000)) and 2000 (IQR (2000–2500)) mg/d], respectively. The median duration of treatment was 4 (2–7) days. At D2 or D3, the median value of first serum vancomycin concentration was 19.8 (IQR (16.0–25.1)) with serum vancomycin concentration between 20–25 mg/L reported in 43 patients (24%). Time spent in the ICU before vancomycin initiation was the only risk factor of non-therapeutic concentration at D2 or D3. Acute kidney injury occurred significantly more when vancomycin concentration was supra therapeutic at D2 or D3. At D28, 44 (26%) patients had died. These results emphasize the need of appropriate loading dose and regular monitoring to improve vancomycin efficacy and avoid renal toxicity.
1. Introduction
Septic shock is one of the main causes of admission into intensive care units (ICUs) with a related mortality rate of approximately 40% [1]. Among gram-positive pathogens, methicillin-resistant Staphylococcus aureus (MRSA) is a risk factor for mortality [2]. As early administration of adequate doses of effective antibiotics is a priority in the management of infected ICU patients, vancomycin remains a cornerstone choice in antibiotic treatment, particularly in the context of potentially resistant Gram-positive pathogens [3]. The risk of suboptimal drug dosing has led to recommendations for high vancomycin dosing coupled with therapeutic drug monitoring (TDM) for critically ill patients [4,5].
Vancomycin’s pharmacodynamics are best described using the area under the curve to minimum inhibitory concentration (AUC/MIC) ratio, with a value >400 recommended to ensure good patient outcome (cure of infection and avoidance of mortality) [6,7,8]. In clinical practice, because of difficulties in establishing the AUC/MIC at bedside, French guidelines and the previous 2009 American guidelines recommended a trough of 15–20 mg/L in serious patients when vancomycin was given by intermittent infusion [5,9]. In the new 2020 American guidelines, published after the completion of this study, only a target based on AUC is recommended when using an intermittent infusion [8]. However, continuous infusion (CI) has been advocated, even though only one study has reported a better patient outcome [10]. The arguments in favour of continuous infusions are a faster attainment of target concentrations, less variability in serum vancomycin concentrations, and less nephrotoxicity [11,12,13]. Moreover, it is cheaper and logistically easier to complete TDM and AUC calculations [14]. Hence, the 2020 American guidelines recommend a trough of 20–25 mg/L in serious patients when vancomycin is given by continuous infusion [8].
Recent studies reported that target serum concentrations were not reached in 30 to 50% of ICU patients given a vancomycin CI infusion [15,16,17]. These findings could be explained by the alterations in vancomycin pharmacokinetics in ICU patients, especially in patients with an augmented renal clearance (ARC) >130 mL/min [18,19]. Because the inflammatory response leads to an increased volume of distribution, a loading dose of at least 25–30 mg/kg over 1 h is recommended [5,9,18]. For the CI daily dose, there is no formal recommendation, leading to heterogeneous dosing. Some authors have proposed determining the dosing according to the measured creatinine renal clearance, which is correlated with vancomycin renal clearance, and further dosing guided by TDM [17,20,21].
Because these studies were very heterogeneous, the present study aimed to assess the proportion of patients with adequate vancomycin concentration after 24–48 h when applying the vancomycin dosing procedure in an institutional ICU (31 beds) over a 3-year period (intravenous bolus followed by CI). The secondary objectives were to determine the factors associated with an appropriate vancomycin serum concentration at 24–48 h, with the occurrence of acute kidney injury (AKI) and mortality.
2. Methods
2.1. Design
This study was performed according to the current French law [22] in a 31-bed French ICU [23]. It was a retrospective analysis of a prospectively designed database (2016) with data collected during a 3-year period: 1st January 2016–31st December 2018. The study was conducted in accordance with the Declaration of Helsinki, and the Institutional Review Board approved the study on November 15th 2016 (# 16.11.06). As the present study only reported the concentrations of serum vancomycin concentrations routinely collected in the ICU, the Institutional Review Board waived the patient’s informed consent. However, the patient and/or his (her) relative was verbally informed of the study and could refuse to participate. STROBE recommendations were followed for performing this observational study [24].
2.2. Patients
Inclusion criteria: all admitted adult patients for whom vancomycin therapy using continuous infusion had been clinically indicated by the physician in charge could be included in the present study.
Non-inclusion criteria:
- -
- Patients <18 years of age and pregnant.
- -
- Patients with previous renal replacement therapy (RRT) for chronic renal failure.
- -
- Patients for whom vancomycin treatment was ongoing.
- -
- Patients for whom vancomycin intermittent infusion was used.
- -
- Patients requiring >14-day vancomycin therapy (such as endocarditis requiring longer treatment duration and higher plasma level).
- -
- Patients who had previously participated to the present study.
- -
- Patients for whom withdrawing care would be decided in the next 48 h.
- -
- Patients not receiving intravenous dosing.
- -
- Patients for whom a refusal was expressed.
Exclusion criteria: Patients without a measured vancomycin serum concentration.
2.3. Vancomycin Administration
The international and French recommendations on infections and septic shock were applied for the included patients [3,9,25,26]. For vancomycin administration (Vancomycine, Sandoz®, Levallois-Peret, France), a loading dose and a subsequent CI left to the physician’s decision is the current standard of practice within the unit. Dosing was adapted according to the vancomycin serum concentration, which is classically performed between 24 h (Day 2 (D2)) and 48 h (Day 3 (D3)) after vancomycin initiation and according to physician’s decision, with a serum concentration target of 20–25 mg/L. Prescription of vancomycin serum concentration measurement throughout the day was left to the discretion of the physician. Dosing adaptation modalities following a non-therapeutic vancomycin concentration were left to the discretion of the physician.
2.4. Measured Parameters
The following parameters were measured:
- Demographic characteristics: age, sex, height, total body weight with calculated body mass index, and previous stable serum creatinine concentration.
- Medical history, initial reason for ICU admission and Simplified Acute Physiology Score II (SAPS II) at ICU admission [27].
- The patient inclusion was defined as the day of vancomycin initiation (D1). During the inclusion period (vancomycin initiation day and 14 following days) the following data were captured: source of infection and anti-infective therapy, including type of infection, anti-infective agent(s) administered, and microbiological cultures collected.
- Clinical parameters including urine output, which was assessed at 08:00 h daily.
- The Sequential Organ Failure Assessment (SOFA) [28] and the Kidney Disease: Improving Global Outcome (KDIGO) [29] scores were calculated at inclusion (D1), daily until Day 4, and at D10 and D15.
- Requirement for vasopressor support, renal replacement therapy (RRT), mechanical ventilation, and/or sedation.
- Co-prescription of nephrotoxic drugs (e.g., aminoglycosides, diuretics, non-steroid anti-inflammatory (NSAI), iodinated contrast products).
- Biological parameters: serum creatinine concentration, calculation of creatinine clearance by chronic kidney disease-epidemiology CKD-EPI formula [30].
- Vancomycin dosing: the loading dose and the continuous infusion regimen (daily dose) and its potential alterations were recorded.
- Vancomycin assays: vancomycin serum concentrations were measured using automated immunoassays (Kinetic Interaction of Microparticles in Solution, COBAS 8000®, Roche Diagnostics). Blood samples for vancomycin monitoring were collected at 8:00 AM if prescribed.
The different times of measurement are shown in Table 1.

Table 1.
Time of measurement of the different studied parameters.
2.5. Objectives and Assessment Criteria
2.5.1. Main Outcome
The proportion of patients with the first-available vancomycin serum concentration between 20–25 mg/L at 24 h (D2) or 48 (D3).
2.5.2. Secondary Outcomes
- The proportion of patients with vancomycin serum concentration <10 mg/L at D2 or D3 (considered as a risk factor of failure and resistance emergence) [5].
- Factors associated with vancomycin serum concentration between 20–25 mg/L at D2–D3, and the factors associated with vancomycin serum concentration under 20 mg/L at D2–D3.
- Patient outcome separating (Test of Cure):
- -
- Clinical cure: resolution of clinical signs and symptoms compared with baseline, and no requirement for additional antibacterial treatment.
- -
- Clinical failure: Persistence or progression of baseline signs and symptoms after at least 2 days of treatment, consistent with active infection. When the same pathogen was found, it was considered as a relapse, whereas the presence of another pathogen was considered as a reinfection.
- -
- Indeterminate: Extenuating circumstances preclude classification to one of the above.
- Patient survival at ICU discharge, hospital discharge and at D28. At D28, patients with and without an appropriate serum vancomycin concentration at D2–D3 were compared.
- The occurrence of AKI (defined as a KDIGO ≥1) during the vancomycin administration, including pre-existing or beginning AKI at vancomycin initiation [29].
2.5.3. Statistical Analysis
Baseline characteristics, primary outcome, and secondary outcomes were first reported. Quantitative variables were expressed as mean ± standard deviation (SD) or median (interquartile range (IQR)). Qualitative variables were expressed by their number and frequency.
In order to analyse relationships between out of target (20–25 mg/L) vancomycin serum concentration at D2–D3 (first available) and several variables of interest, logistic regression was used. Univariate analyses were first considered, and the variables for which p-values were under 0.20 were considered for a multivariate analysis using a stepwise selection of variables. Then, the variables with a p-value <0.05 in the multivariate model were considered statistically significant. The same type of analysis was performed to predict the risk factors of a vancomycin serum concentration lower than 20 mg/L at D2–D3 (first available), after exclusion of overdosed patients (>25 mg/L). Statistical analysis was performed with R 3.1.1 (The R Foundation for Statistical Computing) and SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Study Population
From January 1st 2016 to December 31st 2018, 367 out of 3894 admitted ICU patients were given vancomycin. One hundred sixty seven patients did not meet inclusion criteria and 21 did not have systemic vancomycin dosing, leading to their exclusion. Therefore, 179 patients were included in this study (Figure 1).
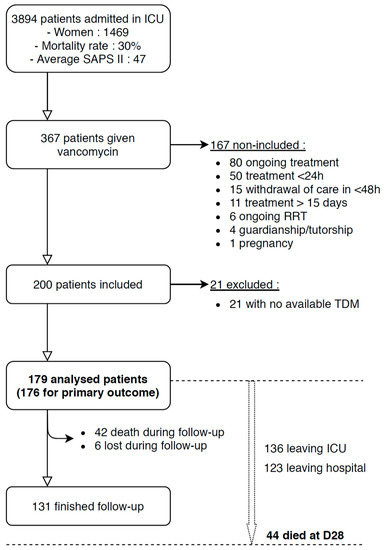
Figure 1.
Flow chart showing patient inclusions and exclusions. TDM: therapeutic drug monitoring. Follow-up was the 15 consecutive days after vancomycin initiation. D1 was the day of vancomycin initiation.
Patient characteristics are shown in Table 2. Infection-related parameters and causative pathogens are shown in Table 3. A causative pathogen (documented infection) was isolated in 81% of patients. Intra-abdominal, bloodstream, and lung were the most frequent infection sites. Five percent of causative pathogens were MRSA. All of the pathogens had a vancomycin MIC ≤ 1.

Table 2.
Patient characteristics.

Table 3.
Infection and causative pathogen patterns.
3.2. Vancomycin Administration
Vancomycin was initiated 1 (0–5) day after patient admission. A loading dose of 1000 (1000–1500) mg (corresponding to 15.6 (12.5–20.8) mg/kg of total body weight) was given in 151/179 patients (84%). The median daily dose of vancomycin infusion for D1 and D2 were 2000 (1600–2000) mg (corresponding to 27.6 (21.3–31.9) mg/kg/d) and 2000 (2000–2500) mg (corresponding to 28.4 (23.8–34.8) mg/kg/d), respectively. The median duration of vancomycin administration was 4 (2–7) days. The identification and the analysis of the isolated pathogen(s) led to cessation of vancomycin infusion in 94 patients (53%).
3.3. Main Outcome
Figure 2 shows the serum vancomycin concentration during its administration. At D2 or D3 (first available), the median value of serum vancomycin concentration was 19.8 (16.0–25.1) mg/L with serum vancomycin concentration between 20–25 mg/L reported in 43 patients (24%).
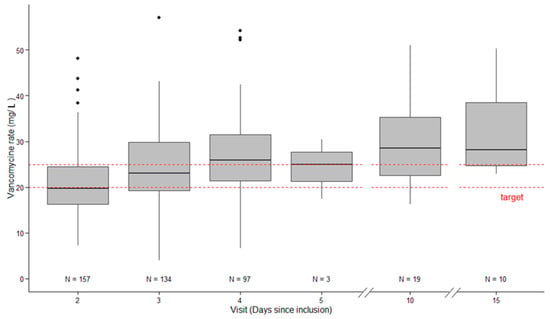
Figure 2.
Vancomycin serum concentration over time (D2, D3, D4, D10, and D15).
3.4. Secondary Outcomes
Serum vancomycin concentrations <20 mg/L or >25 mg/L were recorded in 89 (51%) and 44 (25%) patients, respectively. A serum vancomycin concentration <10 mg/L was recorded in 5/176 patient (3%) at D2 or D3 and never after D3. In 37 patients with RRT, 12 (32%) reached the target of serum vancomycin concentration.
In a multivariate analysis, the only parameter associated with a serum vancomycin <20 or >25 mg/L was a longer time between admission and vancomycin initiation (OR = 1.1 (1.005–1.205)) (Table 4).

Table 4.
Multivariate analysis for risk factors of being out of target.
Moreover, AKI at the vancomycin initiation was significantly associated with a lower incidence of serum vancomycin concentration <20 mg/L at D2–D3 (OR = 0.426 (0.199–0.912)) (Table 5). In contrast, the time between patient admission and the vancomycin initiation was associated with an increased likelihood of a serum vancomycin concentration <20 mg/L at D2–D3.

Table 5.
Multivariate analysis for risk factors of being under the target.
3.5. Patient Outcome
A clinical cure was reported in 116 patients (65%). A relapse of infection occurred in 5 patients (3%), a reinfection with another pathogen occurred in 12 patients (7%), and a clinical failure without identification was reported in 23 patients (13%). The test of cure could not be assessed in 23 patients (13%). Median duration before ICU and hospital discharge in alive patients was 6 (3–13), and 23 (14–47) days, respectively. By D28, 44 (26%) patients had died. Of the 42 patients with serum vancomycin concentration between 20 and 25 mg/L at D2–D3, 8 (19%) died by D28 whereas 37 out of 132 patients (28%) with serum vancomycin concentration <20 mg/L or >25 mg/L at D2–D3 died by D28 (p = 0.22).
AKI during vancomycin administration occurred in 107 (60%) patients. Among those, 49 patients (27%) required RRT after vancomycin initiation. AKI occurred in 34 out of 44 patients (77%) with serum vancomycin concentration >25 mg/L at D2–D3 leading to RRT requirement in 16 patients (36%). Of the 131 patients with serum vancomycin serum concentration <25 mg/L, only 72 (55%) developed AKI (p = 0.009) and 33 (25%) required RRT (p = 0.153).
4. Discussion
In the present study, a therapeutic target of serum vancomycin concentration was reached in 24% patients. A prolonged ICU stay before vancomycin initiation was associated with a higher risk of non-therapeutic exposure (i.e., <20 mg/L or >25 mg/L), especially an increased risk of subtherapeutic exposure. In patients with serum vancomycin concentration >25 mg/L, the occurrences of AKI and an RRT requirement were higher than in other included patients. A serum vancomycin concentration <10 mg/L was reported in 5/176 patient (3%) at D2 or D3 and never after D3, meaning that our dosing regimen was suitable for avoiding low vancomycin exposures.
Vancomycin remains a cornerstone for the management of patients with documented or suspected infection with MRSA or Enterococcus faecium even if the incidence of such resistant pathogens has decreased in Europe and in France in the last two decades [31,32]. The AUC/MIC index has the strongest association with efficacy, with a minimum threshold described around 400 [6,7], whereas AUC >700 has been associated with greater renal toxicity [33]. Because vancomycin has time-dependent pharmacodynamic characteristics, CI is used in one third of ICUs [34]. CI allows for more convenient TDM and is associated with less variability in serum vancomycin concentrations, faster target concentrations achievement, and less nephrotoxicity [11,12,13,14]. However, only one study has reported a reduction in mortality [10]. The 2009 American Society of Health-System Pharmacists (ASHP), the Infectious Diseases Society of America (IDSA), and the Society of Infectious Diseases Pharmacists (SIDP) recommend a trough target ≥15 mg/L with intermittent infusion in severe infections, whereas two French societies (Société Française d’Anesthésie et Réanimation (SFAR) and Société de Réanimation de Langue Française (SRLF)) recommend CI with a target steady-state concentration of 20 mg/L [5,9]. In the present study and in our institution, a therapeutic range of 20–25 mg/L is used. This target during CI is now recommended by the 2020 revised consensus guidelines by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists on therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections (guidelines published after the completion of this study) [8]. Some authors have also proposed a therapeutic target of 20–30 mg/L [15,16,17]. The reason for these high targets is to increase the likelihood of achieving therapeutic exposures for methicillin-resistant Staphylococcus sp. with MIC >1 mg/L [21].
However, until a MIC = 1, a target >16.5 mg/L in continuous infusion (AUC/MIC > 396) could be sufficient. Considering the extremely low rate of MRSA with MIC >1 in our institution (local data not shown), an effective vancomycin concentration was possibly given in a majority of patients (Figure 2).
The present study included a similar patient population to previous studies, including >100 patients [16,21]. The mortality rate (~30%) and a documented infection incidence (~80%) were also similar to previous studies. However, the present therapeutic target was 20–25 mg/L whereas Ocampos et al., Cristallini et al., and Baptista et al. targeted a broader target range: 20–30 mg/L [16,17,21]. With our narrower therapeutic target, only 24% patients met this range. Using a therapeutic target between 20–30 mg/L, previous studies reported an appropriate vancomycin serum concentration in about 50% of patients [16,17,21], which is consistent with our results (Figure 2).
Fifty-one percent of patients were in a subtherapeutic range at D2–3, including five at a very low range (<10 mg/L). An explanation of this result could be a suboptimal use of the loading dose, performed in only 84% of the population and often insufficient (median = 15.6 mg/kg (12.5–20.8)) relative to the recommended dose (25–30 mg/kg) [5,15,35]. However, this criterion was not retrieved in this study as a risk factor of underdosing in multivariate analysis.
At Day 2, more than 30% of patients were supratherapeutic (>25 mg/L) and at D10 or D14, nearly 50% of patients still on therapy manifested concentrations >30 mg/L (Figure 2). These results provide an incentive to maintain regular vancomycin concentration monitoring until the end of the treatment. Moreover, the occurrence of new AKI and an RRT requirement was higher in patients with a serum vancomycin concentration >25 mg/L on D1 or D2 than those with vancomycin <25 mg/L (77 and 36%, respectively). However, our study is not able to determine a causality, and AKI could have happened first and led to higher vancomycin rates by accumulation. This point needs to be explored in further studies, but it is difficult to define vancomycin-caused AKI, especially in the ICU where AKI is multifactorial.
In our analysis the only factor found to be associated with non-therapeutic exposure was a prolonged duration of stay in the ICU before initiating vancomycin administration (i.e., <20 mg/L or >25 mg/L). This result has previously been reported, in a septic population [36], and could be explained by an increased volume of distribution, by overload, and renal dysfunction [18]. Alternatively, the difficulty in calculating vancomycin doses from creatinine clearance due to unstable serum creatinine concentrations or low creatinine production may explain this observation. The presence of AKI at the initiation of vancomycin therapy was associated with a lower proportion of patients with subtherapeutic concentrations, as AKI could blunt the low dosing regimen used in our study. Previous studies reported an association between creatinine clearance and vancomycin through concentration variations [16,37]. The present study did not report similar findings as we used only calculated creatinine clearance, which is poorly correlated with measured creatinine clearance in the ICU population [38,39].
Some limitations could be relevant for the present study. First, this was a single centre study and some findings may not be extrapolated well to other centres. However, the present study included more than 150 patients in a 3-year period and confirmed the main results of previous studies in terms of low achievement of therapeutic exposures as well as the importance of loading doses and assessment of renal clearance for choosing more consistently therapeutic and safer dosing regimens. Second, the present study was only focused on the therapeutic target at D2–D3 in patients in whom the physician in charge of the patient chose vancomycin administration. The extrapolation of patient outcomes should be taken with caution as the analysis of the isolated pathogen(s) led to cessation of vancomycin infusion in 53% of patients after a median CI duration of 4 days. Third, the real MIC was not determined. In our institution, nearly all MRSA MIC are <1, meaning that an effective vancomycin concentration was probably reached in more patients than currently reported (Figure 2). Determining vancomycin MIC for each MRSA could lead to a greater proportion of patients with appropriate serum concentration. In the same way, knowledge of the MIC could lead to a reduction in vancomycin dosing to avoid too high serum concentrations and side effects. Fourth, we also included patients requiring renal replacement therapy, who likely to have different dosing requirements to other patients [40]. Fifth, the occurrence of KDIGO = 1 defined a vancomycin-associated AKI that is different from vancomycin-induced AKI, as many confounding factors could lead to AKI in such circumstances, explaining the high rate of AKI in the present study. Given this, it is perhaps unsurprising that we observed a higher rate of nephrotoxicity than previously reported [41].
5. Conclusions
The present study confirmed the major findings that have been recently reported in different studies: (1) The importance of loading dose: in the present study, a loading dose of 15.6 (12.5–20.8) mg/kg led to a nearly 50% vancomycin serum concentration at D2. Therefore, a loading of 25–30 mg/kg should be encouraged. (2) The importance of measuring creatinine clearance for anticipating the need of increased infusion rate. (3) The need of TDM, because of persistent difficulties to predict vancomycin serum concentration in ICU patients, and for avoiding a too high vancomycin serum concentration that could impair renal function.
Author Contributions
Conceptualization and methodology J.-Y.L. and N.P., data analysis P.G.C., G.M. and N.M., investigation N.P., resources J.-P.L. and A.E., writing—original draft preparation N.P. and J.-Y.L., writing—reviewing and editing—C.R., L.M., J.-P.L., J.A.R. and J.L., visualisation C.B., S.J. and S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank Loubna Elotmani, Sophie Lloret, Emeline Villemont, and Audrey Ambert for their help in collecting data.
Conflicts of Interest
The authors declare that they have no competing interest.
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Hanberger, H.; Walther, S.; Leone, M.; Barie, P.S.; Rello, J.; Lipman, J.; Marshall, J.C.; Anzueto, A.; Sakr, Y.; Pickkers, P.; et al. Increased mortality associated with methicillin-resistant Staphylococcus aureus (MRSA) infection in the intensive care unit: Results from the EPIC II study. Int. J. Antimicrob. Agents 2011, 38, 331–335. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Rybak, M.J.; Akins, R.L. Emergence of methicillin-resistant Staphylococcus aureus with intermediate glycopeptide resistance: Clinical significance and treatment options. Drugs 2001, 61, 1–7. [Google Scholar] [CrossRef]
- Rybak, M.; Lomaestro, B.; Rotschafer, J.C.; Moellering, R.; Craig, W.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health. Syst. Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef]
- Moise-Broder, P.A.; Forrest, A.; Birmingham, M.C.; Schentag, J.J. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 2004, 43, 925–942. [Google Scholar] [CrossRef]
- Zelenitsky, S.; Rubinstein, E.; Ariano, R.; Iacovides, H.; Dodek, P.; Mirzanejad, Y.; Kumar, A. Cooperative Antimicrobial Therapy of Septic Shock-CATSS Database Research Group Vancomycin pharmacodynamics and survival in patients with methicillin-resistant Staphylococcus aureus-associated septic shock. Int. J. Antimicrob. Agents 2013, 41, 255–260. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. AJHP Off. J. Am. Soc. Health Syst. Pharm. 2020, 77, 835–864. [Google Scholar] [CrossRef]
- Bretonnière, C.; Leone, M.; Milési, C.; Allaouchiche, B.; Armand-Lefevre, L.; Baldesi, O.; Bouadma, L.; Decré, D.; Figueiredo, S.; Gauzit, R.; et al. Strategies to reduce curative antibiotic therapy in intensive care units (adult and paediatric). Intensive Care Med. 2015, 41, 1181–1196. [Google Scholar] [CrossRef]
- Rello, J.; Sole-Violan, J.; Sa-Borges, M.; Garnacho-Montero, J.; Muñoz, E.; Sirgo, G.; Olona, M.; Diaz, E. Pneumonia caused by oxacillin-resistant Staphylococcus aureus treated with glycopeptides. Crit. Care Med. 2005, 33, 1983–1987. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.-J.; Chen, H.; Zhou, J.-X. Continuous versus intermittent infusion of vancomycin in adult patients: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2016, 47, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, M.; Delatour, F.; Faurisson, F.; Rauss, A.; Pean, Y.; Misset, B.; Thomas, F.; Timsit, J.F.; Similowski, T.; Mentec, H.; et al. Continuous versus intermittent infusion of vancomycin in severe Staphylococcal infections: Prospective multicenter randomized study. Antimicrob. Agents Chemother. 2001, 45, 2460–2467. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, M.A.; Tacconelli, E.; Grilli, E.; Pea, F.; Petrosillo, N. Continuous versus intermittent infusion of vancomycin for the treatment of Gram-positive infections: Systematic review and meta-analysis. J. Antimicrob. Chemother. 2012, 67, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Waineo, M.F.; Kuhn, T.C.; Brown, D.L. The pharmacokinetic/pharmacodynamic rationale for administering vancomycin via continuous infusion. J. Clin. Pharm. Ther. 2015, 40, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Taccone, F.S.; Udy, A.A.; Vincent, J.-L.; Jacobs, F.; Lipman, J. Vancomycin dosing in critically ill patients: Robust methods for improved continuous-infusion regimens. Antimicrob. Agents Chemother. 2011, 55, 2704–2709. [Google Scholar] [CrossRef]
- Ocampos-Martinez, E.; Penaccini, L.; Scolletta, S.; Abdelhadii, A.; Devigili, A.; Cianferoni, S.; de Backer, D.; Jacobs, F.; Cotton, F.; Vincent, J.-L.; et al. Determinants of early inadequate vancomycin concentrations during continuous infusion in septic patients. Int. J. Antimicrob. Agents 2012, 39, 332–337. [Google Scholar] [CrossRef]
- Baptista, J.P.; Roberts, J.A.; Sousa, E.; Freitas, R.; Deveza, N.; Pimentel, J. Decreasing the time to achieve therapeutic vancomycin concentrations in critically ill patients: Developing and testing of a dosing nomogram. Crit. Care Lond. Engl. 2014, 18, 654. [Google Scholar] [CrossRef]
- Jamal, J.-A.; Roger, C.; Roberts, J.A. Understanding the impact of pathophysiological alterations during critical illness on drug pharmacokinetics. Anaesth. Crit. Care Pain Med. 2018, 37, 515–517. [Google Scholar] [CrossRef]
- Baptista, J.P.; Roberts, J.A.; Udy, A.A. Augmented renal clearance: A real phenomenon with an uncertain cause. Anaesth. Crit. Care Pain Med. 2019, 38, 335–336. [Google Scholar] [CrossRef]
- Pea, F.; Furlanut, M.; Negri, C.; Pavan, F.; Crapis, M.; Cristini, F.; Viale, P. Prospectively validated dosing nomograms for maximizing the pharmacodynamics of vancomycin administered by continuous infusion in critically ill patients. Antimicrob. Agents Chemother. 2009, 53, 1863–1867. [Google Scholar] [CrossRef]
- Cristallini, S.; Hites, M.; Kabtouri, H.; Roberts, J.A.; Beumier, M.; Cotton, F.; Lipman, J.; Jacobs, F.; Vincent, J.-L.; Creteur, J.; et al. New Regimen for Continuous Infusion of Vancomycin in Critically Ill Patients. Antimicrob. Agents Chemother. 2016, 60, 4750–4756. [Google Scholar] [CrossRef] [PubMed]
- Toulouse, E.; Masseguin, C.; Lafont, B.; McGurk, G.; Harbonn, A.; A Roberts, J.; Granier, S.; Dupeyron, A.; Bazin, J.E. French legal approach to clinical research. Anaesth. Crit. Care Pain Med. 2018, 37, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Constantin, J.-M.; Dahyot-Fizelier, C.; Duracher-Gout, C.; Joannes-Boyau, O.; Langeron, O.; Legrand, M.; Mahjoub, Y.; Mirek, S.; Mrozek, S.; et al. French intensive care unit organisation. Anaesth. Crit. Care Pain Med. 2018, 37, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. Lond. Engl. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Montravers, P.; Dupont, H.; Leone, M.; Constantin, J.-M.; Mertes, P.-M.; Société française d’anesthésie et de réanimation (Sfar); Société de réanimation de langue française (SRLF); Laterre, P.-F.; Misset, B.; Société de pathologie infectieuse de langue française (SPILF); et al. Guidelines for management of intra-abdominal infections. Anaesth. Crit. Care Pain Med. 2015, 34, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Bouadma, L.; Bouhemad, B.; Brissaud, O.; Dauger, S.; Gibot, S.; Hraiech, S.; Jung, B.; Kipnis, E.; Launey, Y.; et al. Hospital-acquired pneumonia in ICU. Anaesth. Crit. Care Pain Med. 2018, 37, 83–98. [Google Scholar] [CrossRef]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Inter. Suppl. 2012, 2, 1–138. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC Surveillance of Antimicrobial Resistance in Europe 2016. Available online: https://ecdc.europa.eu/sites/portal/files/documents/EARS-Net-report-2017-update-jan-2019.pdf (accessed on 18 September 2019).
- Leone, M.; Roberts, J.A.; Bassetti, M.; Bouglé, A.; Lavigne, J.-P.; Legrand, M.; Neely, M.; Paiva, J.-A.; Payen, D.; Rello, J.; et al. Update in antibiotic therapy in intensive care unit: Report from the 2019 Nîmes International Symposium. Anaesth. Crit. Care Pain Med. 2019. [Google Scholar] [CrossRef]
- Zasowski, E.J.; Murray, K.P.; Trinh, T.D.; Finch, N.A.; Pogue, J.M.; Mynatt, R.P.; Rybak, M.J. Identification of Vancomycin Exposure-Toxicity Thresholds in Hospitalized Patients Receiving Intravenous Vancomycin. Antimicrob. Agents Chemother. 2017, 62, e01684-17. [Google Scholar] [CrossRef] [PubMed]
- Tabah, A.; De Waele, J.; Lipman, J.; Zahar, J.R.; Cotta, M.O.; Barton, G.; Timsit, J.-F.; Roberts, J.A. Working Group for Antimicrobial Use in the ICU within the Infection Section of the European Society of Intensive Care Medicine (ESICM) The ADMIN-ICU survey: A survey on antimicrobial dosing and monitoring in ICUs. J. Antimicrob. Chemother. 2015, 70, 2671–2677. [Google Scholar] [CrossRef]
- De Waele, J.J.; Danneels, I.; Depuydt, P.; Decruyenaere, J.; Bourgeois, M.; Hoste, E. Factors associated with inadequate early vancomycin levels in critically ill patients treated with continuous infusion. Int. J. Antimicrob. Agents 2013, 41, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Chuma, M.; Makishima, M.; Imai, T.; Tochikura, N.; Sakaue, T.; Kikuchi, N.; Kinoshita, K.; Kaburaki, M.; Yoshida, Y. Duration of Systemic Inflammatory Response Syndrome Influences Serum Vancomycin Concentration in Patients With Sepsis. Clin. Ther. 2016, 38, 2598–2609. [Google Scholar] [CrossRef]
- Marsot, A.; Boulamery, A.; Bruguerolle, B.; Simon, N. Vancomycin: A review of population pharmacokinetic analyses. Clin. Pharmacokinet. 2012, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Baptista, J.P.; Udy, A.A.; Sousa, E.; Pimentel, J.; Wang, L.; Roberts, J.A.; Lipman, J. A comparison of estimates of glomerular filtration in critically ill patients with augmented renal clearance. Crit. Care Lond. Engl. 2011, 15, R139. [Google Scholar] [CrossRef] [PubMed]
- Bragadottir, G.; Redfors, B.; Ricksten, S.-E. Assessing glomerular filtration rate (GFR) in critically ill patients with acute kidney injury--true GFR versus urinary creatinine clearance and estimating equations. Crit. Care Lond. Engl. 2013, 17, R108. [Google Scholar] [CrossRef] [PubMed]
- Jamal, J.-A.; Udy, A.A.; Lipman, J.; Roberts, J.A. The impact of variation in renal replacement therapy settings on piperacillin, meropenem, and vancomycin drug clearance in the critically ill: An analysis of published literature and dosing regimens*. Crit. Care Med. 2014, 42, 1640–1650. [Google Scholar] [CrossRef]
- Lodise, T.P.; Lomaestro, B.; Graves, J.; Drusano, G.L. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob. Agents Chemother. 2008, 52, 1330–1336. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
