Antibiotic Dispensation without a Prescription Worldwide: A Systematic Review
Abstract
1. Introduction
2. Results
2.1. Search Results
2.2. Quality Assessment
2.3. Characteristics of the Studies Selected
2.4. Study Outcomes—Articles that Use the Simulated Patient Method
2.4.1. Frequency of Antibiotic Dispensation without a Prescription
2.4.2. Name/Class of Antibiotics Most Often Dispensed without a Prescription
2.4.3. Types of Disease/Symptoms Most Commonly Associated with Dispensation without a Prescription
2.4.4. Level of Insistence by Patients
| Authors | Year of Publication | Country | Study Design | Data Collection Method | Quality Assesment a | Criteria not Fulfilled a |
|---|---|---|---|---|---|---|
| Abubakar U. et al. [24] | 2020 | Nigeria | Cross-sectional prospective study | Pharmacy interviews/questionnaires | High | 7 |
| Abubakar U. [25] | 2020 | Nigeria | Cross-sectional study | Pharmacy interviews/questionnaires | High | 6, 7, 13 |
| Al-Tannir M. et al. [17] | 2020 | Saudi Arabia | Cross-sectional study | Simulated patient method | High | 7, 12, 14 |
| Badro D.A. et al. [26] | 2020 | Lebanon | Cross-sectional study | Pharmacy interviews/questionnaires | High | 3, 7, 14 |
| Bahta M. et al. [27] | 2020 | Eritrea | Cross-sectional study | Simulated patient method | High | 7, 13, 14 |
| Chen J. et al. [28] | 2020 | China | Cross-sectional study | Simulated patient method | High | 6, 7, 14 |
| Gajdács M. et al. [29] | 2020 | Hungary | Cross-sectional study | Pharmacy interviews/questionnaires | High | 3, 7, 14 |
| Halboup A. et al. [30] | 2020 | Yemen | Cross-sectional study | Simulated patient method | High | 14 |
| Shi L. et al. [31] | 2020 | China | Cross-sectional study | Simulated patient method | High | 6, 7, 14 |
| Wang X. et al. [32] | 2020 | China | Cross-sectional study | Simulated patient method | High | 12, 14 |
| Abdelaziz AI et al. [33] | 2019 | Egypt | Cross-sectional study | Simulated patient method | High | 14 |
| Alrasheedy AA. et al. [34] | 2019 | Saudi Arabia | Cross-sectional study | Simulated patient method and pharmacy interviews/qestionnaires | Medium | 6, 7, 12, 14 |
| Chang J. et al. [7] | 2019 | China | Cross-sectional study | Simulated patient method | High | 6, 7, 14 |
| Damisie G et al. [35] | 2019 | Ethiopia | Cross-sectional study | Simulated patient method | Medium | 3, 7, 10, 14 |
| Hallit S et al. [36] | 2019 | Lebanon | Cross-sectional study | Pharmacy interviews/questionnaires | High | 7, 13 |
| Koji EM et al. [37] | 2019 | Ethiopia | Cross-sectional prospective study | Simulated patient method | High | 7, 14 |
| Mengistu G et al. [11] | 2019 | Ethiopia | Cross-sectional study | Simulated patient method and pharmacy interviews/questionnaires | High | 7, 14 |
| Nafade V. et al. [19] | 2019 | India | Cross-sectional study | Simulated patient method | High | 7, 14 |
| Zawahir S et al. [38] | 2019 | Sri Lanka | Cross-sectional study | Simulated patient method | High | 7, 14 |
| Zawahir S. et al. [39] | 2019 | Sri Lanka | Cross-sectional study | Pharmacy interviews/questionnaires | High | 14 |
| Ajie A.A.D. et al. [40] | 2018 | Indonesia | Cross-sectional study | Pharmacy interviews/questionnaires | High | 18 |
| Alhomoud F et al. [41] | 2018 | Saudi Arabia | Qualitative exploratory study | Pharmacy interviews/questionnaires | Medium | 6, 7, 13, 14 |
| Awosan KJ et al. [42] | 2018 | Nigeria | Cross-sectional study | Pharmacy interviews/questionnaires | High | 13, 14 |
| Erku D.A. et al. [43] | 2018 | Ethiopia | Cross-sectional study | Simulated patient method | High | 3, 7, 14 |
| Horumpende PG et al. [20] | 2018 | Tanzania | Cross-sectional study | Simulated patient method | High | 7, 14 |
| Ibrahim IR et al. [12] | 2018 | Iraq | Cross-sectional study | Simulated patient method | High | 6, 7, 14 |
| Mohamed Ibrahim M.I. et al. [44] | 2018 | Qatar | Cross-sectional study | Simulated patient method | High | 7, 14 |
| Paes M.R. et al. [45] | 2018 | India | Cross-sectional study | Pharmacy interviews/questionnaires | Medium | 3, 7, 10, 11, 12, 14 |
| Rehman IU et al. [46] | 2018 | Pakistan | Cross-sectional study | Pharmacy interviews/questionnaires | Medium | 3, 7, 13, 14 |
| Sarwar M.R. et al. [47] | 2018 | Pakistan | Cross-sectional study | Pharmacy interviews/questionnaires | High | 7, 14 |
| Zapata-Cachafeiro M et al. [48] | 2018 | Spain | Cross-sectional study | Simulated patient method | High | 7, 14 |
| Zawahir S et al. [49] | 2018 | Sri Lanka | Cross-sectional study | Simulated patient method | High | 14 |
| Ansari M. [50] | 2017 | Nepal | Cross-sectional prospective study | Pharmacy interviews/questionnaires | High | 7, 14 |
| Barker A.K. et al. [51] | 2017 | India | Cross-sectional study | Pharmacy interviews/questionnaires | High | 7, 14 |
| Chang J. et al. [52] | 2017 | China | Cross-sectional study | Simulated patient method | High | 7, 14 |
| Jaisue S. et al. [21] | 2017 | Thailand | Cross-sectional study | Simulated patient method | Medium | 3, 7, 10, 14, 18 |
| Mansour O. et al. [53] | 2017 | Syria | Cross-sectional study | Pharmacy interviews/questionnaires | High | 7, 13 |
| Marković-Peković V et al. [18] | 2017 | Republic of Srpska and Herzegovina | Cross-sectional study | Simulated patient method | High | 7, 14, 18 |
| Okuyan B. et al. [54] | 2017 | Turkey | Cross-sectional study | Simulated patient method | High | 7, 14, 18 |
| Abegaz T.M. et al. [14] | 2016 | Ethiopia | Cross-sectional study | Simulated patient method | High | 6, 7, 14 |
| Abood EA et al. [55] | 2016 | Yemen | Cross-sectional study | Pharmacy interviews/questionnaires | High | 3, 7, 14 |
| Erku D.A. [56] | 2016 | Ethiopia | Cross-sectional study | Pharmacy interviews/questionnaires | High | 7, 14 |
| Guinovart MC et al. [57] | 2016 | Spain | Prospective study | Simulated patient method | Medium | 3, 7, 10, 14, 18, 20 |
| Ibrahim M.I.B.M. et al. [13] | 2016 | Qatar | Cross-sectional study | Simulated patient method | High | 7, 14 |
| Kalungia AC et al. [58] | 2016 | Zambia | Descriptive cross-sectional study | Pharmacy interviews/questionnaires | High | 7, 14 |
| Khan M.U. et al. [59] | 2016 | Malaysia | Cross-sectional study | Pharmacy interviews/questionnaires | High | 7, 13, 14 |
| Nawab A. et al. [60] | 2016 | Pakistan | Cross-sectional study | Pharmacy interviews/questionnaires | Medium | 6, 7, 10, 12, 14, 18 |
| Satyanarayana S. et al. [61] | 2016 | India | Cross-sectional study | Simulated patient method | High | 6, 7, 14 |
| Almaaytah A et al. [62] | 2015 | Jordan | Prospective study | Simulated patient method | Medium | 7, 10, 14, 18 |
| Bahnassi A. [63] | 2015 | Syria | Cross-sectional study | Pharmacy interviews/questionnaires | High | 7, 10, 18 |
| Dorj G. et al. [64] | 2015 | Mongolia | Cross-sectional study | Pharmacy interviews/questionnaires | High | 7, 14 |
| Shet A et al. [65] | 2015 | India | Cross-sectional study | Simulated patient method | High | 7, 14 |
| Shreya Svitlana A. et al. [66] | 2015 | India | Cross-sectional study | Pharmacy interviews/questionnaires | Low | 6, 7, 9, 10, 11, 12, 14, 18, 20 |
| Alabid A.H.M.A. et al. [67] | 2014 | Malaysia | Cross-sectional study | Simulated patient method | High | 7, 14 |
| Bahnassi A. [68] | 2014 | Saudi Arabia | Cross-sectional study | Pharmacy interviews/questionnaires | High | 7, 14 |
| Farah R et al. [69] | 2014 | Lebanon | Cross-sectional study | Pharmacy interviews/questionnaires | High | 7, 14, 20 |
| Gastelurrutia M.A. et al. [70] | 2014 | Spain | Prospective study | Pharmacy interviews/questionnaires | Medium | 3, 7, 10, 11, 14 |
| Sabry NA et al. [71] | 2014 | Egypt | Cross-sectional study | Pharmacy interviews/questionnaires | High | 7 |
| Zapata-Cachafeiro M. et al. [72] | 2014 | Spain | Cross-sectional study | Pharmacy interviews/questionnaires | High | 7, 9, 14 |
| Abasaeed AE et al. [73] | 2013 | United Arab Emirates | Cross-sectional study | Pharmacy interviews/questionnaires | High | 7, 14 |
| Malik M. et al. [15] | 2013 | Pakistan | Cross-sectional study | Simulated patient method | High | 3, 7, 18 |
| Minzi O et al. [23] | 2013 | Tanzania | Cross-sectional study | Simulated patient method | High | 7, 9, 14 |
| Marković-Peković V et al. al. [74] | 2012 | Republic of Srpska and Herzegovina | Cross-sectional study | Simulated patient method | Medium | 3, 7, 10, 14, 20 |
| Rathnakar U.P. et al. [75] | 2012 | India | Prospective study | Simulated patient method | High | 7, 14, 18 |
| Simó S et al. [76] | 2012 | Spain | Prospective study | Simulated patient method | Medium | 7, 12, 14, 20 |
| Al-Faham Z et al. [77] | 2011 | Syria | Cross-sectional study | Simulated patient method | High | 7, 10, 20 |
| Al-Mohamadi A et al. [78] | 2011 | Saudi Arabia | Cross-sectional study | Simulated patient method | Medium | 7, 9, 14, 18, 20 |
| Puspitasari HP et al. [79] | 2011 | Indonesia | Cross-sectional study | Simulated patient method | High | 7, 14 |
| Hadi U et al. [80] | 2010 | Indonesia | Cross-sectional study | Simulated patient method | High | 7, 14 |
| Llor C et al. [81] | 2010 | Spain | Prospective study | Simulated patient method | Medium | 3, 7, 14, 20 |
| Plachouras D et al. [82] | 2010 | Greece | Quantitative study | Simulated patient method | Medium | 6, 7, 14, 18, 20 |
| Saengcharoen W. et al. [22] | 2010 | Thailand | Cross-sectional study | Simulated patient method | High | 7, 14 |
| Llor C et al. [83] | 2009 | Spain | Prospective study | Simulated patient method | Medium | 3, 7, 14, 20 |
| Rauber C. et al. [84] | 2009 | Brazil | Cross-sectional study | Pharmacy interviews/questionnaires | High | 7, 9, 14 |
| Viberg N et al. [85] | 2009 | Tanzania | Cross-sectional study | Simulated patient method | High | 7, 14, 18 |
| Nyazema N et al. [86] | 2007 | Zimbabwean | Cross-sectional study | Simulated patient method and Pharmacy interviews/questionnaires | High | 7, 13, 14 |
| Caamaño F et al. [87] | 2005 | Spain | Cross-sectional study | Pharmacy interviews/questionnaires | High | 7, 14, 20 |
| Volpato DE et al. [88] | 2005 | Brazil | Cross-sectional study | Simulated patient method | Medium | 7, 9, 10, 20 |
| Caamano Isorna F. et al. [89] | 2004 | Spain | Cross-sectional study | Pharmacy interviews/questionnaires | High | 7, 20 |
| Larson E et al. [90] | 2004 | United States of America | Cross-sectional study | Simulated patient method | Medium | 7, 14, 17, 18, 20 |
| Chalker J et al. [91] | 2002 | Vietnam | Intervention study | Pharmacy interviews/questionnaires | High | 7 |
| Al-Ghamdi MS. [92] | 2001 | Saudi Arabia | Cross-sectional study | Simulated patient method | Medium | 3, 7, 10, 14, 18, 20 |
| Chalker J et al. [16] | 2000 | Vietnam | Cross-sectional study | Simulated patient method | High | 7, 10, 14 |
| Wachter DA et al. [93] | 1999 | Nepal | Cross-sectional study | Simulated patient method | High | 7, 14, 20 |
| Wolffers I. [94] | 1987 | Sri Lanka | Cross-sectional study | Simulated patient method | Low | 3, 5, 7, 9, 10, 11, 12, 14, 18, 20 |
2.4.5. Questions and Advice Made at the Time of Dispensing Antibiotics without a Prescription
2.5. Study Outcomes—Articles that Used the Pharmacy Interviews/Questionnaires Method
2.5.1. Frequency of Antibiotic Dispensation without a Prescription
2.5.2. Name/Class of Antibiotics Most Often Dispensed without a Prescription
2.5.3. Types of Disease/Symptoms Most Commonly Associated with Dispensation without a Prescription
2.5.4. Questions and Advice Made at the Time of Dispensing Antibiotics without a Prescription
2.6. Study Outcomes—Comparison of the Results Obtained by Using the Two Different Methods
| Authors (Year) | Sample Size | Frequency of Antibiotic Dispensation without a Prescription | Name/Class of Antibiotics Most Often Dispensed without a Prescription | Types of Disease/Symptoms Most Commonly Associated with Dispensation without a Prescription | Level of Insistence by Patients and the Percentage of Antibiotic Dispensation without a Prescription at Each Level |
|---|---|---|---|---|---|
| Al-Tannir M. et al. (2020) [17] | 2011: 327 PH | 2011: 77.6%, n = 254 | Sore throat: Amoxicillin/clavulanic acid (n = 32), azithromycin (n = 11) and amoxicillin (n = 9) Acute sinusitis: Amoxicillin/clavulanic acid (n = 22), azithromycin (n = 10), amoxicillin (n = 3), cefaclor (n = 1), ofloxacillin (n = 1) and others (n = 1) Otitis media: Amoxicillin/clavulanic acid (n = 12), amoxicillin (n = 8), cephalexin (n = 4), azithromycin (n = 1), cefaclor (n = 1) and cefixime (n = 1) Acute bronchitis: Amoxicillin/clavulanic acid (n = 15), amoxicillin (n = 9), azithromycin (n = 5) and others (n = 6) Diarrhea: Metronidazole (n = 51), ciprofloxacin (n = 3), cotrimoxazole (n = 1), ofloxacin (n = 1) and others (n = 1) UTI: Ciprofloxacin (n = 39), amoxicillin (n = 2), cefixime (n = 1), clarithromycin (n = 1) and others (n = 1) | Sore throat, acute sinusitis, otitis media, acute bronchitis, diarrhea, and UTI | Three levels of demand: level 1 (Can I have something to relieve my symptoms?), level 2 (Can I have something stronger?), level 3 (I would like to have an antibiotic.) |
| 2018: 327 PH | 2018: 12.5%, n = 41 | Sore throat: Amoxicillin/clavulanic acid (n = 5), azithromycin (n = 1) and amoxicillin (n = 2) Acute sinusitis: Amoxicillin (n = 1) Otitis media: Amoxicillin/clavulanic acid Acute bronchitis: Amoxicillin/clavulanic acid (n = 2), azithromycin (n = 1), amoxicillin (n = 1) Diarrhea: Metronidazole (n = 17) UTI: Ciprofloxacin (n = 4), nitrofurantoin (n = 1), sulfamethoxazole (n = 1) and trimethoprim (n = 1) | Sore throat, acute sinusitis, otitis media, acute bronchitis, diarrhea, and UTI | Three levels of demand: level 1 (Can I have something to relieve my symptoms?), level 2 (Can I have something stronger?), level 3 (I would like to have an antibiotic.). All the obtained antibiotics were dispensed under level 3 | |
| Bahta M. et al. (2020) [27] | 153 V | 87.6% Uncomplicated UTI: 89.2% Acute watery diarrhea: 86.1% | Ciprofloxacin (n = 65, 47.8%), cotrimoxazole (n = 51, 37.5%), amoxicillin (n = 11, 8.1%), doxycycline (n = 5, 3.7%), tinidazole (n = 3, 2.2%) and metronidazole (n = 1, 0.7%). Uncomplicated UTI: Ciprofloxacin (n = 38, 56.7%), cotrimoxazole (n = 14, 20.9%), amoxicillin (n = 11, 16.4%), doxycycline (n = 3, 4.5%) and tinidazole (n = 1, 1.5%) Acute watery diarrhea: Ciprofloxacin (n = 27, 39.1%), cotrimoxazole (n = 37, 53.6%), doxycycline (n = 2, 2.9%), tinidazole (n = 2, 2.9%) and metronidazole (n = 1, 1.4%) | UTI and acute watery diarrhea | Three levels of demand: level 1 (Asked for some drugs to alleviate the symptoms) (81.3%), level 2 (Request for unspecified antibiotics) (11.2%), level 3 (Ask pharmacy attendant for a specific type of antibiotics) (6.7%) |
| Chen J. et al. (2020) [28] | 1106 PH | 83.6%, n = 925 | Penicillins (n = 333, 36.0%), cephalosporins (n = 274, 29.6%), macrolides (n = 250, 27.0%) | Mild upper respiratory tract symptoms (young adult) | Three levels of demand: level 1 (Symptoms only described) (25.2%), level 2 (Asked for antibiotics) (52.1%), level 3 (Asked for penicillin or cephalosporins) (6.3%). |
| Halboup A. et al. (2020) [30] | 1000 PH, 200 each scenario | 73.3%, n = 733 Sore throat: 99.5%, n = 199 Cough: 92%, n = 184 Diarrhea: 75.5%, n = 151 Otitis media: 52%, n = 103 UTI: 48%, n = 96 | Penicillin (48.3%), sulfonamide (12.5%), macrolide (10.6%), fluoroquinolones (8.8%), chloramphenicol (0.3%) | Sore throat, otitis media, cough, diarrhea, and UTI | Three different levels of demand: level 1 (Asked for medications to relieve the symptoms), level 2 (Asked for a stronger medication), level 3 (Asked for an antibiotic) Sore throat: level 1 (n = 195, 98.0%), level 2 (n = 2, 1.0%), level 3 (n = 2, 1.0%) Cough: level 1 (n = 14, 7.6%), level 2 (n = 101, 55.0%), level 3 (n = 69, 37.5%) Diarrhea: level 1 (n = 121, 80%), level 2 (n = 22, 14.5%), level 3 (n = 8, 5.3%) Otitis media: level 1 (n = 78, 75.0%), level 2 (n = 25, 24%), level 3 (n = 1, 1%) UTI Level 1 (n = 75, 78%), level 2 (n = 19, 19.8%), level 3 (n = 2, 2.0%). |
| Shi L. et al. (2020) [31] | 147 PH (Pediatric case: 73; Adult case: 74) | 88.4%, n = 130 Pediatric case: 79.5%, n = 58 Adult case: 97.3%, n = 72 | Pediatric case: Cephalosporin (35.8%), azithromycin (29.6%) and roxithromycin (16.1%) Adult case: Azithromycin (30.0%), cephalosporin (28.0%) and roxithromycin (26.0%) | Pediatric and adult acute cough associated with a common cold | Three levels of demand: level 1 (Client required some medicine for cough) (22.5%), level 2 (Client explicitly expressed the requirement of antibiotics) (60.5%), level 3 (Client specifically required roxithromyci) (5.4%) Pediatric case: level 1 (n = 15, 20.6%), level 2 (n = 39, 53.4%), level 3 (n = 4, 5.5%). Adult case: level 1 (n = 18, 24.3%), level 2 (n = 50, 67.6%), level 3 (n = 4, 5.4%). |
| Wang X. et al. (2020) [32] | 120 PH/V | 73.3%, n = 88 | Norfloxacin (n = 60), gentamicin (n = 13), levofloxacin (n = 8), ciprofloxacin hydrochloride (n = 8), cefotaxime (n = 1), oxytetracycline (n = 1) and trimethoprim (n = 1) | Acute diarrhea | Two levels of demand: level 1 (“Hi, I have suffered from diarrhea since yesterday, please give me some medicine.”) (55%), level 2 (“Hi, I have suffered from diarrhea since yesterday, and I am here to buy antibiotics.”) (91.7%) |
| Abdelaziz AI et al. (2019) [33] | 238 PH (acute bronchitis: 125, common cold: 113) | 98.4% Acute bronchitis: 97.6%, n = 122 Common cold: 99.1%, n = 112 | Amoxicillin: acute bronchitis: 97.6%, common cold: 99.1%. | Acute bronchitis and common cold | |
| Alrasheedy AA. et al. (2019) [34] | 116 PH, 58 each scenario | 92.15% Pharyngitis: 96.6%, n = 56 UTI: 87.7%, n = 50 | Pharyngitis and UTI | Three levels of demand: level 1 (Asked for something to relieve the symptoms), level 2 (Asked for something stronger), level 3 (Simulated patient directly requested an antibiotic) Pharyngitis: level 1 (85.7%), level 2 (5.4%), level 3 (8.9%) UTI: level 1 (74.0%), level 2 (8.0%), level 3 (18.0%) | |
| Chang J. et al. (2019) [7] | 2411 PH, 4822 INT | 59.3% Diarrhoea: 48.5%, n = 1169 URTI: 70.1%, n = 1690 | Amoxicillin and cephalosporins | Paediatric diarrhoea and adult acute URTI | Three levels of demand: level 1 (“Can you give me some medicine to alleviate the patient’s symptoms?”), level 2 (“Can you give me some antibiotics?”), level 3 (“I would like some amoxicillin or cephalosporins.”) Diarrhoea: level 1 (n = 142, 5.9%), level 2 (n = 685, 28.4%), level 3 (n = 342, 14.2%) URTI: level 1 (n = 321, 13.3%), level 2 (n = 888, 36.8%) and level 3 (n = 481, 20.0%) |
| Damisie G et al. (2019) [35] | 18 DS | 94.4%, n = 17 Sore throat: 77.8%, n = 14 Acute diarrhea: 88.9%, n = 16 UTI: 94.4%, n = 17 | Sore throat: Amoxicillin (n = 10, 71.4%), azithromycin (n = 3, 21.4%), amoxicillin/azithromycin (n = 1, 7.2%) Acute diarrhea: Metronidazole (n = 8, 50.0%), rifampicin (n = 4, 25.0%), tinidazole (n = 2, 12.5%) and ciprofloxacillin/tinidazole/metronidazole (n = 2, 12.5%) UTI: Ciprofloxacin (38.90%), norfloxacin (33.30%), cotrimoxazole (16.70%) and amoxicillin (5.60%) | Sore throat, acute diarrhea, and UTI | Three levels of demand: level 1 (Asking something to alleviate his/her symptoms), level 2 (Asking for a stronger medication), level 3 (Clear request for an antibiotic in the case of not achieving the previous two levels of demand) Sore throat: level 1 (n = 14, 100%), level 2, level 3 (0%) Acute diarrhea: level 1 (n = 11, 68.75%), level 2 (n = 3, 18.75%), level 3 (n = 2, 12.5%) UTI: level 1 (n = 16, 94.1%), level 2 (n = 1, 5.9%), level 3 (0%) |
| Koji EM et al. (2019) [37] | 262 PH | 63.4%, n = 166 | Amoxicillin, amoxicillin-clavulanate, azithromycin, trimethoprim/sulfamethoxazole, metronidazole, ceftriaxone, cloxacillin, vancomycin, ampicillin, cefotaxime, gentamicin | Common cold, acute onset diarrhea, pneumonia (child), meningitis (child) | |
| Mengistu G et al. (2019) [11] a | 105 PH | 86.7% | Cotrimoxazole (97.8%) | Acute watery diarrhea (child) | |
| Nafade V. et al. (2019) [19] | 279 PH, 1522 INT (761 each scenario) | 4.0% Adult cases: 4.3%, n = 33 Child cases: 2.9%, n = 22 | Children Fever: Bromhexine (n = 1), cetirizine (n = 1), chlorpheniramine (n = 7), levocetirizine (n = 1), montelukast (n = 1) URTI: Bromhexine (n = 8), cetirizine (n = 11), chlorpheniramine (n = 45), dextromethorphan (n = 45), levocetirizine (n = 2), levosalbutamol (n = 2), montelukast (n = 1), salbutamol (n = 2), terbutaline (n = 1) Diarrhoea: Loperamide (n = 23) AdultsFever: Bromhexine (n = 9), cetirizine (n = 65), chlorpheniramine (n = 53), dextromethorphan (n = 4), levocetirizine (n = 3), nimesulide (n = 23) URTI: Bromhexine (n = 109), cetirizine (n = 8), chlorpheniramine (n = 115), dextromethorphan (n = 62), levocetirizine (n = 13), montelukast (n = 5), salbutamol (n = 1), terbutaline (n = 26) Diarrhoea: Domperidone (n = 2), loperamide (n = 227), omeprazole (n = 3), ranitidine (n = 2) | URTI, uncomplicated acute diarrhoea, and acute febrile illness suggestive of malaria | |
| Zawahir S et al. (2019) [38] | 242 PH | 41.0%, n = 99 Sore throat: 43.0% Common cold: 15.0% Diarrhoea: 50.0% UTI: 55.0% | Sore throat: Erythromycin (n = 17, 65%), azithromycin (n = 7, 27%;), ciprofloxacin (n = 1, 4%), amoxicillin (n = 1, 4%) Common cold: Amoxicillin (89%; n = 8) and others (n = 1, 11%) Diarrhoea: Metronidazole (n = 23, 79%), ciprofloxacin (n = 2, 7%), erythromycin (n = 2, 7%), azithromycin (n = 1, 3%) and others (n = 1, 3%). UTI: Ciprofloxacin (n = 26, 76%), norfloxacin (n = 5, 15%), others (n = 3, 9%) | Viral infections: acute sore throat, common cold (child), and acute diarrhoea and bacterial uncomplicated UTI | Three levels of demand: level 1 (Can I get some medicine to alleviate the symptoms?) (n = 39, 16%), level 2 (Can I get something stronger?) (n = 33, 14%), level 3 (I would like an antibiotic.) (n = 27, 11%). Sore throat: level 1 (n = 11, 18%), level 2 (n = 7, 12%), level 3 (n = 8, 13%) Common cold: level 1 (n = 1, 2%), level 2 (n = 6, 10%), level 3 (n = 2, 3%) Diarrhoea: level 1 (n = 9, 15%), level 2 (n = 11, 18%), level 3 (n = 10, 17%) UTI: level 1 (n = 18, 29%), level 2 (n = 9, 15%), level 3 (n = 7, 11%) |
| Erku D.A. et al. (2018) [43] | 50 CMRO, 100 V | 86.0%, n = 86 Childhood diarrhea: n = 40 (Pharmacy: (75%, n = 21/28); drug store: 86.4%, n = 19/22); URTI: n = 46 (Pharmacy: 85.7%, n = 24/28; drug store: 100%, n = 22/22) | Childhood diarrhea: Cotrimoxazole (n = 11), metronidazole (n = 15) URTI: Amoxicillin (n = 23), amoxicillin-clavulanic acid (n = 19), azithromycin (n = 15), ciprofloxacin (n = 5), cephalexin (n = 1), cefexime (n = 1), levofloxacin (n = 3) | Acute childhood diarrhea and URTI | |
| Horumpende PG et al. (2018) [20] b | 82 PH (26 class I, 56 class II) | 92.3% Fever: 100.0% Diarrhoea: 100.0% Runny nose: 100.0% Painful urination: 88.8% Cough: 75.0% Pharmacies class I: 44.4%; Pharmacies class II: 56.0%; | Amoxycillin (n = 1), ampiclox (n = 3), trimethoprim/sulphamethoxazole (n = 2), cefixime (n = 1), amoxyclav, (n = 1), azithromycin (n = 4), erythromycin (n = 1), metronidazole | Fever, diarrhoea, runny nose, painful urination, and cough | |
| Ibrahim IR et al. (2018) [12] a | 75 PH | 20.0%, n = 15 | Metronidazole (n = 4, 5.3%), furazolidone (n = 3, 4.0%) | Acute diarrhea | |
| Mohamed Ibrahim M.I. et al. (2018) [44] | 25 PH (common cold: 15 PH, 30 V; allergic rhinitis: 10 PH, 20 V) | 5.0% Common cold: 5.0% and Allergic rhinitis: 5.0% | Common cold (signs and symptoms: sore throat, slight cough, tiredness and body aches), and allergic rhinitis (signs and symptoms: running nose or congestion, sneezing, slight sore throat with phlegm and slight cough when in bed) | ||
| Zapata-Cachafeiro M et al. (2018) [48] | 977 PH | 18.8%, n = 184 | Amoxicillin (n = 127, 69.0%), amoxicillin-clavulanic acid (7%), azithromycin (n = 42, 22.8%), cotrimoxazole (n = 7, 3.8%), moxifloxacin (n = 4, 2.2%), cefuroxime (n = 2, 1.1%), clarithromycin (n = 1, 0.5%) and clindamycin (n = 1, 0.5%) | Sore throat, difficulty swallowing, and feeling feverish, in addition to congestion and cough | Four levels of demand: level 1 (Request for medication to relieve the symptoms) (2.97%), level 2 (Request for a stronger medication than that offered) (4.22%), level 3 (Request for an antibiotics) (4.52%), level 4 (Specific request for amoxicillin) (8.54%) |
| Zawahir S et al. (2018) [49] | 242 PH | 61.0%, n = 147 | Ciprofloxacin (n = 44, 70%), erythromycin, metronidazole, amoxicillin (n = 32, 52%) | URTI (adult and child), watery diarrhoea and UTI | |
| Chang J. et al. (2017) [52] | 256 PH | 66.8% Paediatric diarrhoea: 55.9%, n = 143 Adult acute URTI: 77.7%, n = 199 | Paediatric diarrhoea and adult acute URTI | Three levels of demand: level 1 (“Can you give me some drugs to alleviate the symptoms of the disease?”), level 2 (“Can you give me some antibiotics?”), level 3 (“I would like some amoxicillin or cefaclor.”) Paediatric diarrhoea: Level 1 (9%), level 2 (37.5%), level 3 (9.4%) Acute URTI: level 1 (26.2%), level 2 (43.0%), level 3 (8.6%) | |
| Jaisue S. et al. (2017) [21] b | 91 class I PH | 68.1%, n = 62 | Furazolidone (n = 31, 34.1%), nifuroxazide (n = 17, 18.7%), cotrimoxazole (n = 10, 11.0%), metronidazole (n = 2, 2.2%), cephalexin (n = 2, 2.2%), azithromycin (n = 1, 1.1%), norfloxacin (n = 1, 1.1%) | Non-infectious diarrhoea in a 14-month-old child | |
| Marković-Peković V et al. (2017) [18] | 2010: 131 PH | 2010: 58.0%, n = 76 | 2010: Amoxicillin (n = 65, 85.5%), ampicillin (n = 5, 6.6%), cephalexin (n = 2, 2.6%) and doxycycline (n = 4, 5.3%) | URTI | |
| 2015: 383 PH | 2015: 18.5%, n = 71 | 2015: Amoxicillin (n = 57, 80.3%), ampicillin (n = 9, 12.7%), cephalexin (n = 3, 4.2%), azithromycin (n = 1, 1.4%) and amoxicillin and enzyme inhibitor (n = 1, 1.4%) | URTI | ||
| Okuyan B. et al. (2017) [54] | 70 PH | Antibiotic + NSAIDs (n = 15, 21.4%) Antibiotic alone (n = 17, 24.2%) | Cefuroxime alone (n = 5), cefuroxime with other medication (n = 6), amoxicillin-clavulanic acid alone (n = 12), amoxicillin-clavulanic acid with other medication (n = 9) | Acute uncomplicated rhinosinusitis | |
| Abegaz T.M. et al. (2016) [14] a | 113 PH | 51.3%, n = 58 | Cotrimoxazole and metronidazole | Acute diarrhea | |
| Guinovart MC et al. (2016) [57] | 220 PH | 54.1%, n = 119 | β-lactam antibiotic and amoxicillin-clavulanic acid | UTI, sore throat, and acute bronchitis | Four levels of demand: level 1 (Medication to treat the symptoms was required), level 2 (A stronger medication was required), level 3 (An antibiotic was required), level 4 (A specific antibiotic was required: amoxicillin/clavulanic acid for a UTI and amoxicillin for a sore throat and acute bronchitis) |
| Ibrahim M.I.B.M. et al. (2016) [13] a | 30 PH 60 INT, 95 DD | 43.2%, n = 41 | Nifuroxazide (n = 21, 22.11%), metronidazole alone (n = 12, 12.63%), metronidazole with other medication (n = 4, 4.21%), tinidazole (n = 2, 2.11%), furazolidone (n = 1, 1.05%) | Acute gastroenteritis | |
| Satyanarayana S. et al. (2016) [61] | 622 PH, 1200 V | 27.0%, n = 319 | Presumed tuberculosis: Amoxicillin (n = 100), ofloxacin (n = 25), ciprofloxacin (n = 24), azithromycin (n = 23), cefixime (n = 19), levofloxacin (n = 14), ampicillin (n = 8), roxithromycin (n = 8), cloxacillin (n = 6), erythromycin (n = 6) Tuberculosis case with positive sputum report: Amoxicillin (n = 50), azithromycin (n = 16), cefixime (n = 11), levofloxacin (n = 7), ofloxacin (n = 7), ciprofloxacin (n = 5) | Tuberculosis | |
| Almaaytah A et al. (2015) [62] | 202 PH (Sore throat: 41, otitis media: 38, acute sinusitis: 39, diarrhea: 42, UTI: 42) | 74.3%, n = 150 Sore throat: 97.6%, n = 40 Otitis media: 68.4%, n = 26 Acute sinusitis: 48.5%, n = 15 Diarrhea: 81.0%, n = 34 UTI: 83.3%, n = 35 | Sore throat: Penicillins (45%), penicillin/penicillinase inhibitor (32.5%), first-Gen cephalosporins (10%) Otitis media: choramphenicol (19.2%), penicillins (15.4%), macrolides (11.5%) Acute sinusitis: Penicillins (20%), fluoroquinolones (20%), macrolides (20%) Diarrhea: antiprotozoals (79.4%), sulfonamides (5.9%), fluoroquinolones (2.9%) UTI: fluoroquinolones (80%), third-Gen cephalosporins (5.7%) | Sore throat, otitis media, acute sinusitis, diarrhea, and UTI | Three levels of demand: level 1 (Asking for something to alleviate the symptoms) (n = 121, 59.9%), level 2 (Asking for a stronger medication) (n = 4, 2%), level 3 (Clear request for an antibiotic) (n = 25, 12.4%) Sore throat: level 1 (n = 39, 95.1%), level 2 (n = 0), level 3 (n = 1, 2.4%) Otitis media: level 1 (n = 25, 65.8%), level 2 (n = 0), level 3 (n = 1, 2.6%) Acute sinusitis: level 1 (n = 3, 7.7%), level 2 (n = 4, 10.3%), level 3 (n = 8, 20.5%) Diarrhea: level 1 (n = 24, 57.1%), level 2 (n = 0) and level 3 (n = 10, 23.8%) UTI: level 1 (n = 30, 71.4%), level 2 (n = 0), level 3 (n = 5, 11.9%) |
| Shet A et al. (2015) [65] | 261 PH URTI: 115 PH Acute gastroenteritis (child): 146 PH | 66.7%, n = 174 URTI: 71.3%, n = 82; Acute gastroenteritis (child): 63.0%, n = 92 | URTI: Amoxicillin (n = 42, 51.2 %), amoxicillin-clavulanate (n = 2, 2.4%), ampicillin-cloxacillin (n = 4, 4.9%), cephalexin (n = 2, 2.4%), cefixime (n = 2, 2.4%), azithromycin (n = 10, 12.2%), roxithromycin (n = 3, 3.7%), ciprofloxacin (n = 10, 12.2%), levofloxacin (n = 3, 3.7%) and ofloxacin (alone) (n = 4, 4.9%) Acute gastroenteritis (child): Ofloxacin (alone) (n = 7, 7.6%), norflaxacin (alone) (n = 8, 8.7%), norfloxacin + metronidazole (n = 38, 41.3%), ofloxacin + metronidazole (n = 9, 9.8%), ofloxacin + ornidazole (n = 7, 7.6%), metronidazole (alone) (n = 14, 15.2%) and furazolidone (n = 9, 9.8%) | URTI (adult) and acute gastroenteritis (child) | Two levels of demand: level 1 (Request for a “medicine” to alleviate the described symptoms) (55.6%), level 2 (Specifically asked for a “stronger” medicine) (44.4%) URTI infection: level 1 (53.9%), level 2 (17.4%) Acute gastroenteritis (child): level 1 (56.8%), level 2 (6.2%) |
| Alabid A.H.M.A. et al. (2014) [67] | 50 PH/Ph, 100 V | 32.0%, n = 32 | Amoxil and Amoxiclav (n = 11, 11.0%), erythromycin (n = 9, 9.0%), cefalexin (n = 4, 4.0%) | Common cold symptoms (symptoms of URTI) | |
| Malik M. et al. (2013) [15] a | 238 PH/V | Simulated patients were treated in 198 V (83.1%); antibiotics were given in 69 V (34.4%) | Uncomplicated malaria fever | ||
| Minzi O et al. (2013) [23] | 85 ADDO and 60 DLDB | 67.0% ADDO: 79.0% DLDB: 55.0% | Ciprofloxacin, amoxicillin, ampicillin, chloramphenicol, procaine penicillin, tetracycline | Cough, headache and diarrhea (“typhoid”, child), injured on the left hip by a piece of metal, fever and diarrhea (“cholera”, child), vomiting and diarrhea (“typhoid”, child), cough (“Pneumonia”), UTI, complaining of yellowish urethral discharge with a bad smell (“gonorrhea”) | |
| Marković-Peković V et al. (2012) [74] | 131 PH | 58.0%, 76 pharmacies | Amoxicillin (85%), doxycyline (5%), ampicillin (7%), and cefalexin (3%) | URTI | Without insistence in case of refusal |
| Rathnakar U.P. et al. (2012) [75] | 60 PH, 20 for each scenario | 51.7%, n = 31 | Amoxicillin (n = 19, 31.7%), erythromycin (n = 1, 1.7%), ampicillin+cloxacillin (n = 1, 1.7%), azithromycin (n = 4, 6.7%) | URTI, acute bronchitis, and diarrhoea | Two levels of demand: Level 1 (Can I have something for my symptoms?) and level 2 (I would like an antimicrobial agents.) URTI: level 1 (35%), levels 2 and 3 (40%) Acute bronchitis: level 1 (20%), level 2 and level 3 (30%) Diarrhoea: level 1 (20%), levels 2 and 3 (10%) |
| Simó S et al. (2012) [76] | 50 PH | 8.0%, n = 4 | Amoxicillin/clavulanic acid (n = 4, 8%) | URTI symptoms and fever | |
| Al-Faham Z et al. (2011) [77] | 200 PH | 97.0%, n = 194 | Amoxicillin/clavulanic acid 1000 mg (n = 73, 37.6%); amoxicillin (n = 45, 23.1%); amoxicillin/clavulanic acid 625 mg (n = 25, 12.8%); amoxicillin/floxacillin (n = 13, 6.7%), cefodroxil (n = 13, 6.7%), clarithromycin (n = 6, 3.0%), azithromycin (n = 5, 2.5%), ciproflaxacillin (n = 4, 2.0%), Cloxacillin/Ampicillin (n = 4, 2.0%), cefixime (n = 2, 1.0%), cefprodoxime 100mg (n = 2, 1.0%) and cefprodoxime 200mg (n = 2, 1.0%) | Sinusitis (fever, runny nose with clear secretion and a headache in the frontal sinus region) | Two levels of demand: level 1 (without insistence) (n = 174, 87%), level 2 (with insistence) (n = 20, 10%) |
| Al-Mohamadi A et al. (2011) [78] | 60 PH/Ph | 97.9% | Co-amoxiclav (Augmentin), amoxicillin-clavulanic acid, cefaclor | Sore throat | |
| Puspitasari HP et al. (2011) [79] | 264 PH/V, 88 for each scenario | 91.0%, n = 80 | Ciprofloxacin (n = 80, 91%), tetracycline (n = 80, 91%), amoxicillin (n = 74, 84%) | “discomfort on urination”, infected leg wounds and “productive cough, rainy nose, fever and lost of appetite” | |
| Hadi U et al. (2010) [80] | 104 MRO (75 PH, 28 K, 1 DS) | 75.9%, n = 79 | Amoxicillin (n = 15), chloramphenicol (n = 18), ciprofloxacin (n = 14), cotrimoxazole (n = 14), tetracycline 250 mg (n = 15), tetracycline 500 mg (n = 2) | ||
| Llor C et al. (2010) [81] | 197 PH (sore throat: 69, acute bronchitis: 59, UTI: 69) | 45.2%, n = 89 Sore throat: 34.8%, n = 24 Acute bronchitis: 16.9%, n = 10 UTI: 79.7%, n = 55 | Sore throat, acute bronchitis, and UTI | Three levels of demand: level 1 (Asked for something to alleviate the symptoms of the infection), level 2 (“This medication is not very strong, can’t you give me something stronger?”), level 3 (Asking openly for an antibiotic) | |
| Plachouras D et al. (2010) [82] | 174 PH/V (ciprofloxacin: 102, amoxicillin + clavulanic acid: 72) | 72.4%, n = 126 Ciprofloxacin: 53.0%, n = 54 Amoxicillin + clavulanic acid: 100.0%, n = 72 | Ciprofloxacin (n = 54, 53%), amoxicillin/clavulanic acid (n = 72, 100%) | Without insistence in case of refusal | |
| Saengcharoen W. et al. (2010) [22] b | 115 PH (type I: 96 and type II: 19) | Antibiotics + ORS I (non-Ph + Ph): 26.0%, n = 25 I (non-Ph): 30.3%, n = 10 II: 21.1%, n = 4 Antibiotics only I (non-Ph + Ph): 21.9%, n = 21 I (non-Ph): 27.3%, n = 9 II: 26.3%, n = 5 Antibiotics+ combined drugs I (non-Ph + Ph): 1.0%, n = 1 I (non-Ph): 3.0%, n = 1 II: n = 1, 5.3% | Pharmacy personnel: Nifuroxazide, cotrimoxazole, norfloxacin, erythromycin and amoxicillin | Acute childhood diarrhoea | |
| Llor C et al. (2009) [83] | 197 PH (sore throat: 69, acute bronchitis: 59, UTI: 69) | 45.2%, n = 89 Sore throat: 34.8%, n = 24 Acute bronchitis: 16.9%, n = 10 UTI: 79.7%, n = 55 | Sore throat: Amoxicillin (n = 21, 87.5%), amoxicillin/clavulanic acid (n = 2, 8.3%), azithromycin (n = 1) Acute bronchitis: Amoxicillin (n = 10) UTI: Norfloxacin (n = 22, 40.0%), fosfomycin trometamol (n = 20, 36.4%), pipemidic acid (n = 8, 14.5%) | Sore throat, acute bronchitis, and uncomplicated UTI | Three levels of demand: level 1 (“Can you give me something to alleviate the symptoms of the infection?”) (n = 65, 33.0%), level 2 (“Can’t you give me something stronger?”) (n = 17, 8.6%), level 3 (“I would like an antibiotic.”) (n = 7, 3.6%) Sore throat: level 1 (n = 12, 17.4%), level 2 (n = 10, 14.5%), level 3 (n = 2, 2.9%) Acute bronchitis: level 1 (n = 1, 1.7%), level 2 (n = 5, 8.5%), level 3 (n = 4, 6.8%) UTI: level 1 (n = 52, 75.4%), level 2 (n = 2, 2.9%), level 3 (n = 1, 1.4%) |
| Viberg N et al. (2009) [85] | SCM-female: 144 V and SCM-male: 107 V | 55.5% SCM-female: 35.0% SCM-male: 76.0% | SCM-female: Doxycycline (n = 23), amoxicillin (n = 5), sulfamethoxazole/trimethoprim (n = 13), erythromycin (n = 1), ciprofloxacin (n = 15), metronidazole (n = 16), nitrofurantoin (n = 1) SCM-male: Doxyxycline (n = 40), amoxicillin (n = 3), phenoxymethylpenicillin (n = 2), sulfamethoxazole/trimethoprim (n = 13), erythromycin (n = 1), ciprofloxacin (n = 30), metronidazole (n = 12), nitrofurantoin (n = 1) | Abnormal vaginal discharge and itching (SCM-female), urethral discharge (SCM-male) | |
| Nyazema N et al. (2007) [86] | STI female: 57 V STI male: 63 V Acute diarrhoea: 68 V | 8.0% STI female: 7.0% STI male: 8.0% Acute diarrhoea: 9.0% | Vaginal discharge and itching (STI female), urethral discharge (STI male) and acute diarrhoea (child) | ||
| Volpato DE et al. (2005) [88] | 107 PH | 74.0% | Amoxicillin (n = 46, 74%), azythromycin (n = 6, 9.6%), sulfamethoxazole/trimethoprim (n = 5, 8.1%), cephalexin (n = 2, 3.2%), erythromycin (n = 2, 3.2%) and ampicillin (n = 1, 1.6%) | Acute and uncomplicated rhino-sinusitis | Three levels of demand: No insistence (58%), insisting once (13%) or twice (3%) when the antibiotic was denied. |
| Larson E et al. (2004) [90] | 101 DS (PHN: 34, PBNHN: 37, PWNHN:30) | 50.0% PHN:100.0%, n = 34 PBNHN and PWNHN: 0.0% | Ampicillin (n = 26, 76.5%), ampicillin and tetracycline (n = 2, 5.9%), ampicillin and erythromycin; amoxicillin (n = 2, 5.9%), erythromycin (n = 1, 2.9%) | Sore throat | |
| Al-Ghamdi MS. (2001) [92] | 88 PH | 82.0%, n = 72 | Fluoroquinolones (First choice: n = 50, 69% and Second choice: n = 59, 87%), cotrimoxazole (First choice: n = 9, 13% and Second choice: n = 3, 4%), penicillins (First choice: n = 8, 11% and Second choice: n = 3, 4%), cephalosporins (First choice: n = 3, 4% and Second choice: n = 1, 2%), tetracyclins (First choice: n = 2, 3% and Second choice: n = 2, 3%) | Uncomplicated lower, UTI | |
| Chalker J et al. (2000) [16] a | 60 PH, 297 V | 81.5%, n = 242 | Tetracyclines (n = 36), amphenicols (n = 14), β-lactam antibacterials- Penicillins (n = 10), other β- lactam antibacterials (16), sulphonamides/trimethoprim (n = 6), macrolides and lincosamides (n = 15), quinolones (n = 188), metronidazole (n = 3), spectinomycin (n = 4), drugs for treatment of TB (n = 1) | STD | |
| Wachter DA et al. (1999) [93] | 100 PH | 67.5% Dysuria: 38% Diarrhoea: 97% | Dysuria: Norfloxacin (28%), amoxicillin (5%), trimethaprim/sulphamethoxazole (2%), nalidixic acid (2%), ciprofloxacin (1%) Diarrhoea: Metronidazole (62%), metronidazole/diloxanide furoate (24%), metronidazole/nalidixic acid (comb) (9%), metronidazole/nalidixic acid (separate) (2%) | Dysuria and acute watery diarrhoea (child) | |
| Wolffers I. (1987) [94] | 28 PH | 100.0% | Tetracyclin (100%) |
| Authors (Year) | Sample Size | Frequency of Antibiotic Dispensation without a Prescription | Name/Class of Antibiotics Most Often Dispensed without a Prescription | Types of Disease/Symptoms Most Commonly Associated with Dispensation without a Prescription |
|---|---|---|---|---|
| Abubakar U. et al. (2020) [24] | 98 Ph | PD: 74.5% (n = 73) PSD: 67.3% (n = 66) | Penicillin (n = 84, 85.7%), tetracycline (n = 69, 70.4%), cephalosporin (n = 63, 64.3%), quinolone (n = 61, 62.2%), macrolides (n = 54, 55.1%), sulphonamides (n = 47, 48.0%), aminoglycosides (n = 34, 34.7%), carbapenems (n = 13, 13.3%. | UTI, typhoid fever, genital infections (gonorrhea), wound infections, eye infections, ear infections, diarrhea, malaria, toothache, and cold/flu |
| Abubakar U. (2020) [25] | 98 Ph | PD: 96.9% (n = 95) PSD: 60.2% (n = 59) | ||
| Badro D.A. et al. (2020) [26] | 250 Ph | 88.0% acknowledged dispensing medications without a prescription, those medications included antibiotics (60.0%) | ||
| Gajdács M. et al. (2020) [29] | 192 Ph | PD: 26.0% | ||
| Alrasheedy AA. et al. (2019) [34] | 116 PH | 70.7%, n = 82 | ||
| Hallit S et al. (2019) [36] | 280 PH, 202 Ph | 84.6% | Pharyngitis, otitis media, diarrhoea, and vomiting (child) | |
| Mengistu G et al. (2019) [11] a | 105 PH/PS | 50.5% | Acute watery diarrhea (child) | |
| Zawahir S. et al. (2019) [39] | 265 PS | 31.7%, n = 84 Non-Ph: 33.3%, n = 18; Ph: 31.6%, n = 66 | Acute sore throat, common cold, acute diarrhoea, wound infection, or uncomplicated UTI | |
| Ajie A.A.D. et al. (2018) [40] | 190 PH | PD: 92.1% (n = 175) | Amoxicillin (n = 175, 92.1%), cotrimoxazole (n = 175, 92.1% and ciprofloxacin (n = 159, 83.7%) | |
| Alhomoud F et al. (2018) [41] | 20 Ph | 100.0% | Amoxicillin/clavulanic Acid (Augmentin), amoxicillin and azithromycin. | Fever, sore throat, cold/flu, and cough. |
| Awosan KJ et al. (2018) [42] | 197 PS | PD: 91.9% (n = 181) PSD: 10.2% (n = 20) | ||
| Paes M.R. et al. (2018) [45] | 101 Ph | Dispensing without a prescription was 63.4% of the total dispensing encounters, those medications included antibiotics (5.8%) | ||
| Rehman IU et al. (2018) [46] | 181 Ph | PD: 68.0% (n = 123) PSD: 20.4% (n = 37) | ||
| Sarwar M.R. et al. (2018) [47] | 400 Ph | PD: 93.7% (n = 375) PSD: 34.5% (n = 138) | ||
| Ansari M. (2017) [50] | 16 PH | 66.5% | Cephalosporins, penicillins, and macrolides | Respiratory tract complications (e.g., cough), fever, and UTI |
| Barker A.K. et al. (2017) [51] | 24 PS | 100.0% | Colds, viral infections, coughs, and sore throat | |
| Mansour O et al. (2017) [53] | 173 PH | 85.5% | Tonsillitis | |
| Abood EA et al. (2016) [55] | 170 Ph | 25.3%, n = 43 | Amoxicillin (7.3%) | |
| Erku D.A. (2016) [56] | 389 Ph | PD: 90.2% PSD: 5.4% (n = 21) | ||
| Kalungia AC et al. (2016) [58] | 73 PH | 100% | Amoxicillin (n = 38, 52.1%), cotrimoxazole (n = 18, 24.7%), metronidazole (n = 17, 23.3%) | |
| Khan M.U. et al. (2016) [59] | 188 Ph | PD: 63.3% (n = 119) PSD: 5.3% (n = 10) | ||
| Nawab A. Et al. (2016) [60] | 50 PH | 12.2% of 100 drugs dispensed without a prescription | Metronidazole and amoxicillin/clavulanate potassium | |
| Bahnassi A. (2015) [63] | 147 Ph | 100.0% | Amoxicillin, amoxicillin/clavulanic acid, cephalexin | Sore throat and UTI |
| Dorj G. et al. (2015) [64] | 61 PS | PSD: 21.7% (n = 13) PD’:35.0% | Aminopenicillins, oral (n = 73, 29.9%); aminopenicillins, injection (n = 44, 24.0%); quinolone, oral (n = 30, 24.6%); quinolone, injection (n = 13, 21.3%); cefalosporin, oral (n = 14, 23.0%); cefalosporin, injection (n = 10, 16.4%); macrolides, oral (n = 53, 29.0%); macrolides, injection (n = 29, 15.8%); tetracycline, oral (n = 19, 15.6%) and sulfonamid, oral (n = 18, 29.5%). | Mild/moderate community-acquired pneumonia |
| Shreya Svitlana A. et al. (2015) [66] | 100 PH | 100.0% | Cefodoxime (n = 52), amoxicillin (n = 30), doxycycline-doxy (n = 8), cefixime-taxim (n = 10) | Mild toothache |
| Bahnassi A. (2014) [68] | 54 Ph | 100.0% | Amoxicillin, amoxicillin/clavulanic acid, azithromycin | Sore throat, sinusitis (pregnant), UTI, ear infection (child), and skin infection |
| Farah R et al. (2014) [69] | 100 Ph | 32.0% | Gastrointestinal symptoms, Genito-urinary symptoms, and Respiratory symptoms | |
| Gastelurrutia M.A. et al. (2014) [70] | 152 PH | 9.8% of the total number of antibiotics dispensed | ||
| Sabry NA et al. (2014) [71] | 36 PH, 1158 INT | 36.4% Upon pharmacist’s recommendation: 13.1%, n = 152 Upon patient´s request: 23.3%, n = 270 | Upon pharmacist´s recommendation: Amoxicillin/fluoxacillin (n = 10, 6.58%), ampicillin/sulbactam (n = 10, 6.58%), amoxicillin (n = 8, 5.26%), co-amoxiclav (n = 11, 7.24%), cephalexin (n = 21, 13.82%), cephradine (n = 10, 6.58%), cefaclor (n = 7, 4.61%), cefuroxime (n = 5, 3.29%), cefoperazone (n = 8, 5.26%), cefotaxime (n = 7, 4.61%), ceftriaxone (n = 7, 4.61%), oxycycline (n = 7, 4.61%), tetracycline (n = 3, 1.97%), clarithromycin (n = 4, 2.63%), clindamycin (n = 9, 5.92%), co-trimoxazole (n = 5, 3.29%), ciprofluxacin (n = 7, 4.61%), gatifluxacin (n = 8, 5.26%) and moxifloxacin (n = 5, 3.29%). Upon patient´s request: Amoxicillin/fluoxacillin (n = 28, 10.37%), co-amoxiclav (n = 24, 8.89%), amoxycillin (n = 44, 16.30%), azatreonam (n = 4, 1.48%), doxycycline (n = 20, 7.40%), tetracyclin (n = 4, 1.48%), cephadrin (n = 13, 4.81%), cephalexin (n = 3, 1.11%), cefadroxil (n = 12, 4.44%), cefuraxime (n = 2, 0.74%), cefixime (n = 10, 3.70%), cefotaxime (n = 4, 1.48), ceftriaxone (n = 4, 1.48), azithromycin (n = 12, 4.44%), spiramycin (n = 8, 2.96%), roxithromycin (n = 4, 1.48%), erythromycin (n = 8, 2.96%), clindamycin (n = 8, 2.96%), co-trimoxazole (n = 6, 2.22%), ciprofloxacin (n = 6, 2.22%), sparfloxacin (n = 5, 1.85%), moxifloxacin (n = 5, 1.85%), levofloxacin (n = 8, 2.96%), ofloxacin (n = 8, 2.96%), entamycin (n = 4, 1.48%), chloramphenicol (n = 3, 1.11%), fusidic acid (n = 8, 2.96%), neomycin/bacitracin (n = 5, 1.85%). | Upon pharmacist´s recommendation: UTI (n = 25, 17.86%), sore throat (n = 24, 17.10%), cold & flu (n = 16, 11.40%), toothache (n = 13, 9.29%), infected wound (n = 11, 7.86%), rhinitis (n = 8, 5.70%), acne (n = 8, 5.70%), abdominal cramps (n = 7, 5.00%), tonsillitis (n = 5, 3.57%), post nasal discharges (n = 4, 2.90%), burning (n = 4, 2.90%), asthma (n = 4, 2.90%), food poisoning (n = 4, 2.90%), stomachache (n = 4, 2.90%) and fracture (n = 3, 2.14%). Upon patient´s request: Fever (n = 36, 15.52%), sore throat (n = 25, 10.78%), tonsillitis (n = 19, 8.19%), gingivitis (n = 16, 6.9%), UTI (n = 12, 5.17%), ear ache & inflammation (n = 12, 5.17%), acne (n = 9, 3.88%), wound infection (n = 8, 3.45%), toothache (n = 8, 3.45%), headache (n = 7, 3.02%), urticaria (n = 7, 3.02%), vomiting (n = 6, 2.59%), food poison (n = 6, 2.59%), otitis (n = 6, 2.59%), flu (n = 5, 2.16%), vaginites (n = 5, 2.16%), sneezing (n = 4, 1.72%), sinusitis (n = 4, 1.72%), cough (n = 4, 1.72%), difficulty in breathing (n = 4, 1.72%), skin infection (n = 4, 1.72%), dandruff (n = 4, 1.72%), endometritis (n = 4, 1.72%), prostatitis (n = 4, 1.72%), dizziness (n = 4, 1.72%), nones/joint infection (n = 4, 1.72%), abortion (n = 3, 1.29%), and irritation (n = 2, 0.86%). |
| Zapata-Cachafeiro M. et al. (2014) [72] | 286 Ph | 64.7%, n = 185 | Urinary and dental infections | |
| Abasaeed AE et al. (2013) [73] | 20 Ph, 1645 INT | 26.4% | Ceftriaxone (53.3%), amoxicillin (47.8%), and co-amoxiclav (33.6%) | Cough, influenza, respiratory tract infections, STD, and Helicobacter pylori |
| Rauber C. et al. (2009) [84] | 46 Ph | 85.0% | Diseases: Throat infection, UTI, ear infection, sinusitis, pharyngitis, upper airway infection, pneumonia, fever, dental infection, throat plaque, clear and simple infection, skin infection, intestinal infection, cough with secretion, oral infection, and acne. Symptoms: High fever, formation of throat plaques, pus, pain, sore throat, sinusitis, headache, edema, non-effective anti-inflammatory, intense redness, severe cramps, diarrhea, inflammation, symptoms for more than seven days, and mucus. | |
| Nyazema N et al. (2007) [86] | 59 PH, 73 PS | PD: 31.0% | Amoxicillin (77%), cotrimoxazole (60%), erythromycin (30%), doxycycline (48%) | |
| Caamaño F et al. (2005) [87] | 123 PH, 164 Ph | 65.9% | Clamoxyl® (Amoxicillin) | |
| Caamano Isorna F. et al. (2004) [89] | 123 PH, 164 Ph | 65.9% | Clamoxyl® (Amoxicillin) | |
| Chalker J et al. (2002) [91] | 44 PH (22 control and 22 intervention) | 51.0% Intervention: 57.0%, n = 12.5 and Control: 45.0%, n = 10 | Cefalexin (Intervention: 57% and Control: 45%) | Simple URTI in a child < 5 years old with a mild cough. |
| Authors (Year) | Questions Asked at the Time of Dispensation | Advice Given at the Time of Dispensation |
|---|---|---|
| Simulated patient method | ||
| Al-Tannir M. et al. (2020) [17] | 2011 In all scenarios presented (sore throat, acute sinusitis, otitis media, acute bronchitis, diarrhea, and UTI), none of the pharmacies asked about the drug allergy history | 2011 In all scenarios presented (sore throat, acute sinusitis, otitis media, acute bronchitis, diarrhea, and UTI), none of the pharmacies provided information on potential drug–drug interactions |
| 2018 Asked about history of drug allergies (n = 4, 9.8%) | 2018 Provided information regarding potential drug-drug interactions (n = 21, 51.2%) | |
| Halboup A. et al. (2020) [30] | Sore throat Asked whether the woman was pregnant (n = 31, 15.6%) Cough Asked whether the woman was pregnant (n = 20, 10.8%); Asked about the type of cough (productive or dry) (n = 166, 83.0%). Diarrhea Asked whether the woman was pregnant (=11, 7.3%) Otitis media Asked whether the woman was pregnant (=3, 2.0%) | Sore throat Explained how to use antibiotics (n = 172, 86.4%) Educated the patient about treatment duration (n = 145, 72.9%) Cough Explained how to use antibiotics (n = 72, 39.1%) Educated the patient about treatment duration (n = 12, 6.5%) Diarrhea Explained how to use antibiotics (n = 117, 75.9%) Educated the patient about treatment duration (n = 78, 50.6%) Otitis media Explained how to use antibiotics (n = 98, 95.1%) Educated the patient about treatment duration (n = 97, 95.1%) UTI Explained how to use antibiotics (n = 85, 88.5%) Educated the patient about treatment duration (n = 90, 61.2%) |
| Alrasheedy AA. et al. (2019) [34] | ______ | Pharyngitis Education and counseling about the importance of adherence and appropriate use of antibiotics (50.0%) UTI Education and counseling about the importance of adherence and appropriate use of antibiotics (52.0%) |
| Chang J. et al. (2019) [7] | Diarrhoea Asked about drug allergy history (n = 188, 16.1%) URTI Asked about drug allergy history (n = 494, 29.2%) | Diarrhoea Provided medication advice (n = 251, 21.5%) URTI Provided medication advice (n = 403, 23.8%) |
| Damisie G et al. (2019) [35] | ______ | Sore throat Explained how to take the antibiotics (n = 9, 64.3%) Explained how to take the antibiotics and duration of treatment (n = 1, 7.1%) Explained instruction on side effects (n = 1, 7.1%) Providing no counseling (n = 3, 21.4%) Acute diarrhea Explained how to take the antibiotics (n = 5, 31.2%) Explained how to take the antibiotics and duration of treatment (n = 4, 25.0%) Explained instruction on side effects (n = 1, 6.2%) Provided no counseling (n = 3, 18.7%) UTI Explained how to take the antibiotics (n = 15, 88.2%) Explained instruction on side effects (n = 1, 5.9%) Provided no counseling (n = 1, 5.9%) |
| Koji EM et al. (2019) [37] | Asked about drug allergy history (n = 19); Asked whether a doctor’s visit had taken place (n = 104); Asked about child’s symptoms (n = 70) | ______ |
| Mengistu G et al. (2019) [11] | None of the pharmacists asked about medication history and nutrition condition | None of the pharmacists provided infomed information about side effects and major interactions |
| Zawahir S et al. (2019) [38] | Further questioned about their symptoms or concurrent medical conditions (n = 36, 36.0%) Questions related to action that has already been taken (n = 12, 12.0%) Questions related to drug allergies (n = 10, 10.0%) Questions related to concurrent medicines used (n = 2, 2.0%) | In 18.0% (n = 44) of the instances, pseudo patients were recommended to see a physician, in about a quarter of them (n = 11, 25.0%) an antibiotic was still provided Explained how to take (n = 59, 60.0%) Explained how often to take (n = 47, 47.0%) Explained when to stop taking (n = 22, 22.0%) |
| Erku D.A. et al. (2018) [43] | Asked about drug allergies (n = 7, 8.1%) Queries about past medical and medication history (n = 18, 20.9%) | Instruction on dose and duration (n = 36, 41.9%) Instruction on side effects (n = 24, 27.9%) Advice to visit physician (n = 9, 10.6%) Non-pharmacological advice (n = 12, 14.0%) |
| Horumpende PG et al. (2018) [20] | ______ | None of the pharmacies/retailers voluntarily explained the possible side effects |
| Chang J. et al. (2017) [52] | Paediatric diarrhoea Asked further questions about the patient’s condition (n = 58, 40.6%) Asked about drug allergies (=85, 59.4%) Enquired about other symptoms (n = 6, 4.2%) Asked whether the patient had taken other drugs (n = 3, 2.1%) Adult acute URTI Further enquired regarding patient’s condition (n = 160, 80.4%) Asked whether had other symptoms or not (n = 64, 32.2%) Asked whether had taken other drugs or not (n = 13, 6.5%) Asked about the drug allergy history (n = 82, 41.2%) | Paediatric diarrhoea Provided medication advice (n = 25, 17.5%) Adult acute URTI Provided medication advice (n = 19, 9.6%) |
| Marković-Peković V et al. (2017) [18] | 2010 Patient information given (Written) (n = 59, 77.6%) Patient information given (Oral) (n = 72, 94.7%) Patient information given (Both) (n = 57, 75.0%) Patient information given (None) (n = 2, 2.6%) Asked about penicillin allergy (n = 59, 77.6%) Asked about taking other medicines (n = 19, 25.0%) | ______ |
| 2015 Patient information given (Written) (n = 36, 50.7%) Patient information given (Oral) (n = 46, 64.8%) Patient information given (Both) (n = 32, 45.1%) Patient information given (None) (n = 21, 29.6%) Asked about penicillin allergy (n = 45, 64.3%) Asked about taking other medicines (n = 16, 22.5%) | ______ | |
| Okuyan B. et al. (2017) [54] | None of the pharmacists asked about drug allergies | None of the pharmacists provided any information about other medications that could be used if an unusual condition occurred or if the patient forgot to take the medication |
| Guinovart MC et al. (2016) [57] | In 88 cases (73.9%) the patient was not asked about background of allergies to any antibiotics In none of the cases was the patient asked if she was pregnant Asked whether the patient was taking contraceptive treatment (n = 2, 1.7%) | Advice to visit a physician (36.1%) Explained the duration and treatment (n = 114, 95.8%) |
| Almaaytah A et al. (2015) [62] | Asked about drug allergy (n = 26, 17.3%) Asked about the concomitant use of other drugs (n = 8, 5.3%) | Explained how to take the antibiotic (n = 143, 95.3%) Explained the duration of treatment (n = 25, 16.7%) Recommended consulting a physician (n = 6, 4.0%) |
| Shet A et al. (2015) [65] | None of the pharmacists asked about drug allergies | None of the pharmacies provided counseling on expected side effects Instructions regarding the dose of the antimicrobial drugs (n = 101, 58.0%) Instructions regarding the duration of the antimicrobial drugs (n = 89, 51.1%) |
| Alabid A.H.M.A. et al. (2014) [67] | Asked “What symptoms have you got?” (n = 29) Asked “How long have you had the symptoms?” (n = 21) Concerning soliciting information about the colour of sputum (n = 11 (23.9%) Asked “Is there any blood in sputum?” (n = 2) Asked “How many times/year you presented the same complaint?” (n = 1) What medicines have you used before for? (n = 4) Concerning soliciting information about allergies to medicines (n = 14) | ______ |
| Marković-Peković V et al. (2012) [74] | ______ | Instructions for use given to the patients were oral (95%), written (78%), both (75.0%), and none (3.0%) |
| Rathnakar U.P. et al. (2012) [75] | None of the pharmacists asked about drug allergies | Frequency advised without asking (n = 18) Frequency advised after asking (n = 13) Duration advised without asking (n = 19) Duration advised after asking (n = 12) |
| Simó S et al. (2012) [76] | None of the pharmacies asked about drug allergies | None of the pharmacies explained the adverse effects |
| Puspitasari HP et al. (2011) [79] | In all the scenarios presented (product request for ciprofloxacin 10 tablets 500 mg; product request for 2 capsules tetracycline 250 mg and amoxicillin dry syrups 125 mg per 5 mL), none of the respondents asked about allergies In 2 of 3 scenarios (product request for ciprofloxacin 10 tablets 500 mg and product request for 2 capsules tetracycline 250 mg), none of the respondents asked about other medications taken by the patient | In the scenarios (product request for ciprofloxacin 10 tablets 500 mg and amoxicillin dry syrups 125 mg per 5 mL), none of the pharmacists informed about side effects, precautions/interactions/contra-indications and the risks of the medicine if not taken |
| Hadi U et al. (2010) [80] | The patients were never questioned or referred to a physician | ______ |
| Plachouras D et al. (2010) [82] | No comment was made by the pharmacist and no reason for the intended antibiotic use was requested (n = 107, 85.0%) | In the Amoxicillin + clavulanic acid case: n = 3 (4.2%) cases of dispensing, the collaborator was informed by the pharmacist about adverse events or asked whether such events had occurred in the past when the buyer had used antibiotics |
| Llor C et al. (2009) [83] | Asked patient about other symptoms (n = 61, 68.5%) Asked about drug allergies (n = 15, 16.9%) Asked patient whether she might be pregnant (this question was only necessary in the cases of UTI because the other clinical cases were presented by men) (n = 2, 3.6%) | Explained how often to take the antibiotic (n = 74, 83.1%) Explained how long the antibiotic should be taken (n = 62, 69.7%) Recommended that patient should see a physician if there was not any improvement (n = 4, 4.5%) |
| Wachter DA et al. (1999) [93] | In both scenarios, none of the pharmacies asked about drug allergies In both scenarios, none of the pharmacies asked about pre-existing medical conditions | ______ |
| Pharmacy interview/questionnaire method | ||
| Hallit S et al. (2019) [36] | Age (80.1%) Weight (80.7%) | Instructed the parents to shake the bottle before each administration (81.2%) Dilute the antibiotic to the indicated line (37.1%) Store the antibiotic in the refrigerator (64.4%) Give the exact dose (53.0%) Administer it on time (46.5%) and for a defined duration of treatment (47.5%) Do not stop the antibiotic before consulting a physician or pharmacist (32.2%) |
| Kalungia AC et al. (2016) [58] | Asked the indication for using the specific antibiotic requested (94.0%) | Counselled on dosage instructions (n = 70, 95.9%) Counselled on common side effects (n = 22, 30.1%) No advice (n = 3, 4.1%) Were involved in suggesting changes to the antibiotic choice or brand (97.0%) |
| Bahnassi A. (2015) [63] | Asking for the antibiotic indication (36.0%) | No counseling (66.0%) Dosing directions (34.0%) |
| Bahnassi A. (2014) [68] | Provided an antibiotic without asking for the indication (36.0%) | Finish the antibiotic even when symptoms are relieved (28.0%) Discussed interactions with other medications (32.0%) Discussed possible adverse reactions (58.0%) Discussed dose and dosing regimens (82.0%) |
| Gastelurrutia M.A. et al. (2014) [70] | ______ | Just dispensed (56.9%) The doctor referred (12.1%) |
| Sabry NA et al. (2014) [71] | Upon pharmacist´s recommendation: None of the pharmacies asked about drug allergies Upon patient´s request: None of the pharmacies asked about drug allergies | Upon pharmacist´s recommendation: The pharmacist provided advice and usage instructions to 124 patients (77.5%) Upon the patient´s request: The dispensing pharmacist advised the patient to see the doctor (n = 8) |
| Country | Simulated Patient Method | Pharmacy Interview/Questionnaire Method |
|---|---|---|
| Saudi Arabia | Al-Tannir M. et al. (2020) [17]: [2011: 77.6%; 2018: 12.5%] Alrasheedy AA. et al. (2019) [34]: 92.2% Al-Mohamadi A et al. (2011) [78]: 97.9% Al-Ghamdi MS. (2001) [92]: 82.0% | Alrasheedy AA. et al. (2019) [34]: 70.7% Alhomoud F et al. (2018) [41]: 100.0% Bahnassi A. (2014) [68]: 100.0% |
| Yemen | Halboup A. et al. (2020) [30]: 73.3% | Abood EA et al. (2016) [55]: 25.3% |
| Egypt | Abdelaziz AI et al. (2019) [33]: 98.4% | Sabry NA et al. (2014) [71]: 18.2% |
| Ethiopia | Damisie G et al. (2019) [35]: 94.4% Koji EM et al. (2019) [37]: 63.4% Mengistu G et al. (2019) [11]: 86.7% Erku D.A. et al. (2018) [43]: 86.0% Abegaz T.M. et al. (2016) [14]: 51.3% | Mengistu G et al. (2019) [11]: 50.5% Erku D.A. (2016) [56]: 90.2% |
| India | Nafade V. et al. (2019) [19]: 4.0% Satyanarayana S. et al. (2016) [61]: 27.0% Shet A et al. (2015) [65]: 66.7% Rathnakar U.P. et al. (2012) [75]: 51.7% | Barker A.K. et al. (2017) [51]: 100.0% Shreya Svitlana A. et al. (2015) [66]: 100.0% |
| Sri Lanka | Zawahir S et al. (2019) [38]: 41.0% Zawahir S et al. (2018) [49]: 61.0% Wolffers I. (1987) [94]: 100.0% | Zawahir S. et al. (2019) [39]: 31.7% |
| Indonesia | Puspitasari HP et al. (2011) [79]: 91.0% Hadi U et al. (2010) [80]: 75.9% | Ajie A.A.D. et al. (2018) [40]: 92.1% |
| Pakistan | Malik M. et al. (2013) [15]: 28.57% | Rehman IU et al. (2018) [46]: 68.0% Sarwar M.R. et al. (2018) [47]: 93.7% Nawab A. et al. (2016) [60]: 12.2% |
| Spain | Zapata-Cachafeiro M et al. (2018) [48]: 18.8% Guinovart MC et al. (2016) [57]: 54.1% Simó S et al. (2012) [76]: 8.0% Llor C et al. (2010) [81]: 45.2% Llor C et al. (2009) [83]: 45.2% | Gastelurrutia M.A. et al. (2014) [70]: 9.8% Zapata-Cachafeiro M. et al. (2014) [72]: 64.7% Caamaño F et al. (2005) [87]: 65.9% Caamano Isorna F. et al. (2004) [89]: 65.9% |
| Nepal | Wachter DA et al. (1999) [93]: 67.5% | Ansari M. (2017) [50]: 66.5% |
| Syria | Al-Faham Z et al. (2011) [77]: 97.0% | Mansour O. et al. (2017) [53]: 85.5% Bahnassi A. (2015) [63]: 100.0% |
| Malaysia | Alabid A.H.M.A. et al. (2014) [67]: 32.0% | Khan M.U. et al. (2016) [59]: 63.3% |
| Brazil | Volpato DE et al. (2005) [88]: 74.0% | Rauber C. et al. (2009) [84]: 85.0% |
| Vietnam | Chalker J et al. (2000) [16]: 81.5% | Chalker J et al. (2002) [91]: 51.0% |
| Zimbabwe | Nyazema N et al. (2007) [86]: 8.0% | Nyazema N et al. (2007) [86]: 31.0% |
3. Discussion
4. Materials and Methods
4.1. Search Strategy/Search Methods for Identification of Studies
4.2. Study Inclusion Criteria
4.3. Quality Assessment
4.4. Data Extraction
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Antibiotic Resistance. Newsroom, 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 24 September 2020).
- World Health Organization (WHO). Antimicrobial Resistance. Newsroom, 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 24 September 2020).
- United Nations. UN Health Agency Steps Up Fight against ‘Invisible Pandemic’ of Antimicrobial Resistance. UN News, 2019. Available online: https://news.un.org/en/story/2019/06/1040741 (accessed on 24 September 2020).
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling drug-resistant infections globally: Final report and recmmendations. Rev. Antimicrob. Resist. 2016. [Google Scholar] [CrossRef]
- Abu Al-Halawa, D.; Sarama, R.; Abdeen, Z.; Qasrawi, R. Knowledge, attitudes, and practices relating to antibiotic resistance among pharmacists: A cross-sectional study in the West Bank, Palestine. Lancet 2019, 393, S7. [Google Scholar] [CrossRef]
- Chang, J.; Xu, S.; Zhu, S.; Li, Z.; Yu, J.; Zhang, Y.; Zu, J.; Fang, Y.; Ross-Degnan, D. Assessment of non-prescription antibiotic dispensing at community pharmacies in China with simulated clients: A mixed cross-sectional and longitudinal study. Lancet Infect. Dis. 2019, 19, 1345–1354. [Google Scholar] [CrossRef]
- (PDF) Antibiotic Resistance—The Faceless Threat. Available online: https://www.researchgate.net/publication/228636583_Antibiotic_resistance-The_faceless_threat (accessed on 24 September 2020).
- Grigoryan, L.; Haaijer-Ruskamp, F.M.; Burgerhof, J.G.M.; Mechtler, R.; Deschepper, R.; Tambic-Andrasevic, A.; Andrajati, R.; Monnet, D.L.; Cunney, R.; Di Matteo, A.; et al. Self-medication with antimicrobial drugs in Europe. Emerg. Infect. Dis. 2006, 12, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Björnsdottir, I.; Granas, A.G.; Bradley, A.; Norris, P. A systematic review of the use of simulated patient methodology in pharmacy practice research from 2006 to 2016. Int. J. Pharm. Pract. 2020, 28, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Mengistu, G.; Gietnet, K.; Amare, F.; Sisay, M.; Hagos, B.; Misganaw, D. Self-Reported and Actual Involvement of Community Pharmacy Professionals in the Management of Childhood Diarrhea: A Cross-Sectional and Simulated Patient Study at two Towns of Eastern Ethiopia. Clin. Med. Insights Pediatr. 2019, 13. [Google Scholar] [CrossRef]
- Ibrahim, I.R.; Palaian, S.; Ibrahim, M.I.M. Assessment of diarrhea treatment and counseling in community pharmacies in Baghdad, Iraq: A simulated patient study. Pharm. Pract. (Granada) 2018, 16. [Google Scholar] [CrossRef]
- Ibrahim, M.I.B.M.; Palaian, S.; Al-Sulaiti, F.; El-Shami, S. Evaluating community pharmacy practice in Qatar using simulated patient method: Acute gastroenteritis management. Pharm. Pract. (Granada) 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Abegaz, T.M.; Belachew, S.A.; Abebe, T.B.; Gebresilassie, B.M.; Teni, F.S.; Woldie, H.G. Management of children’s acute diarrhea by community pharmacies in five towns of Ethiopia: Simulated client case study. Clin. Risk Manag. 2016, 12, 515–526. [Google Scholar] [CrossRef]
- Malik, M.; Hassali, M.A.; Shafie, A.A.; Hussain, A.; Aljadhey, H.; Saleem, F. Manejo de casos de malaria en farmacias comunitarias de Pakistán: Amenaza al uso racional de medicamentos. Pharm. Pract. (Granada) 2013, 11, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Chalker, J.; Chuc, N.T.K.; Falkenberg, T.; Do, N.T.; Tomson, G. STD management by private pharmacies in Hanoi: Practice and knowledge of drug sellers. Sex. Transm. Infect. 2000, 76, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Al-Tannir, M.; Altannir, Y.; Altannir, M.; AlFayyad, I. Community pharmacy sales of non-prescribed antibiotics in Riyadh, Saudi Arabia: A simulated patient study. Int. J. Clin. Pharm. 2020, 42, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Marković-Peković, V.; Grubiša, N.; Burger, J.; Bojanić, L.; Godman, B. Initiatives to Reduce Nonprescription Sales and Dispensing of Antibiotics: Findings and Implications. J. Res. Pharm. Pract. 2017, 120–125. [Google Scholar] [CrossRef]
- Nafade, V.; Huddart, S.; Sulis, G.; Daftary, A.; Miraj, S.S.; Saravu, K.; Pai, M. Over-the-counter antibiotic dispensing by pharmacies: A standardised patient study in Udupi district, India. BMJ Glob. Heal. 2019, 4. [Google Scholar] [CrossRef]
- Horumpende, P.G.; Sonda, T.B.; van Zwetselaar, M.; Antony, M.L.; Tenu, F.F.; Mwanziva, C.E.; Shao, E.R.; Mshana, S.E.; Mmbaga, B.T.; Chilongola, J.O. Prescription and non-prescription antibiotic dispensing practices in part I and part II pharmacies in Moshi Municipality, Kilimanjaro Region in Tanzania: A simulated clients approach. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Jaisue, S.; Phomtavong, S.; Eua-anant, S.; Borlace, G.N. Dispensing pattern for acute non-infectious diarrhoea in children at community pharmacies in Thailand. J. Pharm. Pract. Res. 2017, 47, 383–388. [Google Scholar] [CrossRef]
- Saengcharoen, W.; Lerkiatbundit, S. Practice and attitudes regarding the management of childhood diarrhoea among pharmacies in Thailand. Int. J. Pharm. Pract. 2010, 18, 323–331. [Google Scholar] [CrossRef]
- Minzi, O.M.; Manyilizu, V.S. Application of basic pharmacology and dispensing practice of antibiotics in accredited drug-dispensing outlets in tanzania. Drug. Healthc. Patient Saf. 2013, 5, 5–11. [Google Scholar] [CrossRef]
- Abubakar, U.; Tangiisuran, B. Knowledge and practices of community pharmacists towards non-prescription dispensing of antibiotics in Northern Nigeria. Int. J. Clin. Pharm. 2020, 42, 756–764. [Google Scholar] [CrossRef]
- Abubakar, U. Practices and Perception of Nigerian Community Pharmacists Towards Antimicrobial Stewardship Program. Int. J. Pharm. Pharm. Sci. 2020, 37–42. [Google Scholar] [CrossRef]
- Badro, D.A.; Sacre, H.; Hallit, S.; Amhaz, A.; Salameh, P. Good pharmacy practice assessment among community pharmacies in Lebanon. Pharm. Pract. (Granada) 2020, 18. [Google Scholar] [CrossRef]
- Bahta, M.; Tesfamariam, S.; Weldemariam, D.G.; Yemane, H.; Tesfamariam, E.H.; Alem, T.; Russom, M. Dispensing of antibiotics without prescription and associated factors in drug retail outlets of Eritrea: A simulated client method. PLoS ONE 2020, 15. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Chen, X.; Hesketh, T. Widespread illegal sales of antibiotics in Chinese pharmacies—A nationwide cross-sectional study. Antimicrob. Resist. Infect. Control 2020, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Paulik, E.; Szabó, A. Knowledge, attitude and practice of community pharmacists regarding antibiotic use and infectious diseases: A cross-sectional survey in Hungary (KAPPhA-HU). Antibiotics 2020, 9, 41. [Google Scholar] [CrossRef]
- Halboup, A.; Abdi, A.; Ahmed, M.; Al-Qadasi, F.; Othman, G.Q. Access to antibiotics without prescription in community pharmacies in Yemen during the political conflict. Public Health 2020, 183, 30–35. [Google Scholar] [CrossRef]
- Shi, L.; Chang, J.; Liu, X.; Zhai, P.; Hu, S.; Li, P.; Hayat, K.; Kabba, J.A.; Feng, Z.; Yang, C.; et al. Dispensing antibiotics without a prescription for acute cough associated with common cold at community pharmacies in Shenyang, Northeastern China: A cross-sectional study. Antibiotics 2020, 9, 163. [Google Scholar] [CrossRef]
- Wang, X.; Xuan, Z.; Storella, T.H.; Zhou, X. Determinants of non-prescription antibiotic dispensing in Chinese community pharmacies from socio-ecological and health system perspectives. Soc. Sci. Med. 2020, 256, 113035. [Google Scholar] [CrossRef]
- Abdelaziz, A.I.; Tawfik, A.G.; Rabie, K.A.; Omran, M.; Hussein, M.; Abou-Ali, A.; Ahmed, A.S.F. Quality of community pharmacy practice in antibiotic self-medication encounters: A simulated patient study in upper Egypt. Antibiotics 2019, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Alrasheedy, A.A.; Alsalloum, M.A.; Almuqbil, F.A.; Almuzaini, M.A.; Aba Alkhayl, B.S.; Albishri, A.S.; Alharbi, F.F.; Alharbi, S.R.; Alodhayb, A.K.; Alfadl, A.A.; et al. The impact of law enforcement on dispensing antibiotics without prescription: A multi-methods study from Saudi Arabia. Expert Rev. Anti. Infect. Ther. 2019, 18, 87–97. [Google Scholar] [CrossRef]
- Damisie, G.; Hambisa, S.; Yimam, M. Over the Counter Sale of Antibiotics at Drug Stores Found in Mizan-Aman Town, Southwest Ethiopia: A Cross-Sectional Simulated Client Visit Study. J. Pharm. 2019, 2019, 1–6. [Google Scholar] [CrossRef]
- Hallit, S.; Zahreddine, L.; Saleh, N.; Shakaroun, S.; Lahoud, N. Practice of parents and pharmacists regarding antibiotics use in pediatrics: A 2017 cross-sectional study in Lebanese community pharmacies. J. Eval. Clin. Pract. 2019, 26, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Koji, E.M.; Gebretekle, G.B.; Tekle, T.A. Practice of over-the-counter dispensary of antibiotics for childhood illnesses in Addis Ababa, Ethiopia: A simulated patient encounter study. Antimicrob. Resist. Infect. Control 2019, 8. [Google Scholar] [CrossRef]
- Zawahir, S.; Lekamwasam, S.; Aslani, P. Community pharmacy staff’s response to symptoms of common infections: A pseudo-patient study. Antimicrob. Resist. Infect. Control 2019, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Zawahir, S.; Lekamwasam, S.; Aslani, P. A cross-sectional national survey of community pharmacy staff: Knowledge and antibiotic provision. PLoS ONE 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Ajie, A.A.D.; Andrajati, R.; Radji, M. Factors affecting the sale of non-prescribed antibiotics in Jakarta, Indonesia: A cross-sectional study. Int. J. Appl. Pharm. 2018, 10, 243–247. [Google Scholar] [CrossRef][Green Version]
- Alhomoud, F.; Almahasnah, R.; Alhomoud, F.K. “You could lose when you misuse”—Factors affecting over-the-counter sale of antibiotics in community pharmacies in Saudi Arabia: A qualitative study 11 Medical and Health Sciences 1117 Public Health and Health Services. BMC Health Serv. Res. 2018, 18. [Google Scholar] [CrossRef]
- Awosan, K.J.; Ibitoye, P.K.; Abubakar, A.K. Knowledge, risk perception and practices related to antibiotic resistance among patent medicine vendors in Sokoto metropolis, Nigeria. Niger. J. Clin. Pract. 2018, 21, 1476–1483. [Google Scholar] [CrossRef]
- Erku, D.A.; Aberra, S.Y. Non-prescribed sale of antibiotics for acute childhood diarrhea and upper respiratory tract infection in community pharmacies: A 2 phase mixed-methods study. Antimicrob. Resist. Infect. Control 2018, 7. [Google Scholar] [CrossRef]
- Mohamed Ibrahim, M.I.; Awaisu, A.; Palaian, S.; Radoui, A.; Atwa, H. Do community pharmacists in Qatar manage acute respiratory conditions rationally? A simulated client study. J. Pharm. Heal. Serv. Res. 2018, 9, 33–39. [Google Scholar] [CrossRef]
- Rachel Paes, M.; De Sa, S. Drug dispensing practices in private pharmacies in Goa. Natl. J. Physiol. Pharm. Pharmacol. 2018. [Google Scholar] [CrossRef]
- Rehman, I.; Asad, M.; Bukhsh, A.; Ali, Z.; Ata, H.; Dujaili, J.; Blebil, A.; Khan, T. Knowledge and Practice of Pharmacists toward Antimicrobial Stewardship in Pakistan. Pharmacy 2018, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.R.; Saqib, A.; Iftikhar, S.; Sadiq, T. Knowledge of community pharmacists about antibiotics, and their perceptions and practices regarding antimicrobial stewardship: A cross-sectional study in Punjab, Pakistan. Infect. Drug Resist. 2018, 11, 133–145. [Google Scholar] [CrossRef]
- Zapata-Cachafeiro, M.; Piñeiro-Lamas, M.; Guinovart, M.C.; López-Vázquez, P.; Vázquez-Lago, J.M.; Figueiras, A. Magnitude and determinants of antibiotic dispensing without prescription in Spain: A simulated patient study. J. Antimicrob. Chemother. 2018, 74, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Zawahir, S.; Lekamwasam, S.; Aslani, P. Antibiotic dispensing practice in community pharmacies: A simulated client study. Res. Soc. Adm. Pharm. 2018, 15, 584–590. [Google Scholar] [CrossRef]
- Ansari, M. Evaluation of community pharmacies regarding dispensing practices of antibiotics in two districts of central Nepal. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Barker, A.K.; Brown, K.; Ahsan, M.; Sengupta, S.; Safdar, N. What drives inappropriate antibiotic dispensing? A mixed-methods study of pharmacy employee perspectives in Haryana, India. BMJ Open 2017, 7. [Google Scholar] [CrossRef]
- Chang, J.; Ye, D.; Lv, B.; Jiang, M.; Zhu, S.; Yan, K.; Tian, Y.; Fang, Y. Sale of antibiotics without a prescription at community pharmacies in urban China: A multicentre cross-sectional survey. J. Antimicrob. Chemother. 2017, 72, 1235–1242. [Google Scholar] [CrossRef]
- Mansour, O.; Al-Kayali, R. Community pharmacists’ role in controlling bacterial antibiotic resistance in Aleppo, Syria. Iran. J. Pharm. Res. 2017, 16, 1612–1620. [Google Scholar] [CrossRef]
- Okuyan, B.; Savan, M.A.; Izzettin, F.V.; Sancar, M. Evaluation of rational antibiotic dispensing in the community pharmacy setting: A simulated patient study. Acta Pharm. Sci. 2017, 55, 7–16. [Google Scholar] [CrossRef][Green Version]
- Abood, E.A.; Wazaify, M. Abuse and Misuse of Prescription and Nonprescription Drugs from Community Pharmacies in Aden City—Yemen. Subst. Use Misuse 2016, 51, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Erku, D.A. Antimicrobial Stewardship: A Cross-Sectional Survey Assessing the Perceptions and Practices of Community Pharmacists in Ethiopia. Interdiscip. Perspect. Infect. Dis. 2016, 2016. [Google Scholar] [CrossRef]
- Guinovart, M.C.; Figueras, A.; Llor, C. Selling antimicrobials without prescription—Far beyond an administrative problem. Enferm. Infecc. Microbiol. Clin. 2016, 36, 290–292. [Google Scholar] [CrossRef]
- Kalungia, A.C.; Burger, J.; Godman, B.; Costa, J.d.O.; Simuwelu, C. Non-prescription sale and dispensing of antibiotics in community pharmacies in Zambia. Expert Rev. Anti Infect. Ther. 2016, 14, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.; Hassali, M.A.A.; Ahmad, A.; Elkalmi, R.M.; Zaidi, S.T.R.; Dhingra, S. Perceptions and Practices of Community Pharmacists towards Antimicrobial Stewardship in the State of Selangor, Malaysia. PLoS ONE 2016, 11, e0149623. [Google Scholar] [CrossRef]
- Nawab, A.; Kalim, M.; Sheikh, J.; Shoukat, N. DISPENSING OF MEDICATION WITHOUT PRESCRIPTION IN KARACHI, PAKISTAN. Int. Res. J. Pharm. 2016, 7, 39–43. [Google Scholar] [CrossRef]
- Satyanarayana, S.; Kwan, A.; Daniels, B.; Subbaraman, R.; McDowell, A.; Bergkvist, S.; Das, R.K.; Das, V.; Das, J.; Pai, M. Use of standardised patients to assess antibiotic dispensing for tuberculosis by pharmacies in urban India: A cross-sectional study. Lancet Infect. Dis. 2016, 16, 1261–1268. [Google Scholar] [CrossRef]
- Almaaytah, A.; Mukattash, T.L.; Hajaj, J. Dispensing of non-prescribed antibiotics in Jordan. Patient Prefer. Adherence 2015, 9, 1389–1395. [Google Scholar] [CrossRef]
- Bahnassi, A. A qualitative analysis of pharmacists’ attitudes and practices regarding the sale of antibiotics without prescription in Syria. J. Taibah Univ. Med. Sci. 2015, 10, 227–233. [Google Scholar] [CrossRef]
- Dorj, G.; Hendrie, D.; Parsons, R.W.; Sunderland, B. A questionnaire study of injections prescribed and dispensed for patients diagnosed with mild/moderate community-acquired pneumonia in Mongolia. PeerJ 2015, 2015. [Google Scholar] [CrossRef][Green Version]
- Shet, A.; Sundaresan, S.; Forsberg, B.C. Pharmacy-based dispensing of antimicrobial agents without prescription in India: Appropriateness and cost burden in the private sector. Antimicrob. Resist. Infect. Control 2015, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Study on Medicines Delivered by Pharmacists Relating to Dental Pain, without Prescriptions. Available online: https://www.jpsr.pharmainfo.in/Documents/Volumes/vol8Issue09/jpsr08091624.pdf (accessed on 24 September 2020).
- Alabid, A.H.M.A.; Ibrahim, M.I.M.; Hassali, M.A. Antibiotics dispensing for URTIs by community pharmacists and general medical practitioners in Penang, Malaysia: A comparative study using simulated patients. J. Clin. Diagn. Res. 2014, 8, 119–123. [Google Scholar] [CrossRef]
- Bahnassi, A. Pharmacists views and practices in regard to sales of antibiotics without a prescription in Madinah, Saudi Arabia. J. Patient Saf. 2014, 12, 159–164. [Google Scholar] [CrossRef]
- Farah, R.; Lahoud, N.; Salameh, P.; Saleh, N. Antibiotic dispensation by Lebanese pharmacists: A comparison of higher and lower socio-economic levels. J. Infect. Public Health 2014, 8, 37–46. [Google Scholar] [CrossRef] [PubMed]
- (PDF) Assessment of the First 10 Years Institutional Programme on Rational Use of Antibiotics in Gipuzkoa: 1999–2009. Available online: https://www.researchgate.net/publication/289112591_Assessment_of_the_first_10_years_institutional_programme_on_rational_use_of_antibiotics_in_Gipuzkoa_1999-2009 (accessed on 24 September 2020).
- Sabry, N.A.; Farid, S.F.; Dawoud, D.M. Antibiotic dispensing in Egyptian community pharmacies: An observational study. Res. Soc. Adm. Pharm. 2014, 10, 168–184. [Google Scholar] [CrossRef]
- Zapata-Cachafeiro, M.; González-González, C.; Váquez-Lago, J.M.; López-Vázquez, P.; López-Durán, A.; Smyth, E.; Figueiras, A. Determinants of antibiotic dispensing without a medical prescription: A cross-sectional study in the north of Spain. J. Antimicrob. Chemother. 2014, 69, 3156–3160. [Google Scholar] [CrossRef]
- A Comparative Study between Prescribed and over-the-Counter Antibiotics. Available online: https://www.researchgate.net/publication/258065195_A_comparative_study_between_prescribed_and_over-the-counter_antibiotics (accessed on 24 September 2020).
- Marković-Peković, V.; Grubiša, N. Self-medication with antibiotics in the Republic of Srpska community pharmacies: Pharmacy staff behavior. Pharmacoepidemiol. Drug Saf. 2012, 21, 1130–1133. [Google Scholar] [CrossRef]
- (PDF) A Study on the Sale of Antimicrobial Agents without Prescriptions in Pharmacies in an Urban Area in South India. Available online: https://www.researchgate.net/publication/234155875_A_Study_on_the_Sale_of_Antimicrobial_Agents_without_Prescriptions_in_Pharmacies_in_an_Urban_Area_in_South_India (accessed on 24 September 2020).
- Simó, S.; Fraile, D.; Sánchez, A.; García-Algar, O. Dispensación de medicamentos sin prescripción médica en oficinas de farmacia. An. Pediatr. 2012, 79, 10–14. [Google Scholar] [CrossRef]
- Al-Faham, Z.; Habboub, G.; Takriti, F. The sale of antibiotics without prescription in pharmacies in Damascus, Syria. J. Infect. Dev. Ctries. 2011, 5, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Al-Mohamadi, A.; Badr, A.; Bin Mahfouz, L.; Samargandi, D.; Al Ahdal, A. Dispensing medications without prescription at Saudi community pharmacy: Extent and perception. Saudi Pharm. J. 2011, 21, 13–18. [Google Scholar] [CrossRef]
- Puspitasari, H.P.; Faturrohmah, A.; Hermansyah, A. Do Indonesian community pharmacy workers respond to antibiotics requests appropriately? Trop. Med. Int. Heal. 2011, 16, 840–846. [Google Scholar] [CrossRef]
- Hadi, U.; van den Broek, P.; Kolopaking, E.P.; Zairina, N.; Gardjito, W.; Gyssens, I.C. Cross-sectional study of availability and pharmaceutical quality of antibiotics requested with or without prescription (Over The Counter) in Surabaya, Indonesia. BMC Infect. Dis. 2010, 10, 203. [Google Scholar] [CrossRef]
- Llor, C.; Monnet, D.L.; Cots, J.M. Small pharmacies are more likely to dispense antibiotics without a medical prescription than large pharmacies in Catalonia, Spain. Eurosurveillance 2010, 15, 1–4. [Google Scholar] [CrossRef]
- Plachouras, D.; Kavatha, D.; Antoniadou, A.; Giannitsioti, E.; Poulakou, G.; Kanellakopoulou, K.; Giamarellou, H. Dispensing of antibiotics without prescription in Greece, 2008: Another link in the antibiotic resistance chain. Eurosurveillance 2010, 15, 1–4. [Google Scholar] [CrossRef]
- Llor, C.; Cots, J.M. The sale of antibiotics without prescription in pharmacies in catalonia, Spain. Clin. Infect. Dis. 2009, 48, 1345–1349. [Google Scholar] [CrossRef]
- Rauber, C.; Feltrin, M.R.; Piovezan, A.P. Evaluation of antibiotics dispensing profile in Tubarão, Santa Catarina, Brazil. Braz. J. Pharm. Sci. 2009, 45, 787–793. [Google Scholar] [CrossRef]
- Viberg, N.; Mujinja, P.; Kalala, W.; Kumaranayake, L.; Vyas, S.; Tomson, G.; Lundborg, C.S. STI management in Tanzanian private drugstores: Practices and roles of drug sellers. Sex. Transm. Infect. 2009, 85, 300–307. [Google Scholar] [CrossRef]
- Nyazema, N.; Viberg, N.; Khoza, S.; Vyas, S.; Kumaranayake, L.; Tomson, G.; Lundborg, C.S. Low sale of antibiotics without prescription: A cross-sectional study in Zimbabwean private pharmacies. J. Antimicrob. Chemother. 2007, 59, 718–726. [Google Scholar] [CrossRef]
- Caamaño, F.; Tomé-Otero, M.; Takkouche, B.; Gestal-Otero, J.J. Influence of pharmacists’ opinions on their dispensing medicines without requirement of a doctor’s prescription. Gac. Sanit. 2005, 19, 9–14. [Google Scholar] [CrossRef]
- Volpato, D.E.; de Souza, B.V.; Dalla Rosa, L.G.; Melo, L.H.; Daudt, C.A.S.; Deboni, L. Use of antibiotics without medical prescription. Braz. J. Infect. Dis. 2005, 9, 288–291. [Google Scholar] [CrossRef]
- Caamaño Isorna, F.; Tomé-Otero, M.; Takkouche, B.; Figueiras, A. Factors related with prescription requirement to dispense in Spain. Pharmacoepidemiol. Drug Saf. 2004, 13, 405–409. [Google Scholar] [CrossRef]
- Larson, E.; Grullon-Figueroa, L. Availability of antibiotics without prescription in New York City. J. Urban Heal. 2004, 81, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Chalker, J.; Chuc, N.T.K.; Falkenberg, T.; Tomson, G. Private pharmacies in Hanoi, Vietnam: A randomized trial of a 2-year multi-component intervention on knowledge and stated practice regarding ARI, STD and antibiotic/steroid requests. Trop. Med. Int. Heal. 2002, 7, 803–810. [Google Scholar] [CrossRef]
- Al-Ghamdi, M.S. Empirical treatment of uncomplicated urinary tract infection by community pharmacist in the eastern province of Saudi Arabia. Saudi Med. J. 2001, 22, 1105–1108. [Google Scholar]
- Wachter, D.A.; Joshi, M.P.; Rimal, B. Antibiotic dispensing by drug retailers in Kathmandu, Nepal. Trop. Med. Int. Heal. 1999, 4, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Wolffers, I. Drug information and sale practices in some pharmacies of Colombo, Sri Lanka. Soc. Sci. Med. 1987, 25, 319–321. [Google Scholar] [CrossRef]
- Jamshed, S.; Padzil, F.; Shamsudin, S.; Bux, S.; Jamaluddin, A.; Bhagavathula, A.; Azhar, S.; Hassali, M. Antibiotic Stewardship in Community Pharmacies: A Scoping Review. Pharmacy 2018, 6, 92. [Google Scholar] [CrossRef]
- Alhomoud, F.; Aljamea, Z.; Almahasnah, R.; Alkhalifah, K.; Basalelah, L.; Alhomoud, F.K. Self-medication and self-prescription with antibiotics in the Middle East—do they really happen? A systematic review of the prevalence, possible reasons, and outcomes. Int. J. Infect. Dis. 2017, 57, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Sulis, G.; Adam, P.; Nafade, V.; Gore, G.; Daniels, B.; Daftary, A.; Das, J.; Gandra, S.; Pai, M. Antibiotic prescription practices in primary care in low- And middle-income countries: A systematic review and meta-analysis. PLoS Med. 2020, 17, 1–20. [Google Scholar] [CrossRef]
- Thompson, W.; Tonkin-Crine, S.; Pavitt, S.H.; McEachan, R.R.C.; Douglas, G.V.A.; Aggarwal, V.R.; Sandoe, J.A.T. Factors associated with antibiotic prescribing for adults with acute conditions: An umbrella review across primary care and a systematic review focusing on primary dental care. J. Antimicrob. Chemother. 2019, 74, 2139–2152. [Google Scholar] [CrossRef]
- Md Rezal, R.S.; Hassali, M.A.; Alrasheedy, A.A.; Saleem, F.; Md Yusof, F.A.; Godman, B. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: A systematic review of the literature. Expert Rev. Anti. Infect. Ther. 2015, 13, 665–680. [Google Scholar] [CrossRef]
- Jacobs, T.G.; Robertson, J.; van den Ham, H.A.; Iwamoto, K.; Bak Pedersen, H.; Mantel-Teeuwisse, A.K. Assessing the impact of law enforcement to reduce over-the-counter (OTC) sales of antibiotics in low- and middle-income countries; a systematic literature review. BMC Health Serv. Res. 2019, 19, 536. [Google Scholar] [CrossRef] [PubMed]
- Caamaño, F.; Ruano, A.; Figueiras, A.; Gestal-Otero, J.J. Data collection methods for analyzing the quality of the dispensing in pharmacies. Pharm. World Sci. 2002, 24, 217–223. [Google Scholar] [CrossRef]
- Lescure, D.; Paget, J.; Schellevis, F.; van Dijk, L. Determinants of self-medication with antibiotics in European and Anglo-Saxon countries: A systematic review of the literature. Front. Public Heal. 2018, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Goodman, C. Performance of retail pharmacies in low- and middle-income Asian settings: A systematic review. Health Policy Plan. 2016, 31, 940–953. [Google Scholar] [CrossRef]
- Istúriz, R.E.; Carbon, C. Antibiotic Use in Developing Countries. Infect. Control Hosp. Epidemiol. 2000, 21, 394–397. [Google Scholar] [CrossRef]
- Servia-Dopazo, M.; Figueiras, A. Determinants of antibiotic dispensing without prescription: A systematic review. J. Antimicrob. Chemother. 2018, 73, 3244–3253. [Google Scholar] [CrossRef] [PubMed]
- O’neill, J. Antimicrobials in Agriculture and the Environment: Reducing Unnecessary Use and Waste the Review on Antimicrobial Resistance. Available online: https://amr-review.org/sites/default/files/Antimicrobials%20in%20agriculture%20and%20the%20environment%20-%20Reducing%20unnecessary%20use%20and%20waste.pdf (accessed on 22 October 2020).
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Downes, M.J.; Brennan, M.L.; Williams, H.C.; Dean, R.S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016, 6. [Google Scholar] [CrossRef]
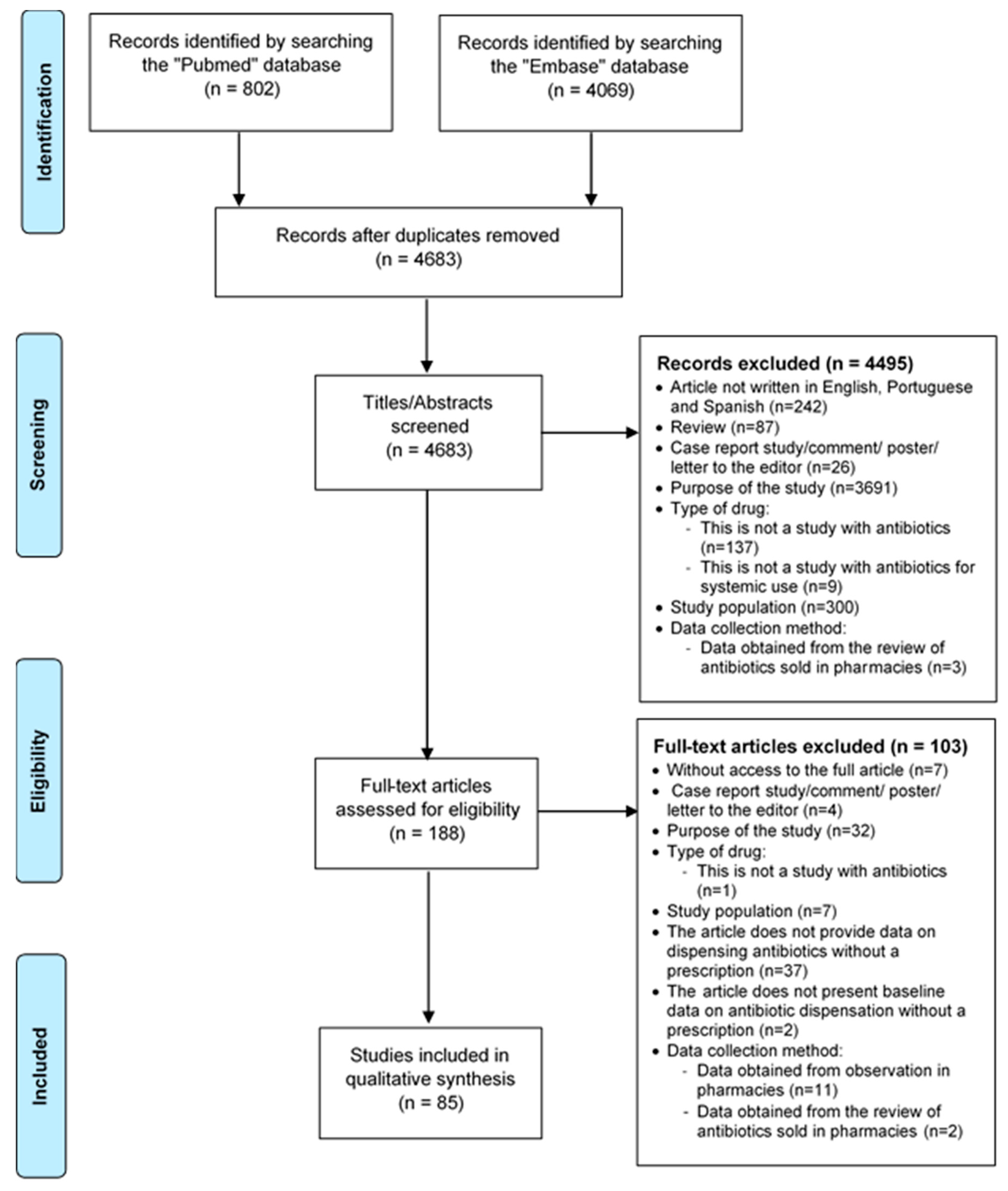
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, A.D.; A. Rodrigues, D.; Figueiras, A.; Zapata-Cachafeiro, M.; Roque, F.; Herdeiro, M.T. Antibiotic Dispensation without a Prescription Worldwide: A Systematic Review. Antibiotics 2020, 9, 786. https://doi.org/10.3390/antibiotics9110786
Batista AD, A. Rodrigues D, Figueiras A, Zapata-Cachafeiro M, Roque F, Herdeiro MT. Antibiotic Dispensation without a Prescription Worldwide: A Systematic Review. Antibiotics. 2020; 9(11):786. https://doi.org/10.3390/antibiotics9110786
Chicago/Turabian StyleBatista, Ana Daniela, Daniela A. Rodrigues, Adolfo Figueiras, Maruxa Zapata-Cachafeiro, Fátima Roque, and Maria Teresa Herdeiro. 2020. "Antibiotic Dispensation without a Prescription Worldwide: A Systematic Review" Antibiotics 9, no. 11: 786. https://doi.org/10.3390/antibiotics9110786
APA StyleBatista, A. D., A. Rodrigues, D., Figueiras, A., Zapata-Cachafeiro, M., Roque, F., & Herdeiro, M. T. (2020). Antibiotic Dispensation without a Prescription Worldwide: A Systematic Review. Antibiotics, 9(11), 786. https://doi.org/10.3390/antibiotics9110786







