Point-of-Care Testing for Pharyngitis in the Pharmacy
Abstract
1. Introduction
2. Inaccuracy of Clinical Assessment for Bacterial Sore Throat
3. Effect of POC Testing on Appropriate Antibiotic Prescribing in Primary Care
4. POC Testing in General Practice
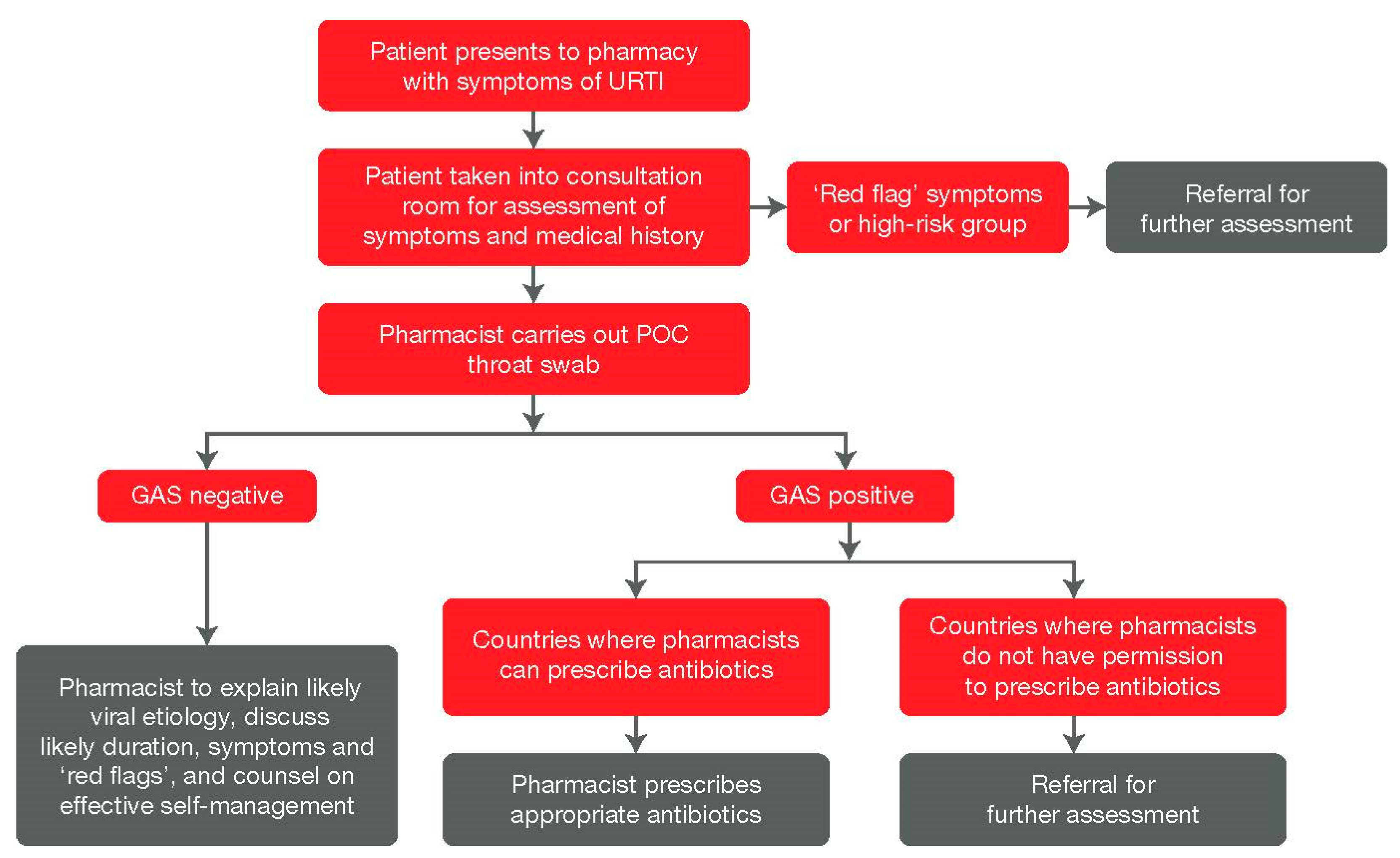
5. POC Testing in the Pharmacy Setting
6. POC Testing and COVID-19
7. Pharmacist’s Contribution to Antimicrobial Stewardship and to Deliver POC Testing
8. Implementation Considerations and Challenges for Pharmacies
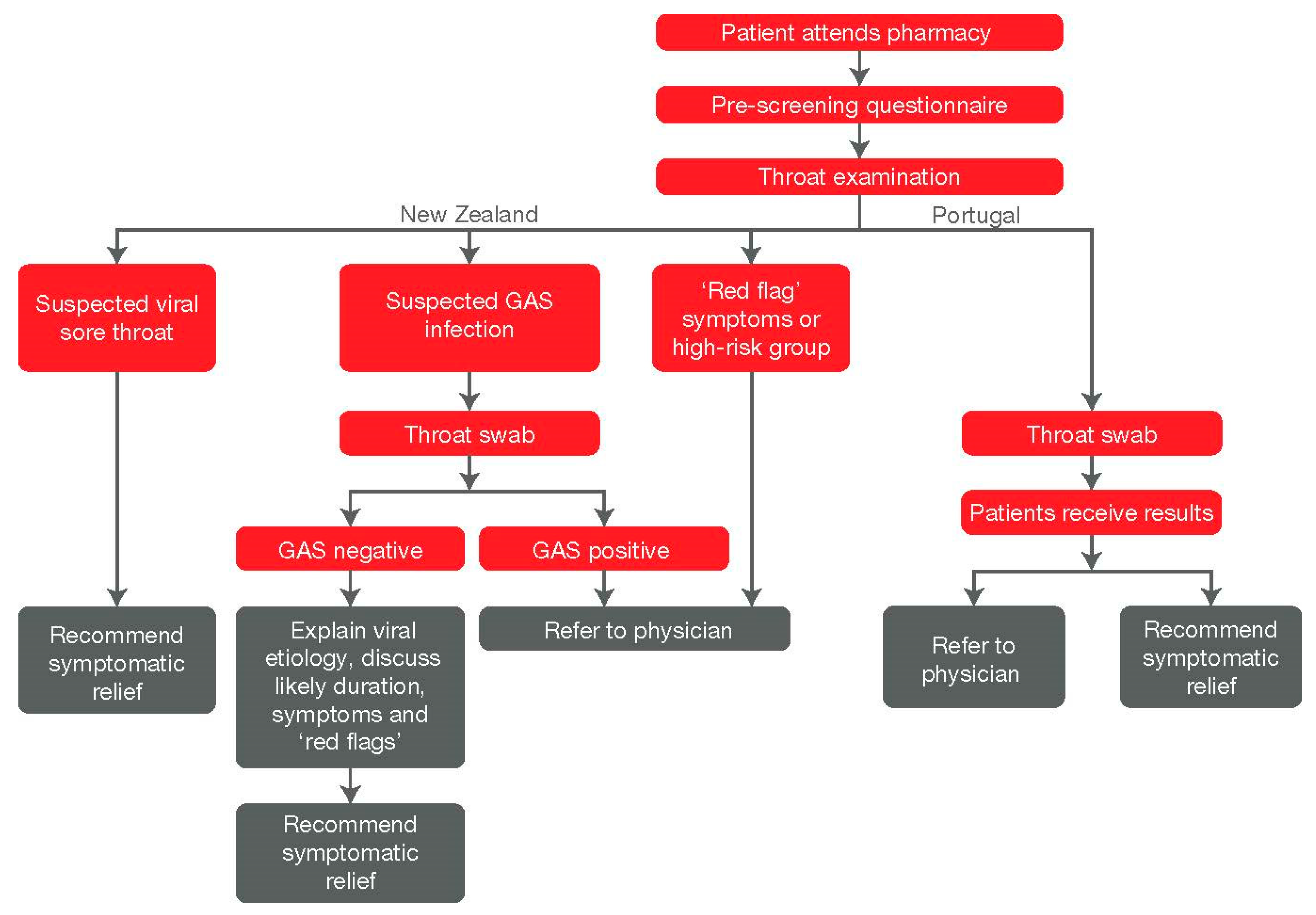
9. Country Examples and Recent/Ongoing Initiatives
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Finley, C.R.; Chan, D.S.; Garrison, S.; Korownyk, C.; Kolber, M.R.; Campbell, S.; Eurich, D.T.; Lindblad, A.J.; Vandermeer, B.; Allan, G.M. What are the most common conditions in primary care? Systematic review. Can. Fam. Physician 2018, 64, 832–840. [Google Scholar] [PubMed]
- Addey, D.; Shephard, A. Incidence, causes, severity and treatment of throat discomfort: A four-region online questionnaire survey. BMC Ear Nose Throat Disord. 2012, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, M.L.; De Sutter, A.; Deveugele, M.; Peersman, W.; Butler, C.C.; De Meyere, M.; De Maeseneer, J.; Christiaens, T. Are sore throat patients who hope for antibiotics actually asking for pain relief? Ann. Fam. Med. 2006, 4, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Shephard, A. A questionnaire-based study in 12 countries to investigate the drivers of antibiotic-seeking behavior for sore throat. J. Fam. Med. Community Health 2014, 1, 1014. [Google Scholar]
- Worrall, G.J. Acute sore throat. Can. Fam. Physician 2007, 53, 1961–1962. [Google Scholar]
- Bisno, A.L. Acute pharyngitis. N. Engl. J. Med. 2001, 344, 205–211. [Google Scholar] [CrossRef]
- Ebell, M.H.; Smith, M.A.; Barry, H.C.; Ives, K.; Carey, M. The rational clinical examination. Does this patient have strep throat? JAMA 2000, 284, 2912–2918. [Google Scholar] [CrossRef]
- Spinks, A.; Glasziou, P.P.; Del Mar, C.B. Antibiotics for sore throat. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Luo, R.; Sickler, J.; Vahidnia, F.; Lee, Y.C.; Frogner, B.; Thompson, M. Diagnosis and Management of Group a Streptococcal Pharyngitis in the United States, 2011–2015. BMC Infect. Dis. 2019, 19, 193. [Google Scholar] [CrossRef]
- Hawker, J.I.; Smith, S.; Smith, G.E.; Morbey, R.; Johnson, A.P.; Fleming, D.M.; Shallcross, L.; Hayward, A.C. Trends in antibiotic prescribing in primary care for clinical syndromes subject to national recommendations to reduce antibiotic resistance, UK 1995–2011: Analysis of a large database of primary care consultations. J. Antimicrob. Chemother. 2014, 69, 3423–3430. [Google Scholar] [CrossRef]
- Gulliford, M.C.; Dregan, A.; Moore, M.V.; Ashworth, M.; Staa, T.V.; McCann, G.; Charlton, J.; Yardley, L.; Little, P.; McDermott, L. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: Survey of 568 UK general practices. BMJ Open 2014, 4, e006245. [Google Scholar] [CrossRef] [PubMed]
- Kenealy, T.; Arroll, B. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Worrall, G.; Hutchinson, J.; Sherman, G.; Griffiths, J. Diagnosing streptococcal sore throat in adults: Randomized controlled trial of in-office aids. Can. Fam. Physician 2007, 53, 666–671. [Google Scholar]
- Dallas, A.; van Driel, M.; Morgan, S.; Tapley, A.; Henderson, K.; Ball, J.; Oldmeadow, C.; Davey, A.; Mulquiney, K.; Davis, J.; et al. Antibiotic prescribing for sore throat: A cross-sectional analysis of the ReCEnT study exploring the habits of early-career doctors in family practice. Fam. Pract. 2016, 33, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Goossens, H.; Ferech, M.; Vander Stichele, R.; Elseviers, M. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Antimicrobial Resistance Fact Sheet. Available online: http://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 21 July 2020).
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef] [PubMed]
- Riedel, S.; Beekmann, S.E.; Heilmann, K.P.; Richter, S.S.; Garcia-de-Lomas, J.; Ferech, M.; Goosens, H.; Doern, G.V. Antimicrobial use in Europe and antimicrobial resistance in Streptococcus pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis 2007, 26, 485–490. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Action Plan on Antimicrobial Resistance. Available online: http://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/ (accessed on 10 June 2020).
- Shulman, S.T.; Bisno, A.L.; Clegg, H.W.; Gerber, M.A.; Kaplan, E.L.; Lee, G.; Martin, J.M.; Van Beneden, C. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin. Infect. Dis 2012, 55, e86–e102. [Google Scholar] [CrossRef]
- Cohen, J.F.; Pauchard, J.Y.; Hjelm, N.; Cohen, R.; Chalumeau, M. Efficacy and safety of rapid tests to guide antibiotic prescriptions for sore throat. Cochrane Database Syst. Rev. 2020, 6, CD012431. [Google Scholar] [CrossRef]
- Thompson, T.Z.; McMullen, A.R. Group A Streptococcus Testing in Pediatrics: The Move to Point-of-Care Molecular Testing. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef]
- Melton, B.L.; Lai, Z. Review of community pharmacy services: What is being performed, and where are the opportunities for improvement? Integr. Pharm. Res. Pract. 2017, 6, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Goodman, C. Performance of retail pharmacies in low- and middle-income Asian settings: A systematic review. Health Policy Plan. 2016, 31, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Eflein, J. Number of Coronavirus (COVID-19) Tests Performed in the Most Impacted Countries Worldwide as of 13 July 2020. Available online: https://www.statista.com/statistics/1028731/covid19-tests-select-countries-worldwide/ (accessed on 14 July 2020).
- Buck, D. Testing Times: The Government’s Approach to Covid-19 Testing. Available online: https://www.kingsfund.org.uk/publications/governments-approach-covid-19-testing (accessed on 3 July 2020).
- Gubbins, P.O.; Klepser, M.E.; Dering-Anderson, A.M.; Bauer, K.A.; Darin, K.M.; Klepser, S.; Matthias, K.R.; Scarsi, K. Point-of-care testing for infectious diseases: Opportunities, barriers, and considerations in community pharmacy. J. Am. Pharm. Assoc. 2014, 54, 163–171. [Google Scholar] [CrossRef]
- Fine, A.M.; Nizet, V.; Mandl, K.D. Large-scale validation of the Centor and McIsaac scores to predict group A streptococcal pharyngitis. Arch. Intern. Med. 2012, 172, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Willis, B.H.; Coomar, D.; Baragilly, M. Comparison of Centor and McIsaac scores in primary care: A meta-analysis over multiple thresholds. Br. J. Gen. Pract 2020, 70, e245–e254. [Google Scholar] [CrossRef]
- Shephard, A.; Smith, G.; Aspley, S.; Schachtel, B.P. Randomised, double-blind, placebo-controlled studies on flurbiprofen 8.75 mg lozenges in patients with/without group A or C streptococcal throat infection, with an assessment of clinicians’ prediction of ‘strep throat’. Int. J. Clin. Pract. 2015, 69, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Orda, U.; Mitra, B.; Orda, S.; Fitzgerald, M.; Gunnarsson, R.; Rofe, G.; Dargan, A. Point of care testing for group A streptococci in patients presenting with pharyngitis will improve appropriate antibiotic prescription. Emerg. Med. Australas. 2016, 28, 199–204. [Google Scholar] [CrossRef]
- Parker, K.G.; Gandra, S.; Matushek, S.; Beavis, K.G.; Tesic, V.; Charnot-Katsikas, A. Comparison of 3 Nucleic Acid Amplification Tests and a Rapid Antigen Test with Culture for the Detection of Group A Streptococci from Throat Swabs. J. Appl. Lab. Med. 2019, 4, 164–169. [Google Scholar] [CrossRef]
- National Institute of Health and Care Excellence (NICE). Rapid Tests for Group A Streptococcal Infections in People with a Sore Throat. Available online: https://www.nice.org.uk/guidance/dg38 (accessed on 21 July 2020).
- Lean, W.L.; Arnup, S.; Danchin, M.; Steer, A.C. Rapid diagnostic tests for group A streptococcal pharyngitis: A meta-analysis. Pediatrics 2014, 134, 771–781. [Google Scholar] [CrossRef]
- Stewart, E.H.; Davis, B.; Clemans-Taylor, B.L.; Littenberg, B.; Estrada, C.A.; Centor, R.M. Rapid antigen group A streptococcus test to diagnose pharyngitis: A systematic review and meta-analysis. PLoS ONE 2014, 9, e111727. [Google Scholar] [CrossRef]
- Cohen, J.F.; Bertille, N.; Cohen, R.; Chalumeau, M. Rapid antigen detection test for group A streptococcus in children with pharyngitis. Cochrane Database Syst. Rev. 2016, 7, CD010502. [Google Scholar] [CrossRef]
- Oliver, J.; Malliya Wadu, E.; Pierse, N.; Moreland, N.J.; Williamson, D.A.; Baker, M.G. Group A Streptococcus pharyngitis and pharyngeal carriage: A meta-analysis. PLoS Negl. Trop Dis. 2018, 12, e0006335. [Google Scholar] [CrossRef] [PubMed]
- Llor, C.; Bjerrum, L.; Munck, A.; Cots, J.M.; Hernandez, S.; Moragas, A.; Investigators, H.A. Access to point-of-care tests reduces the prescription of antibiotics among antibiotic-requesting subjects with respiratory tract infections. Respir. Care 2014, 59, 1918–1923. [Google Scholar] [CrossRef] [PubMed]
- Bird, C.; Winzor, G.; Lemon, K.; Moffat, A.; Newton, T.; Gray, J. A Pragmatic Study to Evaluate the Use of a Rapid Diagnostic Test to Detect Group A Streptococcal Pharyngitis in Children With the Aim of Reducing Antibiotic Use in a UK Emergency Department. Pediatr. Emerg. Care 2018. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Berg, B.; Quezada, T.; Fader, R.; Walker, K.; Tang, S.; Cowen, U.; Duncan, D.; Sickler, J. Diagnosis and antibiotic treatment of group a streptococcal pharyngitis in children in a primary care setting: Impact of point-of-care polymerase chain reaction. BMC Pediatr. 2019, 19, 24. [Google Scholar] [CrossRef]
- Little, P.; Hobbs, R.; Moore, M.; Mant, D.; Williamson, I.; McNulty, C. Clinical score and rapid antigen detection test to guide antibiotic use for sore throats: Randomised controlled trial of PRISM (primary care streptococcal management). BMJ 2013, 347, f5806, Erratum in 2018, 360, k1068. [Google Scholar] [CrossRef]
- Mitsakakis, K.; Kaman, W.E.; Elshout, G.; Specht, M.; Hays, J.P. Challenges in identifying antibiotic resistance targets for point-of-care diagnostics in general practice. Future Microbiol. 2018, 13, 1157–1164. [Google Scholar] [CrossRef]
- Butler, C.C.; Simpson, S.; Wood, F. General practitioners’ perceptions of introducing near-patient testing for common infections into routine primary care: A qualitative study. Scand J. Prim. Health Care 2008, 26, 17–21. [Google Scholar] [CrossRef]
- Demore, B.; Tebano, G.; Gravoulet, J.; Wilcke, C.; Ruspini, E.; Birge, J.; Boivin, J.M.; Henard, S.; Dieterling, A.; Munerol, L.; et al. Rapid antigen test use for the management of group A streptococcal pharyngitis in community pharmacies. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1637–1645. [Google Scholar] [CrossRef]
- Papastergiou, J.; Trieu, C.R.; Saltmarche, D.; Diamantouros, A. Community pharmacist-directed point-of-care group A Streptococcus testing: Evaluation of a Canadian program. J. Am. Pharm Assoc. 2018, 58, 450–456. [Google Scholar] [CrossRef]
- Klepser, D.G.; Klepser, M.E.; Dering-Anderson, A.M.; Morse, J.A.; Smith, J.K.; Klepser, S.A. Community pharmacist-physician collaborative streptococcal pharyngitis management program. J. Am. Pharm Assoc 2016, 56, 323–329 e1. [Google Scholar] [CrossRef]
- Klepser, D.G.; Klepser, M.E.; Smith, J.K.; Dering-Anderson, A.M.; Nelson, M.; Pohren, L.E. Utilization of influenza and streptococcal pharyngitis point-of-care testing in the community pharmacy practice setting. Res. Soc. Adm. Pharm. 2018, 14, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, E.; Evans, A.; Cannings-John, R.; Ahmed, H.; Hood, K.; Reid, N.; Howe, R.; Williams, E.; Way, C. Impact of a pilot NHS-funded sore throat test and treat service in community pharmacies on provision and quality of patient care. BMJ Open Qual. 2020, 9, e000833. [Google Scholar] [CrossRef]
- Thornley, T.; Marshall, G.; Howard, P.; Wilson, A.P. A feasibility service evaluation of screening and treatment of group A streptococcal pharyngitis in community pharmacies. J. Antimicrob. Chemother. 2016, 71, 3293–3299. [Google Scholar] [CrossRef] [PubMed]
- Essack, S.; Blocker, A.; Dongen, M. Global Call to Healthcare Providers Treating COVID-19 Patients to Implement Diagnostic Stewardship/Microbial Diagnostics and Exercise Prudence in Prescribing Antibiotics. Available online: https://www.amr-insights.eu/call-to-healthcare-providers-treating-covid-19-patients-to-implement-diagnostic-stewardship-and-exercise-prudence-in-prescribing-antibiotics/ (accessed on 10 June 2020).
- Rawson, T.M.; Moore, L.S.P.; Castro-Sanchez, E.; Charani, E.; Davies, F.; Satta, G.; Ellington, M.J.; Holmes, A.H. COVID-19 and the potential long-term impact on antimicrobial resistance. J. Antimicrob. Chemother. 2020, 75, 1681–1684. [Google Scholar] [CrossRef] [PubMed]
- Merks, P.; Jakubowska, M.; Drelich, E.; Swieczkowski, D.; Bogusz, J.; Bilmin, K.; Sola, K.F.; May, A.; Majchrowska, A.; Koziol, M.; et al. The legal extension of the role of pharmacists in light of the COVID-19 global pandemic. Res. Soc. Adm. Pharm. 2020. [Google Scholar] [CrossRef] [PubMed]
- Essack, S.; Bell, J.; Shephard, A. Community pharmacists-Leaders for antibiotic stewardship in respiratory tract infection. J. Clin. Pharm. Ther. 2018, 43, 302–307. [Google Scholar] [CrossRef]
- National Institute of Health and Care Excellence (NICE). Sore Throat (Acute): Antimicrobial Prescribing. Available online: https://www.nice.org.uk/guidance/ng84/chapter/Recommendations (accessed on 10 July 2020).
- Pelucchi, C.; Grigoryan, L.; Galeone, C.; Esposito, S.; Huovinen, P.; Little, P.; Verheij, T. Guideline for the management of acute sore throat. Clin. Microbiol. Infect. 2012, 18 (Suppl. S1), 1–28. [Google Scholar] [CrossRef]
- Buss, V.H.; Deeks, L.S.; Shield, A.; Kosari, S.; Naunton, M. Analytical quality and effectiveness of point-of-care testing in community pharmacies: A systematic literature review. Res. Soc. Adm. Pharm. 2019, 15, 483–495. [Google Scholar] [CrossRef]
- Dulaney, K.; Hohmeier, K.; Fisher, C.; Cardosi, L.; Wasson, M. Exploring pharmacists’ perceptions regarding influenza and streptococcal testing within a chain pharmacy. J. Am. Pharm. Assoc. 2018, 58, 438–441 e1. [Google Scholar] [CrossRef]
- Mantzourani, E.; Hicks, R.; Evans, A.; Williams, E.; Way, C.; Deslandes, R. Community Pharmacist Views On The Early Stages Of Implementation Of A Pathfinder Sore Throat Test And Treat Service In Wales: An Exploratory Study. Integr. Pharm. Res. Pract. 2019, 8, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Lathia, N.; Sullivan, K.; Tam, K.; Brna, M.; MacNeil, P.; Saltmarche, D.; Agro, K. Cost-minimization analysis of community pharmacy-based point-of-care testing for strep throat in 5 Canadian provinces. Can. Pharm. J. 2018, 151, 322–331. [Google Scholar] [CrossRef]
- International Pharmaceutical Federation (FIP). FIP Statement of Policy: Point of Care Testing in Pharmacies; International Pharmaceutical Federation (FIP): New Orleans, LA, USA, 2004. [Google Scholar]
- Zhou, M.; Desborough, J.; Parkinson, A.; Douglas, K.; McDonald, D.; Boom, K. Barriers to pharmacist prescribing: A scoping review comparing the UK, New Zealand, Canadian and Australian experiences. Int. J. Pharm. Pract. 2019, 27, 479–489. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Role of Pharmacist in Encouraging Prudent Use of Antibiotics and Averting Antimicrobial Resistance: A Review of Policy and Experience in Europe; World Health Organization: Geneva, Switzerland, 2014.
- Courtenay, M.; Rowbotham, S.; Lim, R.; Peters, S.; Yates, K.; Chater, A. Examining influences on antibiotic prescribing by nurse and pharmacist prescribers: A qualitative study using the Theoretical Domains Framework and COM-B. BMJ Open 2019, 9, e029177. [Google Scholar] [CrossRef] [PubMed]
- Alhomoud, F.; Almahasnah, R.; Alhomoud, F.K. “You could lose when you misuse”-factors affecting over-the-counter sale of antibiotics in community pharmacies in Saudi Arabia: A qualitative study. BMC Health Serv. Res. 2018, 18, 915. [Google Scholar] [CrossRef]
- Gauld, N.J. Analysing the landscape for prescription to non-prescription reclassification (switch) in Germany: An interview study of committee members and stakeholders. BMC Health Serv. Res. 2019, 19, 404. [Google Scholar] [CrossRef]
- Duncan, P.; Ridd, M.J.; McCahon, D.; Guthrie, B.; Cabral, C. Barriers and enablers to collaborative working between GPs and pharmacists: A qualitative interview study. Br. J. Gen. Pract. 2020, 70, e155–e163. [Google Scholar] [CrossRef]
- Hindi, A.M.K.; Jacobs, S.; Schafheutle, E.I. Solidarity or dissonance? A systematic review of pharmacist and GP views on community pharmacy services in the UK. Health Soc. Care Community 2019, 27, 565–598. [Google Scholar] [CrossRef]
- Corn, C.E.; Klepser, D.G.; Dering-Anderson, A.M.; Brown, T.G.; Klepser, M.E.; Smith, J.K. Observation of a Pharmacist-Conducted Group A Streptococcal Pharyngitis Point-of-Care Test: A Time and Motion Study. J. Pharm Pract. 2018, 31, 284–291. [Google Scholar] [CrossRef]
- Nordin, N.; Ahmad Hassali, M.A.; Sarriff, A. A Global Picture of Extended Pharmacy Services, Perceptions, and Barriers toward Its Performance: A Systematic Review. Asian J. Pharm. Clin. Res. 2017, 10, 417–427. [Google Scholar] [CrossRef]
- Hassali, M.A.; Nordin, N.; Sarriff, A.; Saleem, F. Community Pharmacy Marketing in the New Era: A Global Picture of Extended Community Pharmacy Services. Marketing 2018, 129. [Google Scholar] [CrossRef]
- Kuupiel, D.; Bawontuo, V.; Mashamba-Thompson, T.P. Improving the Accessibility and Efficiency of Point-of-Care Diagnostics Services in Low- and Middle-Income Countries: Lean and Agile Supply Chain Management. Diagnostics 2017, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.E.; Kelling, S.E.; Bright, D.R. Pharmacy Technicians and Point of Care Testing. J. Pharm. Technol. 2015, 31, 143–148. [Google Scholar] [CrossRef]
- Working Group on Point of Care Testing in Primary and Community Care. Guidelines for Safe and Effective Management and Use of Point of Care Testing in Primary and Community Care. Available online: http://www.hpra.ie/docs/default-source/default-document-library/guidelines-for-point-of-care-testing-02.pdf?sfvrsn=0 (accessed on 21 July 2020).
- Kwon, K.T.; Ko, J.H.; Shin, H.; Sung, M.; Kim, J.Y. Drive-Through Screening Center for COVID-19: A Safe and Efficient Screening System against Massive Community Outbreak. J. Korean Med. Sci. 2020, 35, e123. [Google Scholar] [CrossRef] [PubMed]
- Abu Farha, R.; Abu Hammour, K.; Alefishat, E.; Alsaeed, H.; Alma’aiah, S. Drive-thru pharmacy service: Assessments of awareness, perception and barriers among pharmacists in Jordan. Saudi Pharm. J. 2017, 25, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Odukoya, O.K.; Chui, M.A.; Pu, J. Factors influencing quality of patient interaction at community pharmacy drive-through and walk-in counselling areas. Int. J. Pharm. Pract. 2014, 22, 246–256. [Google Scholar] [CrossRef]
- Global Respiratory Infection Partnership (GRIP). Global Respiratory Infection Partnership Advisory Board 2019 Meeting Report; Unpublished; 2019. [Google Scholar]
- Sessa, A.; (Italian College of General Practitioners and Primary Care, Florence, Italy). Personal communication, 2020.
- Goode, J.V.; Owen, J.; Page, A.; Gatewood, S. Community-Based Pharmacy Practice Innovation and the Role of the Community-Based Pharmacist Practitioner in the United States. Pharmacy 2019, 7, 106. [Google Scholar] [CrossRef]
- Glibreath, M. Point-of-Care Testing: Background Paper Prepared for the 2015–2016 APhA Policy Committee; American Pharmacy Association Policy Committee: Washington, DC, USA, 2014. [Google Scholar]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Malangu, N. The future of community pharmacy practice in South Africa in the light of the proposed new qualification for pharmacists: Implications and challenges. Glob. J. Health Sci. 2014, 6, 226–233. [Google Scholar] [CrossRef]
- Medicines and Related Sustances Act (Act 101 of 1965); South African Health Products Regulatory Authority (SAHPRA): Pretoria, South Africa, 1965; p. 14.
- Republic of South Africa Department of Health. Standard Treatment Guidelines and Essential Medicines List for South Africa, 6th ed.; The National Department of Health: Pretoria, South Africa, 2018.
- Sadek, M.M.; Elnour, A.A.; Al Kalbani, N.M.; Bhagavathula, A.S.; Baraka, M.A.; Aziz, A.M.; Shehab, A. Community pharmacy and the extended community pharmacist practice roles: The UAE experiences. Saudi Pharm. J. 2016, 24, 563–570. [Google Scholar] [CrossRef]
- Shephard, A.; Fung, J.; Cordeiro Pires, A. In In-Pharmacy Administration of Streptococcus Pyogenes Point-of-Care Testing: Data from New Zealand and Portugal. In Proceedings of the FIP World Congress, Seville, Spain, 12–16 September 2021. [Google Scholar]
- Goossens, H. VALUE-Dx Summary. Available online: https://value-dx.eu/wp-content/uploads/2019/11/VALUE-Dx-Summary_October-2019.pdf (accessed on 20 July 2020).
- Jones, L.F.; Owens, R.; Sallis, A.; Ashiru-Oredope, D.; Thornley, T.; Francis, N.A.; Butler, C.; McNulty, C.A.M. Qualitative study using interviews and focus groups to explore the current and potential for antimicrobial stewardship in community pharmacy informed by the Theoretical Domains Framework. BMJ Open 2018, 8, e025101. [Google Scholar] [CrossRef] [PubMed]
- Klepser, D.G.; Bisanz, S.E.; Klepser, M.E. Cost-effectiveness of pharmacist-provided treatment of adult pharyngitis. Am. J. Manag. Care 2012, 18, e145–e154. [Google Scholar] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essack, S.; Bell, J.; Burgoyne, D.; Tongrod, W.; Duerden, M.; Sessa, A.; Altiner, A.; Shephard, A. Point-of-Care Testing for Pharyngitis in the Pharmacy. Antibiotics 2020, 9, 743. https://doi.org/10.3390/antibiotics9110743
Essack S, Bell J, Burgoyne D, Tongrod W, Duerden M, Sessa A, Altiner A, Shephard A. Point-of-Care Testing for Pharyngitis in the Pharmacy. Antibiotics. 2020; 9(11):743. https://doi.org/10.3390/antibiotics9110743
Chicago/Turabian StyleEssack, Sabiha, John Bell, Douglas Burgoyne, Wirat Tongrod, Martin Duerden, Aurelio Sessa, Attila Altiner, and Adrian Shephard. 2020. "Point-of-Care Testing for Pharyngitis in the Pharmacy" Antibiotics 9, no. 11: 743. https://doi.org/10.3390/antibiotics9110743
APA StyleEssack, S., Bell, J., Burgoyne, D., Tongrod, W., Duerden, M., Sessa, A., Altiner, A., & Shephard, A. (2020). Point-of-Care Testing for Pharyngitis in the Pharmacy. Antibiotics, 9(11), 743. https://doi.org/10.3390/antibiotics9110743




