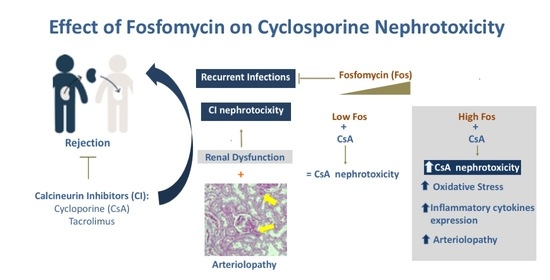
Effect of Fosfomycin on Cyclosporine Nephrotoxicity
Abstract
1. Introduction
2. Results
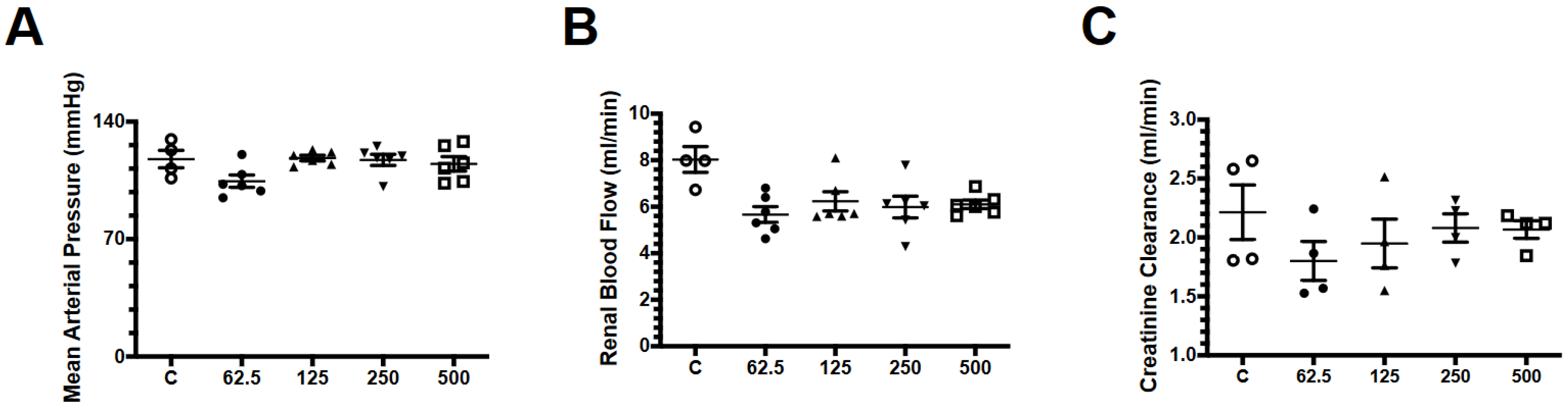
2.1. Effect of Fosfomycin on Renal Function
2.2. Effect of Fosfomycin on Renal Dysfunction Induced by CsA
2.3. Influence of CsA and Fosfomycin on Arteriolopathy, Oxidative, and Endoplasmic Reticulum Stress
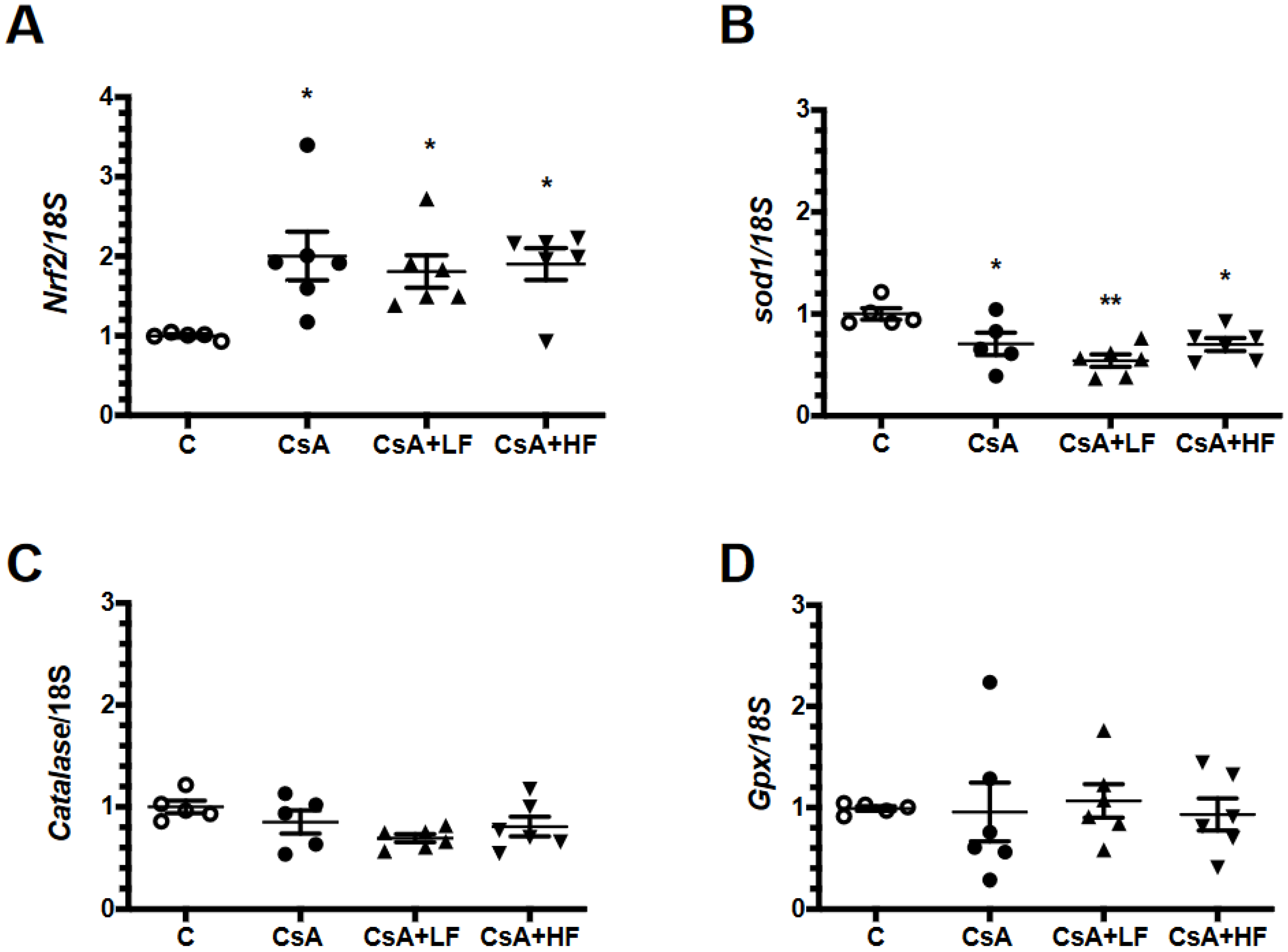
2.4. Renal mRNA Levels of Anti-Oxidative Enzymes during CsA Treatment and Fosfomycin Co-Administration
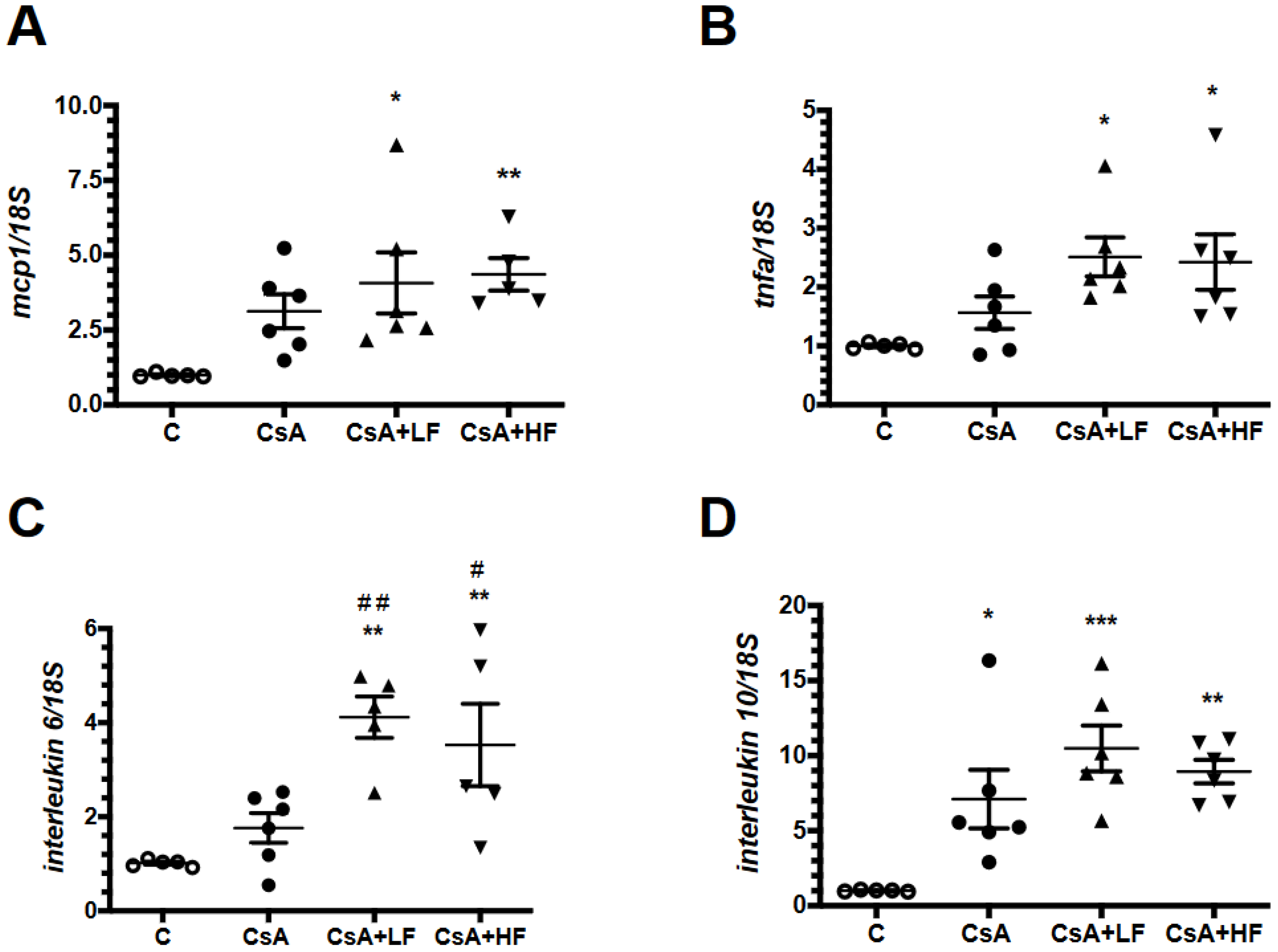
2.5. Effects of CsA and Fosfomycin on Renal Inflammation
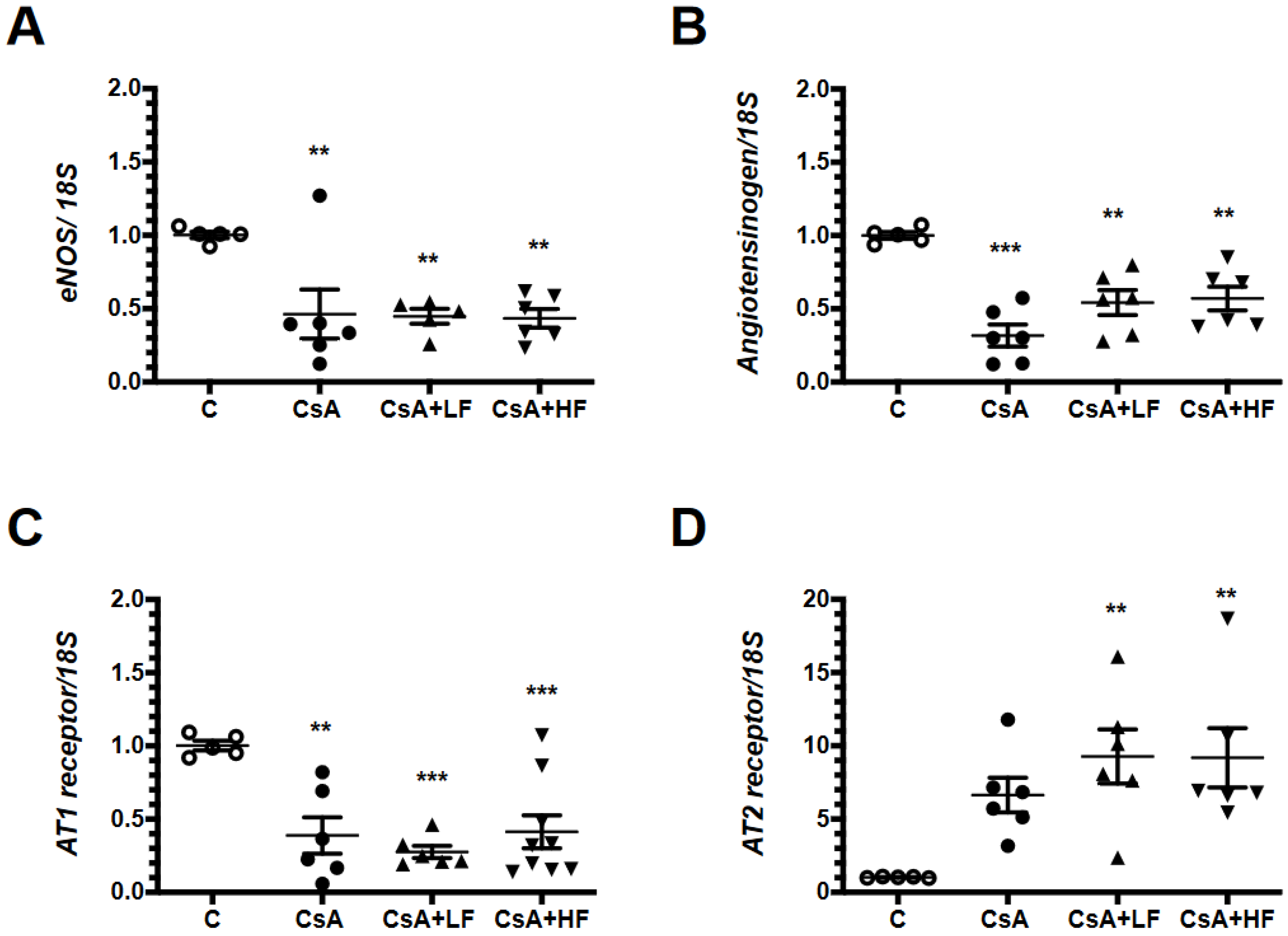
2.6. Renal mRNA Levels of Vasoactive Factors during CsA Treatment and Fosfomycin Co-Administration
2.7. Effects of CsA and Fosfomycin on mRNA Levels of the Endothelin Pathway
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Subject Inclusion, Housing, Randomization, and Size Calculation
4.3. Effect of Different Doses of Fosfomycin on Renal Function
4.4. Effect of Fosfomycin Administration on Cyclosporine Nephrotoxicity
4.5. Functional Parameters
4.6. Light Microscopy Analysis
4.7. Gene Expression Analysis
4.8. Urinary Hydrogen Peroxide Excretion
4.9. Serum Cyclosporin
4.10. Western Blot Analysis
4.11. Urinary 8-OHdG Excretion
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Czarkowska-pączek, B.; Wawiórko, E.; Młynarczyk, G.; Paczek, L. Antibiotic-resistant bacterial colonization increases the number of hospitalizations in patients after solid organ transplantation or with non-communicable diseases. Adv. Clin. Exp. Med. 2020, 29, 307–312. [Google Scholar]
- van Delden, C.; Stampf, S.; Hirsch, H.H.; Manuel, O.; Meylan, P.; Cusini, A.; Hirzel, C.; Khanna, N.; Weisser, M.; Garzoni, C.; et al. Burden and Timeline of Infectious Diseases in the First Year After Solid Organ Transplantation in the Swiss Transplant Cohort Study. Clin. Infect. Dis. 2020, XX, 1–11. [Google Scholar] [CrossRef]
- Aguado, J.M.; Silva, J.T.; Fernández-Ruiz, M.; Cordero, E.; Fortún, J.; Gudiol, C.; Martínez-Martínez, L.; Vidal, E.; Almenar, L.; Almirante, B.; et al. Management of multidrug resistant Gram-negative bacilli infections in solid organ transplant recipients: SET/GESITRA-SEIMC/REIPI recommendations. Transplant. Rev. 2018, 32, 36–57. [Google Scholar] [CrossRef] [PubMed]
- Bodro, M.; Sabé, N.; Tubau, F.; Lladó, L.; Baliellas, C.; Roca, J.; Cruzado, J.M.; Carratalà, J. Risk factors and outcomes of bacteremia caused by drug-resistant ESKAPE pathogens in solid-organ transplant recipients. Transplantation 2013, 96, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Rowe, T.A.; Juthani-Mehta, M. Diagnosis and management of urinary tract infection in older adults. Infect. Dis. Clin. North Am. 2014, 28, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Vouloumanou, E.K.; Samonis, G.; Vardakas, K.Z. Fosfomycin. Clin. Microbiol. Rev. 2016, 29, 321–347. [Google Scholar] [CrossRef] [PubMed]
- López-Medrano, F.; Silva, J.T.; Fernández-Ruiz, M.; Vidal, E.; Origüen, J.; Calvo-Cano, A.; Luna-Huerta, E.; Merino, E.; Hernández, D.; Jironda-Gallegos, C.; et al. Oral fosfomycin for the treatment of lower urinary tract infections among kidney transplant recipients—Results of a Spanish multicenter cohort. Am. J. Transplant. 2020, 20, 451–462. [Google Scholar] [CrossRef]
- Patel, S.S.; Balfour, J.A.; Bryson, H.M. Fosfomycin tromethamine. Drugs 1997, 53, 637–656. [Google Scholar] [CrossRef]
- Arreola-Guerra, J.M.; Rosado-Canto, R.; Alberú, J.; Maravilla, E.; Torres-González, P.; Criollo, E.; Pérez, M.; Mancilla, E.; Arvizu, M.; Morales-Buenrostro, L.E.; et al. Fosfomycin trometamol in the prophylaxis of post-kidney transplant urinary tract infection: A controlled, randomized clinical trial. Transpl. Infect. Dis. 2018, 20, e12980. [Google Scholar] [CrossRef]
- Ekberg, H.; Tedesco-Silva, H.; Demirbas, A.; Víkto, S.; Nashan, B.; Gürkan, A.; Margreiter, R.; Hugo, C.; Grinyó, J.M.; Frei, U.; et al. Reduced exposure to calcineurin inhibitors in renal transplantation [8]. N. Engl. J. Med. 2008, 358, 2562–2575. [Google Scholar] [CrossRef]
- Azzi, J.R.; Sayegh, M.H.; Mallat, S.G. Calcineurin Inhibitors: 40 Years Later, Can’t Live Without …. J. Immunol. 2013, 191, 5785–5791. [Google Scholar] [CrossRef] [PubMed]
- Borel, J.F.; Feurer, C.; Gubler, H.U.; Stähelin, H. Biological effects of cyclosporin A: A new antilymphocytic agent. Agents Actions 1994, 6, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.W.; Zimmer, G.; Chen, J.; Ladd, D.; Li, E.; Alt, F.W.; Wiederrecht, G.; Cryan, J.; O´Neil, E.A.; Seidman, C.E.; et al. T Cell Responses in Calcineurin A Alpha-Deficient Mice. J. Exp. Med. 1996, 183, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Miroux, C.; Moralès, O.; Carpentier, A.; Dharancy, S.; Conti, F.; Boleslowski, E.; Podevin, P.; Auriault, C.; Pancré, V.; Delhem, N. Inhibitory Effects of Cyclosporine on Human Regulatory T Cells In Vitro. Transplant. Proc. 2009, 41, 3371–3374. [Google Scholar] [CrossRef] [PubMed]
- European Multicentre Trial Group Cyclosporin in Cadaveric Renal Transplantation: One-Year Follow-Up of a Multicentre Trial. Lancet 1983, 322, 986–989. [CrossRef]
- Myers, B.D.; Ross, J.; Newton, L.; Luetscher, J.; Perlroth, M. Cyclosporine-Associated Chronic Nephropathy. N. Engl. J. Med. 1984, 311, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.M.; Paller, M.S.; Ferris, T.F. Effect of cyclosporine administration on renal hemodynamics in conscious rats. Kidney Int. 1985, 28, 767–774. [Google Scholar] [CrossRef]
- Pérez-Rojas, J.M.; Derive, S.; Blanco, J.A.; Cruz, C.; De La Maza, L.M.; Gamba, G.; Bobadilla, N.A. Renocortical mRNA expression of vasoactive factors during spironolactone protective effect in chronic cyclosporine nephrotoxicity. Am. J. Physiol. Ren. Physiol. 2005, 289, F1020–F1030. [Google Scholar] [CrossRef]
- Bobadilla, N.A.; Gamba, G. New insights into the pathophysiology of cyclosporine nephrotoxicity: A role of aldosterone. Am. J. Physiol. Ren. Physiol. 2007, 293, 2–9. [Google Scholar] [CrossRef]
- Sack, K.; Schulz, E.; Marre, R.; Kreft, B. Fosfomycin Protects Against Tubulotoxicity Induced by Cis-Diaminedichloroplatin and Cyclosporin A in the rat. Klin. Wochenschr. 1987, 65, 525–527. [Google Scholar] [CrossRef]
- Yanagida, C.; Ito, K.; Komiya, I.; Horie, T. Protective effect of fosfomycin on gentamicin-induced lipid peroxidation of rat renal tissue. Chem. Biol. Interact. 2004, 148, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kumano, N.; Koinumaru, S.; Oizumi, K.; Konno, K. cis-Diamminedichloroplatinum (CDDP)-Fosfomycin (FOM) Combination in Lung Cancer Patients with Respect to the Peripheral T Lymphocyte Subsets. Tohoku J. Exp. Med. 1986, 148, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Hayasahi, M.; Numaguchi, M.; Watabe, H.; Enomoto, H.; Yaoi, Y. Cisplatin-induced nephrotoxicity and the protective effect of fosfomycin on it as demonstrated by using a crossover study of urinary metabolite levels. Acta Obstet. Gynecol. Scand. 1997, 76, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Rojanasthien, N.; Kumsorn, B.; Atikachai, B.; Leotrakul, S.; Thongprasert, S. Protective Effects of Fosfomycin on Cysplatin-Induced Nephrotoxicity in Patients With Lung Cancer. Int. J. Clin. Pharmacol. Ther. 2001, 39, 121–125. [Google Scholar] [CrossRef]
- Khanna, A.; Plummer, M.; Bromberek, C.; Bresnahan, B.; Hariharan, S. Expression of TGF-β and fibrogenic genes in transplant recipients with tacrolimus and cyclosporine nephrotoxicity. Kidney Int. 2002, 62, 2257–2263. [Google Scholar] [CrossRef]
- Nankiwell, B.J.; Borrows, R.J.; Fung, C.L.-S.; O´Connell, P.J.; Allen, R.D.M.; Chapman, J.R. The natural history of chronic allograft nephropathy. N. Engl. J. Med. 2003, 349, 2326–2333. [Google Scholar] [CrossRef]
- Rao, S.R.; Sundararajan, S.; Subbarayan, R.; Murugan Girija, D. Cyclosporine-A induces endoplasmic reticulum stress and influences pro-apoptotic factors in human gingival fibroblasts. Mol. Cell. Biochem. 2017, 429, 179–185. [Google Scholar] [CrossRef]
- Morikawa, K.; Watabe, H.; Araake, M.; Morikawa, S. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob. Agents Chemother. 1996, 40, 1366–1370. [Google Scholar] [CrossRef]
- Bobadilla, N.A.; Tapia, E.; Franco, M.; López, P.; Mendoza, S.; García-Torres, R.; Alvarado, J.A.; Herrera-Acosta, J. Role of nitric oxide in renal hemodynamic abnormalities of cyclosporin nephrotoxicity. Kidney Int. 1994, 46, 773–779. [Google Scholar] [CrossRef]
- Bobadilla, N.A.; Gamba, G.; Tapia, E.; García-Torres, R.; Bolio, A.; López-Zetina, P.; Herrera-Acosta, J. Role of NO in cyclosporin nephrotoxicity: Effects of chronic NO inhibition and NO synthases gene expression. Am. J. Physiol. Ren. Physiol. 1998, 274, 791–798. [Google Scholar] [CrossRef]
- Bergan, T.; Thorsteinsson, S.B.; Albini, E. Pharmacokinetic profile of Fosfomycin Trometamol. Pharmacology 1993, 39, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Faulds, D.; Goa, K.L.; Benfield, P. Cyclosporin: A Review of its Pharmacodynamic and Pharmacokinetic Properties, and Therapeutic Use in Immunoregulatory Disorders. Drugs 1993, 45, 953–1040. [Google Scholar] [CrossRef]
- Ghaznavi, R.; Zahmatkesh, M.; Kadkhodaee, M.; Mahdavi-Mazdeh, M. Cyclosporine Effects on the Antioxidant Capacity of Rat Renal Tissues. Transplant. Proc. 2007, 39, 866–867. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Kokuryo, T.; Hashimoto, Y.; Inui, K. Effects of Fosfomycin and Imipenem ± Cilastatin on the Nephrotoxicity of Vancomycin and Cisplatin in Rats. J. Pharm. Pharmacol. 1999, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Xie, J.; Song, C.; Liu, J.; Zheng, J.; Liu, C.; Zhang, X.; Li, P.; Wang, F. The Activation of Nrf2 and Its Downstream Regulated Genes Mediates the Antioxidative Activities of Xueshuan Xinmaining Tablet in Human Umbilical Vein Endothelial Cells. Evidence Based Complement. Altern. Med. 2015, 2015, 187265. [Google Scholar] [CrossRef]
- Zeitlinger, M.; Marsik, C.; Steiner, I.; Sauermann, R.; Seir, K.; Jilma, B.; Wagner, O.; Joukhadar, C. Immunomodulatory effects of fosfomycin in an endotoxin model in human blood. J. Antimicrob. Chemother. 2007, 59, 219–223. [Google Scholar] [CrossRef]
- Okabayashi, T.; Yokota, S.I.; Yoto, Y.; Tsutsumi, H.; Fujii, N. Fosfomycin suppresses chemokine induction in airway epithelial cells infected with respiratory syncytial virus. Clin. Vaccine Immunol. 2009, 16, 859–865. [Google Scholar] [CrossRef]
- Jang, H.R.; Rabb, H. The innate immune response in ischemic acute kidney injury. Clin. Immunol. 2009, 130, 41–50. [Google Scholar] [CrossRef]
- Rafiee, P.; Johnson, C.P.; Li, M.S.; Ogawa, H.; Heidemann, J.; Fisher, P.J.; Lamirand, T.H.; Otterson, M.F.; Wilson, K.T.; Binion, D.G. Cyclosporine A enhances leukocyte binding by human intestinal microvascular endothelial cells through inhibition of p38 MAPK and iNOS: Paradoxical proinflammatory effect on the microvascular endothelium. J. Biol. Chem. 2002, 277, 35605–35615. [Google Scholar] [CrossRef]
- Yayama, K.; Horii, M.; Hiyoshi, H.; Takano, M.; Okamoto, H.; Kagota, S.; Kunitomo, M. Up-Regulation of Angiotensin II Type 2 Receptor in Rat Thoracic Aorta by Pressure-Overload. J. Pharmacol. Exp. Ther. 2004, 308, 736–743. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Trejo, J.A.; Pérez-Villalva, R.; Arreola-Guerra, J.M.; Ramírez, V.; Sifuentes-Osornio, J.; Bobadilla, N.A. Effect of Fosfomycin on Cyclosporine Nephrotoxicity. Antibiotics 2020, 9, 720. https://doi.org/10.3390/antibiotics9100720
Ortega-Trejo JA, Pérez-Villalva R, Arreola-Guerra JM, Ramírez V, Sifuentes-Osornio J, Bobadilla NA. Effect of Fosfomycin on Cyclosporine Nephrotoxicity. Antibiotics. 2020; 9(10):720. https://doi.org/10.3390/antibiotics9100720
Chicago/Turabian StyleOrtega-Trejo, Juan Antonio, Rosalba Pérez-Villalva, José M. Arreola-Guerra, Victoria Ramírez, José Sifuentes-Osornio, and Norma A Bobadilla. 2020. "Effect of Fosfomycin on Cyclosporine Nephrotoxicity" Antibiotics 9, no. 10: 720. https://doi.org/10.3390/antibiotics9100720
APA StyleOrtega-Trejo, J. A., Pérez-Villalva, R., Arreola-Guerra, J. M., Ramírez, V., Sifuentes-Osornio, J., & Bobadilla, N. A. (2020). Effect of Fosfomycin on Cyclosporine Nephrotoxicity. Antibiotics, 9(10), 720. https://doi.org/10.3390/antibiotics9100720