Prevalence and Epidemiology of Multidrug-Resistant Pathogens in the Food Chain and the Urban Environment in Northwestern Germany
Abstract
1. Introduction
2. Results
2.1. Prevalence of AMR Genes
2.2. Genotyping of Selected Isolates
3. Discussion
4. Materials and Methods
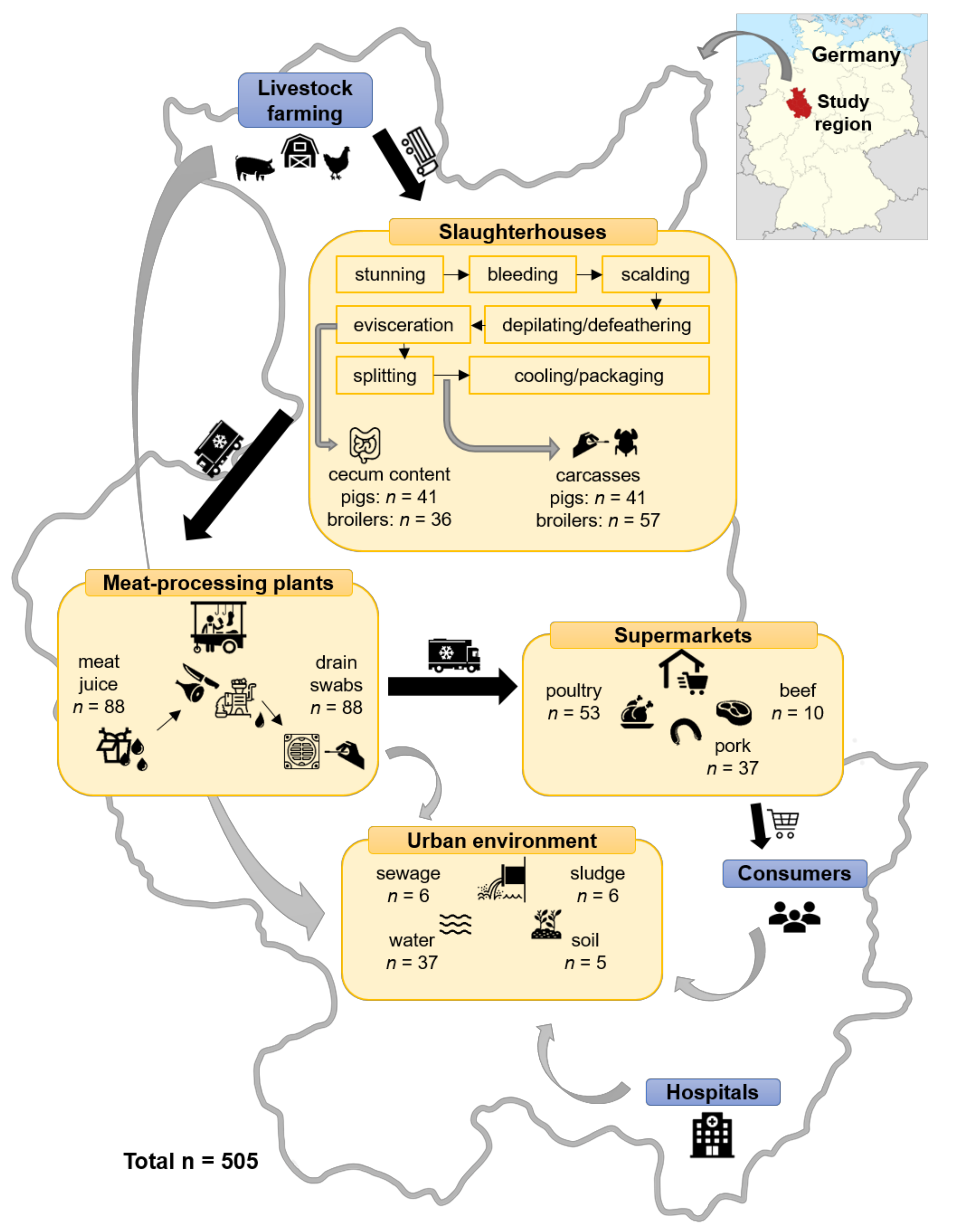
4.1. Sample Collection
4.2. Molecular and Culture-Based Screening for Col-E, CPE, VRE, and ESBL-Producers
4.3. Molecular Typing of Selected Isolates
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwarz, S.; Silley, P.; Simjee, S.; Woodford, N.; van Duijkeren, E.; Johnson, A.P.; Gaastra, W. Assessing the antimicrobial susceptibility of bacteria obtained from animals. Vet. Microbiol. 2010, 141, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, P.M.; Loureiro, L.; Matos, A.J.F. Transfer of multidrug-resistant bacteria between intermingled ecological niches: The interface between humans, animals and the environment. Int. J. Environ. Res. Public Health 2013, 10, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Hille, K.; Ruddat, I.; Mellmann, A.; Köck, R.; Kreienbrock, L. Simultaneous occurrence of MRSA and ESBL-producing Enterobacteriaceae on pig farms and in nasal and stool samples from farmers. Vet. Microbiol. 2017, 200, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Friese, A.; Schulz, J.; Laube, H.; von Salviati, C.; Hartung, J.; Roesler, U. Faecal occurrence and emissions of livestock-associated methicillin-resistant Staphylococcus aureus (laMRSA) and ESBL/AmpC-producing E. coli from animal farms in Germany. Berl. Münch. Tierärztl. Wochenschr. 2013, 126, 175–180. [Google Scholar]
- Beneke, B.; Klees, S.; Stührenberg, B.; Fetsch, A.; Kraushaar, B.; Tenhagen, B.A. Prevalence of methicillin-resistant Staphylococcus aureus in a fresh meat pork production chain. J. Food Prot. 2011, 74, 126–129. [Google Scholar] [CrossRef]
- Schulz, J.; Friese, A.; Klees, S.; Tenhagen, B.A.; Fetsch, A.; Rösler, U.; Hartung, J. Longitudinal study of the contamination of air and of soil surfaces in the vicinity of pig barns by livestock-associated methicillin-resistant Staphylococcus aureus. Appl. Environ. Microbiol. 2012, 78, 5666–5671. [Google Scholar] [CrossRef]
- Köck, R.; Daniels-Haardt, I.; Becker, K.; Mellmann, A.; Friedrich, A.W.; Mevius, D.; Schwarz, S.; Jurke, A. Carbapenem-resistant Enterobacteriaceae in wildlife, food-producing, and companion animals: A systematic review. Clin. Microbiol. Infect. 2018, 24, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Roschanski, N.; Falgenhauer, L.; Grobbel, M.; Guenther, S.; Kreienbrock, L.; Imirzalioglu, C.; Roesler, U. Retrospective survey of mcr-1 and mcr-2 in German pig-fattening farms, 2011–2012. Int. J. Antimicrob. Agents 2017, 50, 266–271. [Google Scholar] [CrossRef]
- Ceccarelli, D.; Van Essen-Zandbergen, A.; Veldman, K.T.; Tafro, N.; Haenen, O.; Mevius, D.J. Chromosome-based blaOXA-48-like variants in Shewanella species isolates from food-producing animals, fish, and the aquatic environment. Antimicrob. Agents Chemother. 2017, 61, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Johnson, A.P. Transferable resistance to colistin: A new but old threat. J. Antimicrob. Chemother. 2016, 71, 2066–2070. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, R.; Schwarz, S.; Wu, C.; Shen, J.; Walsh, T.R.; Wang, Y. Farm animals and aquaculture: Significant reservoirs of mobile colistin resistance genes. Environ. Microbiol. 2020, 22, 2469–2484. [Google Scholar] [CrossRef] [PubMed]
- Przybysz, S.M.; Correa-Martinez, C.; Köck, R.; Becker, K.; Schaumburg, F. SuperPolymyxinTM medium for the screening of colistin-resistant gram-negative bacteria in stool samples. Front. Microbiol. 2018, 9, 2809. [Google Scholar] [CrossRef] [PubMed]
- Remschmidt, C.; Schröder, C.; Behnke, M.; Gastmeier, P.; Geffers, C.; Kramer, T.S. Continuous increase of vancomycin resistance in enterococci causing nosocomial infections in Germany - 10 years of surveillance. Antimicrob. Resist. Infect. Control 2018, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Pauly, N.; Hammerl, J.A.; Grobbel, M.; Tenhagen, B.A.; Käsbohrer, A.; Bisenius, S.; Fuchs, J.; Horlacher, S.; Lingstädt, H.; Mauermann, U.; et al. ChromID® CARBA agar fails to detect carbapenem-resistant Enterobacteriaceae with slightly reduced susceptibility to carbapenems. Front. Microbiol. 2020, 11, 1678. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.; Sib, E.; Gajdiss, M.; Klanke, U.; Lenz-Plet, F.; Barabasch, V.; Albert, C.; Schallenberg, A.; Timm, C.; Zacharias, N.; et al. Dissemination of multi-resistant Gram-negative bacteria into German wastewater and surface waters. FEMS Microbiol. Ecol. 2018, 94. [Google Scholar] [CrossRef]
- Borowiak, M.; Baumann, B.; Fischer, J.; Thomas, K.; Deneke, C.; Hammerl, J.A.; Szabo, I.; Malorny, B. Development of a novel mcr-6 to mcr-9 multiplex PCR and assessment of mcr-1 to mcr-9 occurrence in colistin-resistant salmonella enterica isolates from environment, feed, animals and food (2011–2018) in Germany. Front. Microbiol. 2020, 11, 80. [Google Scholar] [CrossRef]
- Irrgang, A.; Roschanski, N.; Tenhagen, B.A.; Grobbel, M.; Skladnikiewicz-Ziemer, T.; Thomas, K.; Roesler, U.; Käsbohrer, A. Prevalence of mcr-1 in E. coli from livestock and food in Germany, 2010-2015. PLoS ONE 2016, 11, e0159863. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, J.; Hu, Q.; Rao, X. Morganella morganii, a non-negligent opportunistic pathogen. Int. J. Infect. Dis. 2016, 50, 10–17. [Google Scholar] [CrossRef]
- Bundesministerium für Ernährung und Landwirtschaft (BMEL); Bundesamt für Verbraucherschutz und Lebensmittelsicherheit (BVL); Bundesinstitut für Risikobewertung (BfR). Lagebild zur Antibiotikaresistenz im Bereich Tierhaltung und Lebensmittelkette. 2018. Available online: https://www.bmel.de/SharedDocs/Downloads/DE/_Tiere/Tiergesundheit/Tierarzneimittel/Lagebild-Antibiotikaeinsatz-bei-Tieren-Juli-2018.pdf?__blob=publicationFile&v=2 (accessed on 17 September 2020).
- Moennighoff, C.; Thomas, N.; Nienhaus, F.; Hartmann, M.; Menrath, A.; Merkel, J.; Detlefsen, H.; Kreienbrock, L.; Hennig-Pauka, I. Phenotypic antimicrobial resistance in Escherichia coli strains isolated from swine husbandries in North Western Germany - Temporal patterns in samples from laboratory practice from 2006 to 2017. BMC Vet. Res. 2020, 16, 37. [Google Scholar] [CrossRef]
- Schaumburg, F.; Sertic, S.M.; Correa-Martinez, C.; Mellmann, A.; Köck, R.; Becker, K. Acquisition and colonization dynamics of antimicrobial-resistant bacteria during international travel: A prospective cohort study. Clin. Microbiol. Infect. 2019, 25, 1287.e1–1287.e7. [Google Scholar] [CrossRef]
- Terveer, E.M.; Nijhuis, R.H.T.; Crobach, M.J.T.; Knetsch, C.W.; Veldkamp, K.E.; Gooskens, J.; Kuijper, E.J.; Claas, E.C.J. Prevalence of colistin resistance gene (mcr-1) containing Enterobacteriaceae in feces of patients attending a tertiary care hospital and detection of a mcr-1 containing, colistin susceptible E. coli. PLoS ONE 2017, 12, e0178598. [Google Scholar] [CrossRef]
- Saly, M.; Jayol, A.; Poirel, L.; Megraud, F.; Nordmann, P.; Dubois, V. Prevalence of faecal carriage of colistin-resistant gram-negative rods in a university hospital in Western France, 2016. J. Med. Microbiol. 2017, 66, 842–843. [Google Scholar] [CrossRef][Green Version]
- Zurfluh, K.; Stephan, R.; Widmer, A.; Poirel, L.; Nordmann, P.; Nüesch, H.J.; Hächler, H.; Nüesch-Inderbinen, M. Screening for fecal carriage of MCR-producing Enterobacteriaceae in healthy humans and primary care patients. Antimicrob. Resist. Infect. Control 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Bich, V.T.N.; Thanh, L.V.; Thai, P.D.; Van Phuong, T.T.; Oomen, M.; Driessen, C.; Beuken, E.; Hoang, T.H.; Van Doorn, H.R.; Penders, J.; et al. An exploration of the gut and environmental resistome in a community in northern Vietnam in relation to antibiotic use. Antimicrob. Resist. Infect. Control 2019, 8, 194. [Google Scholar] [CrossRef]
- Giani, T.; Sennati, S.; Antonelli, A.; Di Pilato, V.; Di Maggio, T.; Mantella, A.; Niccolai, C.; Spinicci, M.; Monasterio, J.; Castellanos, P.; et al. High prevalence of carriage of mcr-1-positive enteric bacteria among healthy children from rural communities in the Chaco region, Bolivia, september to october 2016. Eurosurveillance 2018, 23, 1800115. [Google Scholar] [CrossRef]
- Trung, N.V.; Matamoros, S.; Carrique-Mas, J.J.; Nghia, N.H.; Nhung, N.T.; Chieu, T.T.B.; Mai, H.H.; van Rooijen, W.; Campbell, J.; Wagenaar, J.A.; et al. Zoonotic transmission of mcr-1 colistin resistance gene from small-scale poultry farms, Vietnam. Emerg. Infect. Dis. 2017, 23, 529–532. [Google Scholar] [CrossRef]
- Mughini-Gras, L.; Dorado-García, A.; van Duijkeren, E.; van den Bunt, G.; Dierikx, C.M.; Bonten, M.J.M.; Bootsma, M.C.J.; Schmitt, H.; Hald, T.; Evers, E.G.; et al. Attributable sources of community-acquired carriage of Escherichia coli containing β-lactam antibiotic resistance genes: A population-based modelling study. Lancet Planet. Health 2019, 3, e357–e369. [Google Scholar] [CrossRef]
- Bager, F.; Madsen, M.; Christensen, J.; Aarestrup, F.M. Avoparcin used as a growth promoter is associated with the occurrence of vancomycin-resistant Enterococcus faecium on Danish poultry and pig farms. Prev. Vet. Med. 1997, 31, 95–112. [Google Scholar] [CrossRef]
- Klare, I.; Badstübner, D.; Konstabel, C.; Böhme, G.; Claus, H.; Witte, W. Decreased incidence of VanA-type vancomycin-resistant enterococci isolated from poultry meat and from fecal samples of humans in the community after discontinuation of avoparcin usage in animal husbandry. Microb. Drug Resist. 1999, 5, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, G.S.; Haaheim, H.; Dahl, K.H.; Kruse, H.; Løvseth, A.; Olsvik, Ø.; Sundsfjord, A. Transmission of vanA-type vancomycin-resistant enterococci and vanA resistance elements between chicken and humans at avoparcin-exposed farms. Microb. Drug Resist. 1998, 4, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Borgen, K.; Sorum, M.; Wasteson, Y.; Kruse, H. VanA-type vancomycin-resistant enterococci (VRE) remain prevalent in poultry carcasses 3 years after avoparcin was banned. Int. J. Food Microbiol. 2001, 64, 89–94. [Google Scholar] [CrossRef]
- Del Grosso, M.; Caprioli, A.; Chinzari, P.; Fontana, M.C.; Pezzotti, G.; Manfrin, A.; Di Giannatale, E.; Goffredo, E.; Pantosti, A. Detection and characterization of vancomycin-resistant enterococci in farm animals and raw meat products in Italy. Microb. Drug Resist. 2000, 6, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.K.; Kim, T.S.; Lee, H.S.; Nam, H.M.; Joo, Y.S.; Koh, H.B. Persistence of vanA-type Enterococcus faecium in Korean livestock after ban on avoparcin. Microb. Drug Resist. 2006, 12, 136–139. [Google Scholar] [CrossRef]
- Heuer, O.E.; Pedersen, K.; Jensen, L.B.; Madsen, M.; Olsen, J.E. Persistence of vancomycin-resistant enterococci (VRE) in broiler houses after the avoparcin ban. Microb. Drug Resist. 2002, 8, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Leinweber, H.; Alotaibi, S.M.I.; Overballe-Petersen, S.; Hansen, F.; Hasman, H.; Bortolaia, V.; Hammerum, A.M.; Ingmer, H. Vancomycin resistance in Enterococcus faecium isolated from Danish chicken meat is located on a pVEF4-like plasmid persisting in poultry for 18 years. Int. J. Antimicrob. Agents 2018, 52, 283–286. [Google Scholar] [CrossRef]
- Sting, R.; Richter, A.; Popp, C.; Hafez, H.M. Occurrence of vancomycin-resistant enterococci in Turkey flocks. Poult. Sci. 2013, 92, 346–351. [Google Scholar] [CrossRef]
- Top, J.; Willems, R.; Bonten, M. Emergence of CC17 Enterococcus faecium: From commensal to hospital-adapted pathogen. FEMS Immunol. Med. Microbiol. 2008, 52, 297–308. [Google Scholar] [CrossRef]
- Savin, M.; Bierbaum, G.; Hammerl, J.A.; Heinemann, C.; Parcina, M.; Sib, E.; Voigt, A.; Kreyenschmidt, J. Antibiotic-resistant bacteria and antimicrobial residues in wastewater and process water from German pig slaughterhouses and their receiving municipal wastewater treatment plants. Sci. Total Environ. 2020, 727, 138788. [Google Scholar] [CrossRef]
- Correa-Martinez, C.L.; Tönnies, H.; Froböse, N.J.; Mellmann, A.; Kampmeier, S. Transmission of vancomycin-resistant enterococci in the hospital setting: Uncovering the patient– environment interplay. Microorganisms 2020, 8. [Google Scholar] [CrossRef]
- Rebelo, A.R.; Bortolaia, V.; Kjeldgaard, J.S.; Pedersen, S.K.; Leekitcharoenphon, P.; Hansen, I.M.; Guerra, B.; Malorny, B.; Borowiak, M.; Hammerl, J.A.; et al. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Eurosurveillance 2018, 23, 1–11. [Google Scholar] [CrossRef]
- Hasman, H.; Agersø, Y.; Hendriksen, R.; Cavaco, L.M.; Guerra-Roman, B. Isolation of ESBL-AmpC-and Carbapenemase-Producing E. coli from Fresh Meat; Laboratory Protocol. Version 4; DTU Foof: Lyngby, Denmark, 2018. [Google Scholar]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Zankari, E.; Garcia-Fernandez, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Moller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
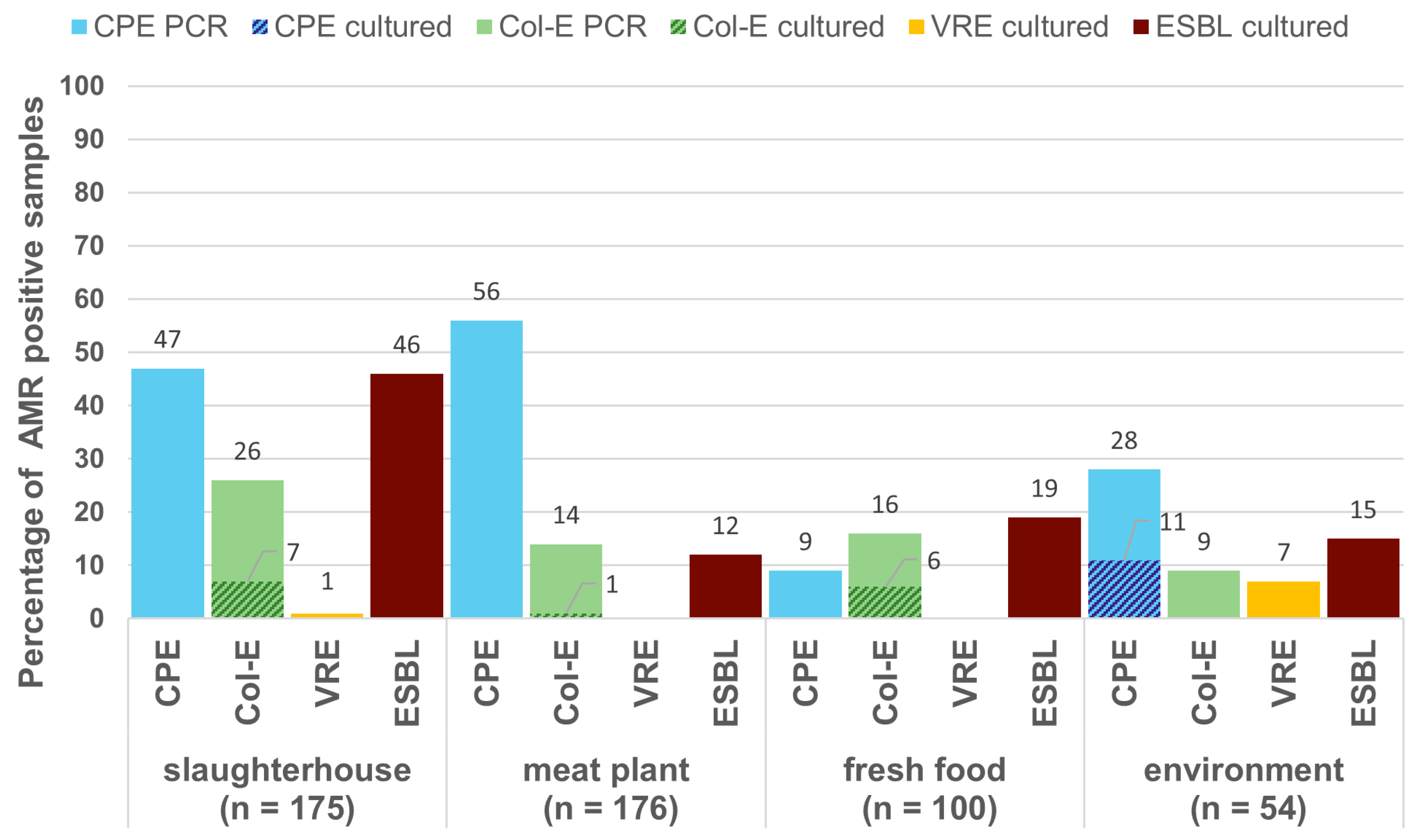
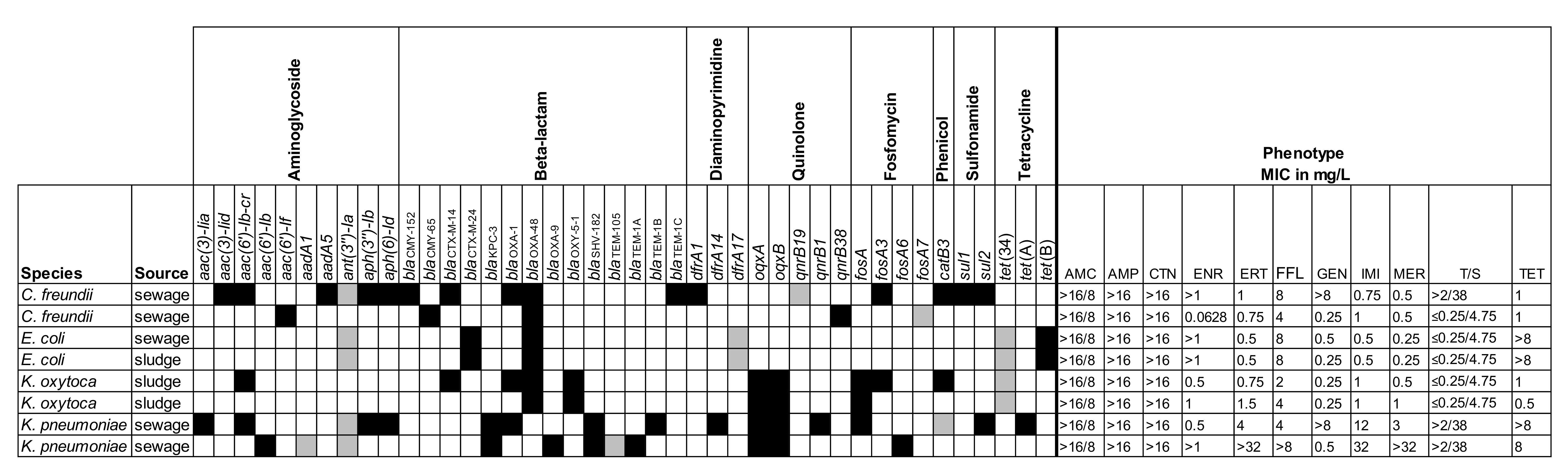

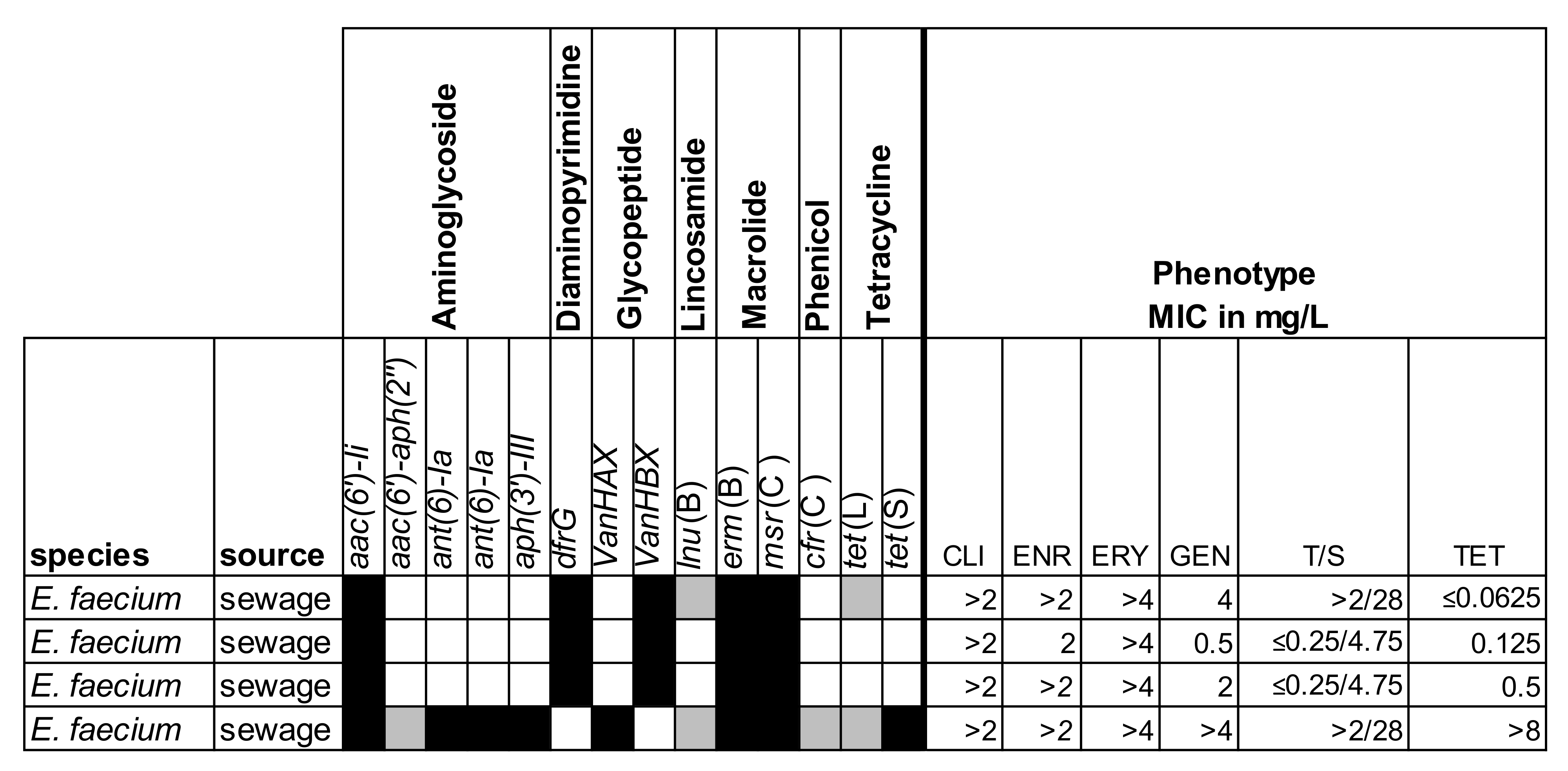
| Specimen | No. of Samples | No. of Positive Samples in Direct PCR | No. of Isolates Cultured from Samples | ||||
|---|---|---|---|---|---|---|---|
| Carbapene-Mases (n) | mcr (n) | CPE | Col-E | VRE | ESBL | ||
| Carcasses broilers | 57 | blaOXA-48 (57) | mcr-1 (20), mcr-5 (22) ** | 0 | mcr-1 (6), mcr-5 (1) | 1 | 8 * |
| Carcasses pigs | 41 | blaOXA-48 (25) | mcr-2 (1), mcr-4 (2) | 0 | 0 | 0 | n.a. |
| Cecum broilers | 36 | – | mcr-1 (6), mcr-5 (1) | 0 | mcr-1 (5) | 0 | 20 |
| Cecum pigs | 41 | – | mcr-1 (3) | 0 | mcr-1 (1) | 0 | 17 |
| Drain swab | 88 | blaOXA-48 (60) | mcr-1 (1), mcr-4 (14) | 0 | mcr-1 (1) | 0 | 8 |
| Meat juice | 88 | blaOXA-48 (38) | mcr-1 (4), mcr-4 (5) | 0 | mcr-1(1) | 0 | 13 |
| Beef | 10 | blaOXA-48 (1) | - | 0 | 0 | 0 | 1 |
| Pork | 37 | blaOXA-48 (4) | mcr-1 (1), mcr-4 (1), mcr-5 (1) | 0 | 0 | 0 | 3 |
| Poultry | 53 | blaOXA-48 (4) | mcr-1 (13) | 0 | mcr-1 (6) | 0 | 15 |
| Water | 37 | blaOXA-48 (6) | mcr-4 (1) | 0 | 0 | 0 | 0 |
| Sewage/Sludge/Soil | 17 | blaOXA-48 (7), blaKPC (2), blaVIM/NDM (3) | mcr-1 (4) | blaOXA-48 (6), blaKPC (2) | 0 | 4 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klees, S.; Effelsberg, N.; Stührenberg, B.; Mellmann, A.; Schwarz, S.; Köck, R. Prevalence and Epidemiology of Multidrug-Resistant Pathogens in the Food Chain and the Urban Environment in Northwestern Germany. Antibiotics 2020, 9, 708. https://doi.org/10.3390/antibiotics9100708
Klees S, Effelsberg N, Stührenberg B, Mellmann A, Schwarz S, Köck R. Prevalence and Epidemiology of Multidrug-Resistant Pathogens in the Food Chain and the Urban Environment in Northwestern Germany. Antibiotics. 2020; 9(10):708. https://doi.org/10.3390/antibiotics9100708
Chicago/Turabian StyleKlees, Sylvia, Natalie Effelsberg, Birgit Stührenberg, Alexander Mellmann, Stefan Schwarz, and Robin Köck. 2020. "Prevalence and Epidemiology of Multidrug-Resistant Pathogens in the Food Chain and the Urban Environment in Northwestern Germany" Antibiotics 9, no. 10: 708. https://doi.org/10.3390/antibiotics9100708
APA StyleKlees, S., Effelsberg, N., Stührenberg, B., Mellmann, A., Schwarz, S., & Köck, R. (2020). Prevalence and Epidemiology of Multidrug-Resistant Pathogens in the Food Chain and the Urban Environment in Northwestern Germany. Antibiotics, 9(10), 708. https://doi.org/10.3390/antibiotics9100708







