Non-Surgical Periodontal Therapy with Adjunctive Amoxicillin/Metronidazole or Metronidazole When No Aggregatibacter actinomycetemcomitans Is Detected—A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
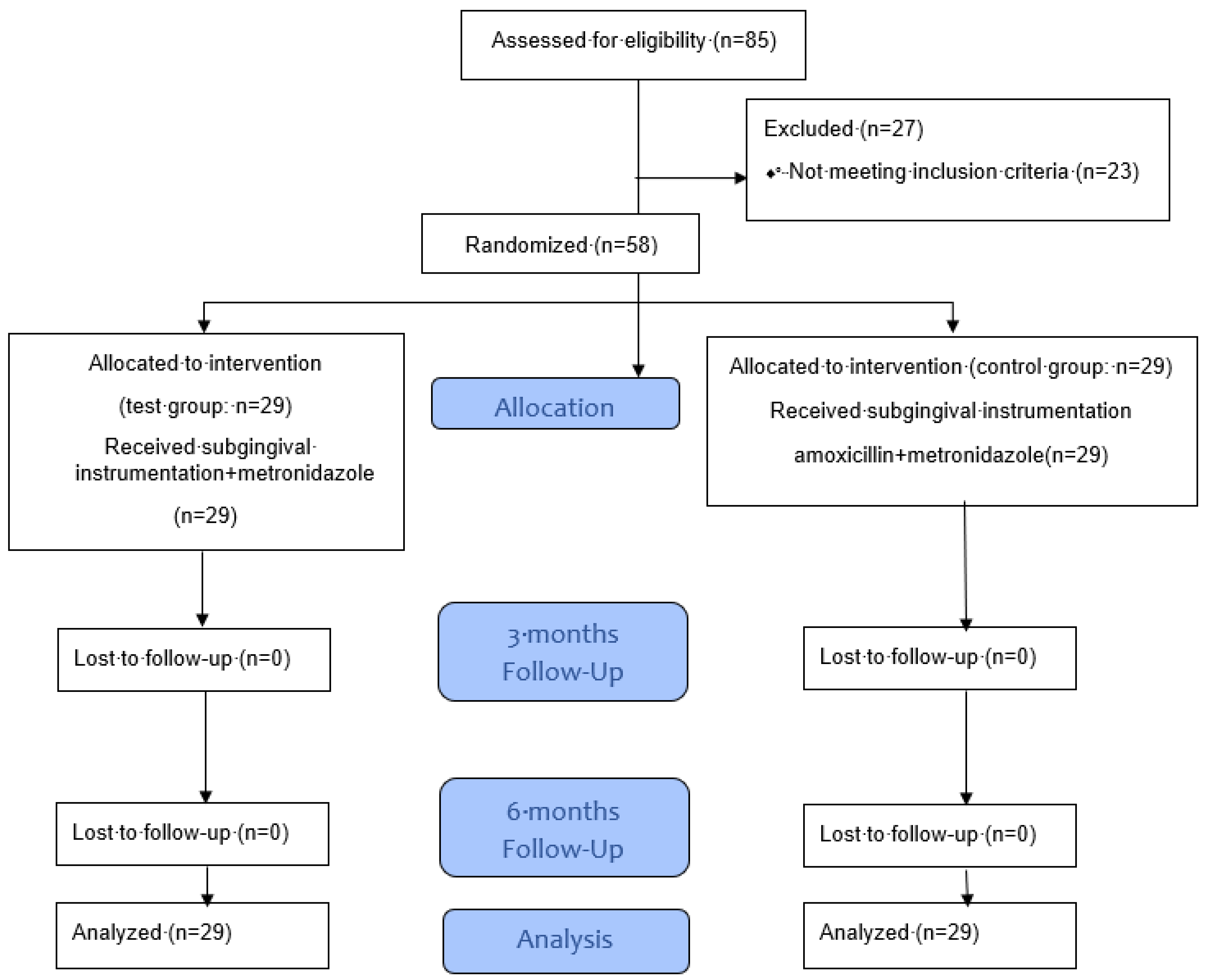
2.2. Therapy and Follow-Up Treatment
2.3. Clinical Variables and Sampling Procedures
2.4. Biomarkers and Microbiological Analysis
2.5. Data Analysis
2.6. Ethics Statements
2.7. Informed Consent
3. Results
3.1. Study Population and Clinical Variables
3.2. Tested Bacteria Associated with Periodontal Disease
3.3. Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontology 2000 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Eke, P.; Dye, B.; Wei, L.; Thornton-Evans, G.; Genco, R. Prevalence of Periodontitis in Adults in the United States: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Severe Periodontitis in 1990–2010. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Eickholz, P.; Loos, B.G.; Papapanou, P.; Van Der Velden, U.; Armitage, G.; Bouchard, P.; Deinzer, R.; Dietrich, T.; Hughes, F.; et al. Principles in prevention of periodontal diseases. J. Clin. Periodontol. 2015, 42, S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.M. Clinical significance of non-surgical periodontal therapy: An evidence-based perspective of scaling and root planing. J. Clin. Periodontol. 2002, 29, 22–32. [Google Scholar] [CrossRef]
- Herrera, D. Scaling and Root Planning is Recommended in the Nonsurgical Treatment of Chronic Periodontitis. J. Évid. Based Dent. Pract. 2016, 16, 56–58. [Google Scholar] [CrossRef]
- Krishna, R.; De Stefano, J.A. Ultrasonic vs. hand instrumentation in periodontal therapy: Clinical outcomes. Periodontology 2000 2016, 71, 113–127. [Google Scholar] [CrossRef]
- Sanz, I.; Alonso, B.; Carasol, M.; Herrera, D.; Sanz, M. Nonsurgical treatment of periodontitis. J. Evid. Based Dent. Pract. 2012, 12, 76–86. [Google Scholar] [CrossRef]
- Smiley, C.J.; Tracy, S.L.; Abt, E.; Michalowicz, B.S.; John, M.T.; Gunsolley, J.; Cobb, C.M.; Rossmann, J.; Harrel, S.K.; Forrest, J.L.; et al. Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J. Am. Dent. Assoc. 2015, 146, 508–524.e5. [Google Scholar] [CrossRef]
- Teughels, W.; Feres, M.; Oud, V.; Martín, C.; Matesanz, P.; Herrera, D. Adjunctive effect of systemic antimicrobials in periodontitis therapy. A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 257–281. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of Stage I-III Periodontitis -The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 4–60. [Google Scholar] [CrossRef] [PubMed]
- Eickholz, P.; Koch, R.; Kocher, T.; Hoffmann, T.; Kim, T.; Meyle, J.; Kaner, D.; Schlagenhauf, U.; Harmsen, D.; Harks, I.; et al. Clinical benefits of systemic amoxicillin/metronidazole may depend on periodontitis severity and patients’ age: An exploratory sub-analysis of the ABPARO trial. J. Clin. Periodontol. 2019, 46, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Åberg, C.H.; Kelk, P.; Johansson, A. Aggregatibacter actinomycetemcomitans: Virulence of its leukotoxin and association with aggressive periodontitis. Virulence 2014, 6, 188–195. [Google Scholar] [CrossRef]
- Wolff, L.F.; Aeppli, D.M.; Pihlstrom, B.; Anderson, L.; Stoltenberg, J.; Osborn, J.; Hardie, N.; Shelburne, C.; Fischer, G. Natural distribution of 5 bacteria associated with periodontal disease. J. Clin. Periodontol. 1993, 20, 699–706. [Google Scholar] [CrossRef]
- Rafiei, M.; Kiani, F.; Sayehmiri, K.; Sayehmiri, F.; Tavirani, M.; Dousti, M.; Sheikhi, A. Prevalence of Anaerobic Bacteria (P.gingivalis) as Major Microbial Agent in the Incidence Periodontal Diseases by Meta-analysis. J. Dent. (Shiraz, Iran) 2018, 19, 232–242. [Google Scholar]
- Holt, S.C.; Ebersole, J.L. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The ’red complex’, a prototype polybacterial pathogenic consortium in periodontitis. Periodontology 2000 2005, 38, 72–122. [Google Scholar] [CrossRef]
- Mombelli, A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontology 2000 2017, 76, 85–96. [Google Scholar] [CrossRef]
- Benso, B. Virulence factors associated with Aggregatibacter actinomycetemcomitans and their role in promoting periodontal diseases. Virulence 2017, 8, 111–114. [Google Scholar] [CrossRef]
- Henderson, B.; Ward, J.M.; Ready, D. Aggregatibacter (Actinobacillus) actinomycetemcomitans: A triple A* periodontopathogen? Periodontology 2000 2010, 54, 78–105. [Google Scholar] [CrossRef]
- Kunz, E.M.K.; Thurnheer, T.; Karygianni, L.; Walter, C.; Sculean, A.; Eick, S. Antibiotic Susceptibility Patterns of Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis Strains from Different Decades. Antibiotics 2019, 8, 253. [Google Scholar] [CrossRef]
- Binta, B.; Patel, M. Detection of cfxA2, cfxA3, and cfxA6 genes in beta-lactamase producing oral anaerobes. J. Appl. Oral Sci. 2016, 24, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Sgolastra, F.; Gatto, R.; Petrucci, A.; Monaco, A. Effectiveness of Systemic Amoxicillin/Metronidazole as Adjunctive Therapy to Scaling and Root Planing in the Treatment of Chronic Periodontitis: A Systematic Review and Meta-Analysis. J. Periodontol. 2012, 83, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Hagenfeld, D.; Koch, R.; Jünemann, S.; Prior, K.; Harks, I.; Eickholz, P.; Hoffmann, T.; Kim, T.-S.; Kocher, T.; Meyle, J.; et al. Do we treat our patients or rather periodontal microbes with adjunctive antibiotics in periodontal therapy? A 16S rDNA microbial community analysis. PLoS ONE 2018, 13, e0195534. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-L.; Wang, M.-S.; Cheng, A.-C.; Pan, K.-C.; Li, C.-F.; Deng, S.-X. A simple and rapid method for extracting bacterial DNA from intestinal microflora for ERIC-PCR detection. World J. Gastroenterol. 2008, 14, 2872–2876. [Google Scholar] [CrossRef]
- Eick, S.; Straube, A.; Guentsch, A.; Pfister, W.; Jentsch, H. Comparison of real-time polymerase chain reaction and DNA-strip technology in microbiological evaluation of periodontitis treatment. Diagn. Microbiol. Infect. Dis. 2011, 69, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, H.F.R.; Heusinger, T.; Weickert, A.; Eick, S. Professional tooth cleaning prior to non-surgical periodontal therapy: A randomized clinical trial. J. Periodontol. 2019, 91, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Haffajee, A.D.; Torresyap, G.; Socransky, S.S. Clinical changes following four different periodontal therapies for the treatment of chronic periodontitis: 1-year results. J. Clin. Periodontol. 2007, 34, 243–253. [Google Scholar] [CrossRef]
- Moher, D.; Schulz, K.F.; Altman, D.G. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001, 357, 1191–1194. [Google Scholar] [CrossRef]
- Mombelli, A.; Almaghlouth, A.; Cionca, N. Differential benefits of amoxicillin-metronidazole in different phases of periodontal therapy in a randomized controlled crossover clinical trial. Zahnmed. up2date 2015, 9, 198. [Google Scholar] [CrossRef]
- Torres, M.J.; Blanca, M. The Complex Clinical Picture of β-Lactam Hypersensitivity: Penicillins, Cephalosporins, Monobactams, Carbapenems, and Clavams. Med. Clin. N. Am. 2010, 94, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Van Winkelhoff, A.J.; Tijhof, C.J.; De Graaff, J. Microbiological and Clinical Results of Metronidazole Plus Amoxicillin Therapy in Actinobacillus actinomycetemcomitans-Associated Periodontitis. J. Periodontol. 1992, 63, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Pavicić, M.J.; Van Winkelhoff, A.J.; Pavicić-Temming, Y.A.; De Graaff, J. Amoxycillin causes an enhanced uptake of metronidazole in Actinobacillus actinomycetemcomitans: A mechanism of synergy. J. Antimicrob. Chemother. 1994, 34, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Zandbergen, D.; Slot, D.E.; Niederman, R.; Van Der Weijden, F.A. The concomitant administration of systemic amoxicillin and metronidazole compared to scaling and root planing alone in treating periodontitis: A systematic review. BMC Oral Health 2016, 16, 27. [Google Scholar] [CrossRef]
- Cosgarea, R.; Juncar, R.; Heumann, C.; Tristiu, R.; Lascu, L.; Arweiler, N.; Stavropoulos, A.; Sculean, A. Adjunctive Use of Systemic Antibiotics (Amoxicillin 500 MG plus Metronidazole 500 MG 3 times a Day for 3 or 7 Days) to Nonsurgical Periodontal Therapy may Improve Clinical Outcomes in Treating Severe Chronic Periodontitis. J. Clin. Periodontol. 2016, 43, 767–77. [Google Scholar] [CrossRef]
- Harks, I.; Koch, R.; Eickholz, P.; Hoffmann, T.; Kim, T.-S.; Kocher, T.; Meyle, J.; Kaner, D.; Schlagenhauf, U.; Doering, S.; et al. Is progression of periodontitis relevantly influenced by systemic antibiotics? A clinical randomized trial. J. Clin. Periodontol. 2015, 42, 832–842. [Google Scholar] [CrossRef]
- Matuliene, G.; Pjetursson, B.E.; Salvi, G.E.; Schmidlin, K.; Bragger, U.; Zwahlen, M.; Lang, N.P. Influence of residual pockets on progression of periodontitis and tooth loss: Results after 11 years of maintenance. J. Clin. Periodontol. 2008, 35, 685–695. [Google Scholar] [CrossRef]
- Boia, S.; Boariu, M.; Baderca, F.; Rusu, D.; Muntean, D.; Horhat, F.; Boia, E.-R.; Borza, C.; Anghel, A.; Stratul, Ş.-I. Clinical, microbiological and oxidative stress evaluation of periodontitis patients treated with two regimens of systemic antibiotics, adjunctive to non-surgical therapy. A placebo-controlled randomized clinical trial. Exp. Ther. Med. 2019, 18, 5001–5015. [Google Scholar] [CrossRef]
- Morales, A.; Gandolfo, A.; Bravo, J.; Carvajal, P.; Silva, N.; Godoy, C.; García-Sesnich, J.; Hoare, A.; Díaz, P.; Gamonal, J. Microbiological and clinical effects of probiotics and antibiotics on nonsurgical treatment of chronic periodontitis: A randomized placebo- controlled trial with 9-month follow-up. J. Appl. Oral Sci. 2018, 26, e20170075. [Google Scholar] [CrossRef]
- Duarte, P.M.; Feres, M.; Yassine, L.L.S.; Soares, G.M.S.; Miranda, T.S.; Faveri, M.; Retamal-Valdes, B.; Figueiredo, L.C. Clinical and microbiological effects of scaling and root planing, metronidazole and amoxicillin in the treatment of diabetic and non-diabetic subjects with periodontitis: A cohort study. J. Clin. Periodontol. 2018, 45, 1326–1335. [Google Scholar] [CrossRef]
- Mombelli, A.; Cionca, N.; Almaghlouth, A.; Décaillet, F.; Courvoisier, D.S.; Giannopoulou, C. Are There Specific Benefits of Amoxicillin Plus Metronidazole in Aggregatibacter actinomycetemcomitans-Associated Periodontitis? Double-Masked, Randomized Clinical Trial of Efficacy and Safety. J. Periodontol. 2013, 84, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Loos, B.; Needleman, I. Endpoints of active periodontal therapy. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 61–71. [Google Scholar] [CrossRef] [PubMed]
- Rooney, J.; Wade, W.G.; Sprague, S.V.; Newcombe, R.G.; Addy, M. Adjunctive effects to non-surgical periodontal therapy of systemic metronidazole and amoxycillin alone and combined. J. Clin. Periodontol. 2002, 29, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Feres, M.; Soares, G.M.S.; Mendes, J.A.V.; Silva, M.P.; Faveri, M.; Teles, R.; Socransky, S.S.; Figueiredo, L.C. Metronidazole alone or with amoxicillin as adjuncts to non?surgical treatment of chronic periodontitis: A 1?year double?blinded, placebo?controlled, randomized clinical trial. J. Clin. Periodontol. 2012, 39, 1149–1158. [Google Scholar] [CrossRef]
- Stone, V.N.; Xu, P. Targeted antimicrobial therapy in the microbiome era. Mol. Oral Microbiol. 2017, 32, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.; Nibali, L.; Lambertenghi, R.; Ready, D.; Suvan, J.; Griffiths, G.S.; Wilson, M.; Tonetti, M.S. Impact of baseline microbiological status on clinical outcomes in generalized aggressive periodontitis patients treated with or without adjunctive amoxicillin and metronidazole: An exploratory analysis from a randomized controlled clinical trial. J. Clin. Periodontol. 2014, 41, 1080–1089. [Google Scholar] [CrossRef]
- Ardila, C.M.; Bedoya-García, J.-A. Antimicrobial resistance of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Tannerella forsythia in periodontitis patients. J. Glob. Antimicrob. Resist. 2020, 22, 215–218. [Google Scholar] [CrossRef]
- Rams, T.E.; Sautter, J.D.; Van Winkelhoff, A.J. Comparative In Vitro Resistance of Human Periodontal Bacterial Pathogens to Tinidazole and Four Other Antibiotics. Antibiotics 2020, 9, 68. [Google Scholar] [CrossRef]
- Kunz, E.M.K.; Lenkeit, K.; Waltimo, T.; Weiger, R.; Walter, C. Combinatorial effects of amoxicillin and metronidazole on selected periodontal bacteria and whole plaque samples. Arch. Oral Biol. 2014, 59, 608–615. [Google Scholar] [CrossRef]
- Eick, S.; Pfister, W. Efficacy of Antibiotics Against Periodontopathogenic Bacteria Within Epithelial Cells: An In Vitro Study. J. Periodontol. 2004, 75, 1327–1334. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Thurnheer, T. Validation of Antibiotic Efficacy on In Vitro Subgingival Biofilms. J. Periodontol. 2014, 85, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Ziegeler, S.; Raddatz, A.; Hoff, G.; Buchinger, H.; Bauer, I.; Stockhausen, A.; Sasse, H.; Sandmann, I.; Horsch, S.; Rensing, H. Antibiotics modulate the stimulated cytokine response to endotoxin in a human ex vivo, in vitro model. Acta Anaesthesiol. Scand. 2006, 50, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Paolillo, R.; Guida, L.; Annunziata, M.; Bevilacqua, N.; Tufano, M.A. Effect of metronidazole and modulation of cytokine production on human periodontal ligament cells. Int. Immunopharmacol. 2010, 10, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Zhao, Y.; Wang, C.; Li, H.; Shi, X.X.; Ren, X.Y. Influence of periodontal non-surgical therapy on serum interleukin 6 expression and carotid artery wall in rats with periodontitis and type 2 diabetes mellitus. Hua Xi Kou Qiang Yi Xue Za Zhi 2019, 37, 589–593. (In Chinese) [Google Scholar]
- Al-Khureif, A.A.; Mohamed, B.A.; Siddiqui, A.Z.; Khan, A.A.; Divakar, D.D. Repeated application of photodynamic and antibiotic therapy as an adjunct to root surface debridement in patients with grade C and stage III or IV aggressive periodontitis. Photodiagnosis Photodyn. Ther. 2020, 29, 101610. [Google Scholar] [CrossRef]
| Variable | MET Group (n = 29) | AMX/MET Group (n = 29) | p |
|---|---|---|---|
| Age (years; mean ± SD) | 55.14 ± 11.48 | 52.17 ± 10.16 | 0.171 |
| Male/female, n | 20/9 | 18/11 | 0.581 |
| Smoker, n | 16 | 15 | 0.792 |
| Variable | Baseline | 3 Months | P (Baseline–3 Months) | 6 Months | P (Baseline–6 Months) | Difference Baseline–3 Months | Difference Baseline–6 Months |
|---|---|---|---|---|---|---|---|
| Mean CAL (mm) | |||||||
| MET | 4.58 (4.21/5.01) | 3.77 (3.59/4.03) | <0.001 * | 3.77 (3.55/4.15) | <0.001 * | 0.72 (0.41/1.20) | 0.61 (0.45/1.11) |
| AMX/MET | 4.67 (4.14/4.0) | 3.73 (3.61/4.08) | <0.001 * | 3.77 (3.64/4.21) | <0.001 * | 0.84 (0.49/1.15) | 0.65 (0.45/1.19) |
| P | 0.988 | 0.988 | 0.630 | 0.852 | 0.822 | ||
| BOP (%) | |||||||
| MET | 75.9 (37.64/100) | 12.8 (10.6/15.9) | <0.001 * | 13.1 (0.9/8.99) | <0.001 * | 64.7 (19.6/87.4) | 59.9 (19.9/86.7) |
| AMX/MET | 81.0 (44.49/100) | 13.7 (11.9/15.9) | <0.001 * | 12.6 (11.7/19.2) | <0.001 * | 56.1 (25.7/86.4) | 53.3 (30.3/87.6) |
| P | 0.955 | 0.262 | 0.785 | 0.780 | 0.697 | ||
| Mean PD (mm) | |||||||
| MET | 4.44 (4.16/4.83) | 3.64 (3.48/3.80) | <0.001 * | 3.67 (3.50/3.79) | <0.001 * | 0.69 (0.47/1.22) | 0.66 (0.46/1.21) |
| AMX/MET | 4.55 (4.15/4.95) | 3.62 (3.47/3.91) | <0.001 * | 3.69 (3.66/3.9) | <0.001 * | 0.92 (0.5/1.1) | 0.83 (0.47/1.18) |
| P | 0.602 | 0.932 | 0.355 | 0.474 | 0.816 | ||
| Number of sites with PD ≥ 6 mm | |||||||
| MET | 27 (16.5/44) | 5 (2/8.5) | 0.001 * | 4 (2/9) | <0.001 * | 20 (14/39) | 21 (13.5/37) |
| AMX/MET | 33 (17/43) | 6 (2/8.5) | <0.001 * | 5 (3.5/9.5) | <0.001 * | 26 (15/37) | 28 (14.5/38) |
| P | 0.641 | 0.956 | 0.894 | 0.549 | 0.570 | ||
| Mean PD of sites with initial PD ≥ 6 mm (mm) | |||||||
| MET | 7.04 (6.47/7.44) | 4.95 (4.65/5.38) | <0.001 | 4.86 (4.45/5.31) | <0.001 * | 2.09 (1.44/2.66) | 2.19 (1.73/2.76) |
| AMX/MET | 7.19 (6.67/7.61) | 4.68 (4.30/5.01) | <0.001 | 4.68 (4.30/5.02) | <0.001 * | 2.51 (2.00/2.76) | 2.51 (2.12/2.88) |
| P | 0.234 | 0.052 | 0.197 | 0.011 # | 0.032 # | ||
| Baseline | 3 Months | P (Baseline–3 Months) | 6 Months | P (Baseline–6 Months) | |
|---|---|---|---|---|---|
| A. actinomycetemcomitans | |||||
| MET | 0 (0/0) | 0 (0/0) | n.a. | 0 (0/0) | n.a. |
| AMX/MET | 0 (0/0) | 0 (0/0) | n.a. | 0 (0/0) | n.a. |
| P | n.a. | n.a. | n.a. | ||
| P. gingivalis | |||||
| MET | 4.36 (0/5.55) | 0(0/3.37) | 0.001 * | 0 (0/3.24) | 0.001 * |
| AMX/MET | 4(0/5.83) | 0 (0/0) | 0.001 * | 0 (0/2.12) | 0.003 * |
| P | 0.647 | 0.288 | 0.353 | ||
| T. forsythia | |||||
| MET | 5.84 (5.5/6.4) | 4.23 (3.77/5.4) | 0.001 * | 4.8 (3.24/5.55) | 0.004 * |
| AMX/MET | 5.76 (5.25/6.31) | 3.56 (2.60/4.68) | <0.001 * | 4.53 (3.73/5.17) | 0.002 * |
| P | 0.652 | 0.042 # | 0.613 | ||
| T. denticola | |||||
| MET | 3.15 (0/4.32) | 2.52 (0/3.56) | 0.140 | 2.28 (0/3.44) | 0.028 * |
| AMX/MET | 3.54 (0/4.8) | 0 (0/3.25) | 0.005 * | 2.78 (0/3.5) | 0.027 * |
| P | 0.358 | 0.075 | 0.416 | ||
| Baseline | 3 Months | P (Baseline–3 Months) | 6 Months | P (Baseline–6 Months) | |
|---|---|---|---|---|---|
| MMP-8 | |||||
| MET | 1794.8 (805.7/2977.0) | 758.52 (359.7/2224.0) | 0.424 | 2358.08 (815.6/6478.1) | 0.471 |
| AMX/MET | 1776.81 (702.5/4123.2) | 722.64 (233.9/8593.0) | 0.096 | 2132.96 (145.2/7099.4) | 0.495 |
| P | 0.886 | 0.528 | 0.461 | ||
| IL-1 | |||||
| MET | 18.24 (7.39/41.11) | 8.51 (2.35/18.16) | 0.949 | 17.62 (6.19/44.98) | 0.471 |
| AMX/MET | 18.17 (11.83/35.71) | 10 (2.92/29) | 0.084 | 9.49 (3.12/41.89) | 0.909 |
| P | 0.702 | 0.414 | 0.268 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jentsch, H.F.R.; Dietrich, M.; Eick, S. Non-Surgical Periodontal Therapy with Adjunctive Amoxicillin/Metronidazole or Metronidazole When No Aggregatibacter actinomycetemcomitans Is Detected—A Randomized Clinical Trial. Antibiotics 2020, 9, 686. https://doi.org/10.3390/antibiotics9100686
Jentsch HFR, Dietrich M, Eick S. Non-Surgical Periodontal Therapy with Adjunctive Amoxicillin/Metronidazole or Metronidazole When No Aggregatibacter actinomycetemcomitans Is Detected—A Randomized Clinical Trial. Antibiotics. 2020; 9(10):686. https://doi.org/10.3390/antibiotics9100686
Chicago/Turabian StyleJentsch, Holger F. R., Martin Dietrich, and Sigrun Eick. 2020. "Non-Surgical Periodontal Therapy with Adjunctive Amoxicillin/Metronidazole or Metronidazole When No Aggregatibacter actinomycetemcomitans Is Detected—A Randomized Clinical Trial" Antibiotics 9, no. 10: 686. https://doi.org/10.3390/antibiotics9100686
APA StyleJentsch, H. F. R., Dietrich, M., & Eick, S. (2020). Non-Surgical Periodontal Therapy with Adjunctive Amoxicillin/Metronidazole or Metronidazole When No Aggregatibacter actinomycetemcomitans Is Detected—A Randomized Clinical Trial. Antibiotics, 9(10), 686. https://doi.org/10.3390/antibiotics9100686






