Antibacterial Activity of Colloidal Silver against Gram-Negative and Gram-Positive Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. In Vitro Susceptibility Testing
2.3. Time–Kill Kinetic Assays
2.4. Determination of Reactive Oxygen Species (ROS)
2.5. Membrane Permeabilization Assay
2.6. Statistical Analysis
3. Results
3.1. Antimicrobial Activity of Colloidal Silver
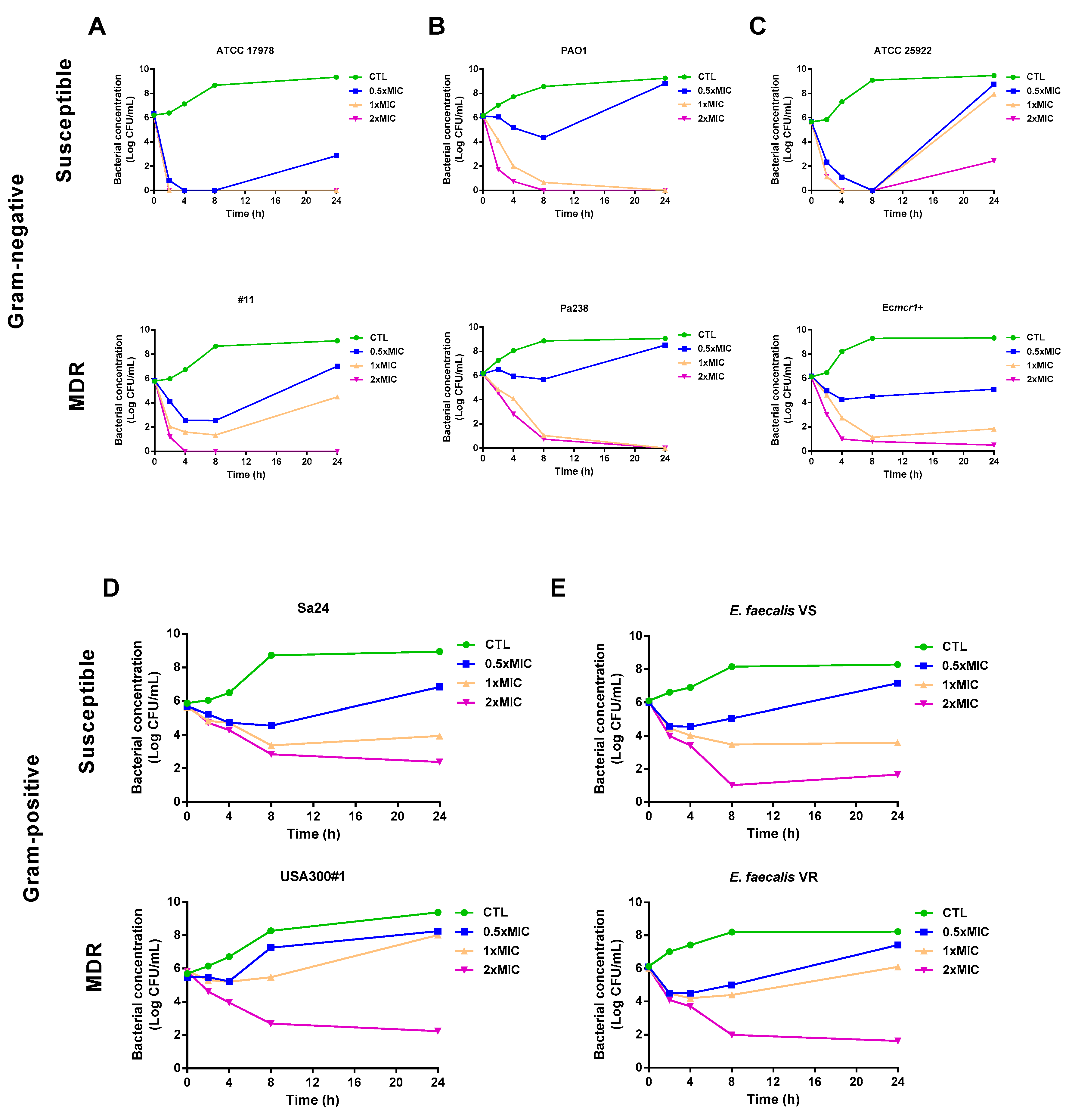
3.2. Time–Kill Curves
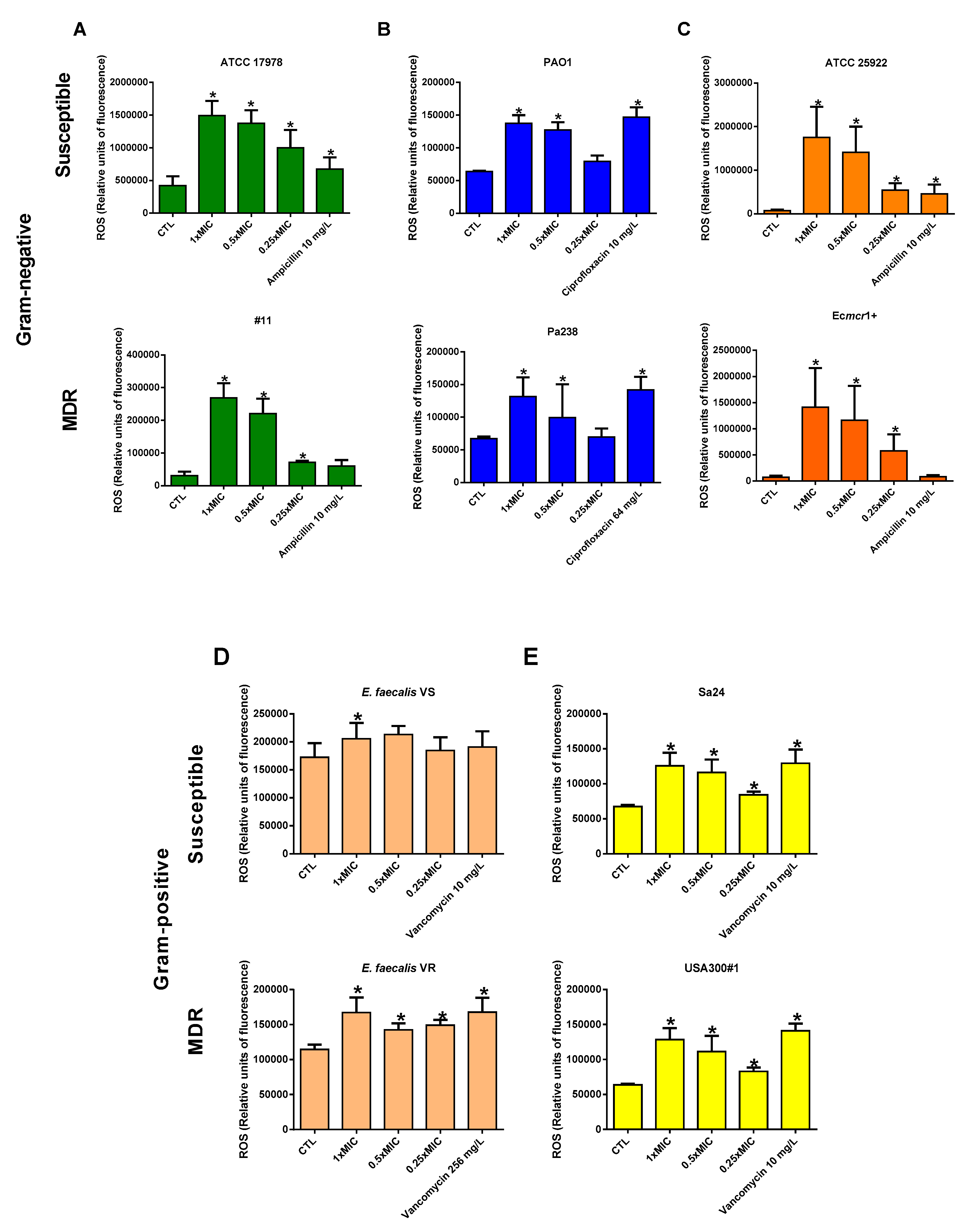
3.3. Colloidal Silver Effect on ROS Production
3.4. Colloidal Silver Effect on Bacterial Membrane Permeability
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shrivastava, S.R.; Shrivastava, P.S.; Ramasamy, J. World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. J. Med. Soc. 2018, 32, 76. [Google Scholar] [CrossRef]
- Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Huynh, E.; Hamood, A.; De Souza, A.; Mehta, D.; Moeller, K.; Morgan, M.; Reid, T.W. The ability of a colloidal silver gel wound dressing to kill bacteria in vitro and in vivo. J. Wound Care 2017, 26, 16. [Google Scholar] [CrossRef] [PubMed]
- Barras, F.; Aussel, L.; Ezraty, B. Silver and Antibiotic, New Facts to an Old Story. Antibiotics 2018, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, Z. Colloidal Silver: The Natural Antibiotic Alternative; Healing Wisdom Publications: New York, NY, USA, 1995; pp. 1–18. [Google Scholar]
- Lansdown, A.B. Silver in Health Care: Antimicrobial Effects and Safety in Use. Exog. Dermatol. 2006, 33, 17–34. [Google Scholar]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver enhances antibiotic activity against gram-negative bacteria. Sci. Transl. Med. 2013, 5, 190ra81. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Gu, M.; Imlay, J.A. The SoxRS response of Escherichia coli is directly activated by redox-cycling drugs rather than by superoxide. Mol. Microbiol. 2011, 79, 1136–1150. [Google Scholar] [CrossRef]
- Russell, A.; Hugo, W. 7 Antimicrobial Activity and Action of Silver. Prog. Med. Chem. 1994, 31, 351–370. [Google Scholar]
- Arakawa, H.; Neault, J.; Tajmir-Riahi, H. Silver(I) complexes with DNA and RNA studied by Fourier transform infrared spectroscopy and capillary electrophoresis. Biophys. J. 2001, 81, 1580–1587. [Google Scholar] [CrossRef]
- Herisse, M.; Duverger, Y.; Martin-Verstraete, I.; Barras, F.; Ezraty, B. Silver potentiates aminoglycoside toxicity by enhancing their uptake. Mol. Microbiol. 2017, 105, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Pitts, B.; Stewart, P.S.; Camper, A.; Yoon, J. Comparison of the Antimicrobial Effects of Chlorine, Silver Ion, and Tobramycin on Biofilm▿. Antimicrob. Agents Chemother. 2008, 52, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Aboelfetoh, E.F.; El-Shenody, R.A.; Ghobara, M.M. Eco-friendly synthesis of silver nanoparticles using green algae (Caulerpa serrulata): Reaction optimization, catalytic and antibacterial activities. Environ. Monit. Assess. 2017, 189, 349. [Google Scholar] [CrossRef] [PubMed]
- Goggin, R.; Jardeleza, C.; Wormald, P.J.; Vreugde, S. Colloidal silver: A novel treatment for Staphylococcus aureus biofilms? Int. Forum Allergy Rhinol. 2014, 4, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Facal, P.; Thomas, N.; Vandecandelaere, I.; Ramezanpour, M.; Cooksley, C.; Prestidge, C.A.; Coenye, T.; Wormald, P.-J.; Vreugde, S. Taking the Silver Bullet Colloidal Silver Particles for the Topical Treatment of Biofilm-Related Infections. ACS Appl. Mater. Interfaces 2017, 9, 21631–21638. [Google Scholar] [CrossRef] [PubMed]
- Ooi, M.L.; Richter, K.; Bennett, C.; Macias-Valle, L.; Vreugde, S.; Psaltis, A.J.; Wormald, P.-J. Topical Colloidal Silver for the Treatment of Recalcitrant Chronic Rhinosinusitis. Front. Microbiol. 2018, 9, 720. [Google Scholar] [CrossRef]
- Villar, M.; Cano, M.E.; Gato, E.; Garnacho-Montero, J.; Miguel Cisneros, J.; Ruíz de Alegría, C.; Fernández-Cuenca, F.; Martínez-Martínez, L.; Vila, J.; Pascual, A.; et al. Epidemiologic and clinical impact of Acinetobacter baumannii colonization and infection: A reappraisal. Medicine 2014, 93, 202–210. [Google Scholar] [CrossRef]
- Fernández-Cuenca, F.; Tomás-Carmona, M.; Caballero-Moyano, F.; Bou, G.; Martínez-Martínez, L.; Vila, J.; Pachón, J.; Cisneros, J.M.; Rodríguez-Baño, J.; Pascual, A. In vitro activity of 18 antimicrobial agents against clinical isolates of Acinetobacter spp.: Multicenter national study GEIH-REIPI-Ab 2010. Enferm. Infecc. Microbiol. Clin. 2013, 31, 4–9. [Google Scholar] [CrossRef]
- Peña, C.; Suarez, C.; Gozalo, M.; Murillas, J.; Almirante, B.; Pomar, V.; Aguilar, M.; Granados, A.; Calbo, E.; Rodríguez-Baño, J.; et al. Prospective multicenter study of the impact of carbapenem resistance on mortality in Pseudomonas aeruginosa bloodstream infections. Antimicrob. Agents Chemother. 2012, 56, 1265–1272. [Google Scholar] [CrossRef]
- Rodríguez Villodres, Á.; Álvarez Marín, R.; Pérez Moreno, M.A.; Roca, C.; Miró Canturri, A.; Pachón Ibáñez, M.E.; Aznar, J.; Pachón, J.; Lepe, J.A.; Smani, Y. Epidemiología clínica y factores pronósticos de la bacteriemia por Klebsiella pneumoniae vs. la bacteriemia por Escherichia coli. In Proceedings of the XXI Congreso de la Sociedad Española de Enfermedades Infecciosas y Microbiología clínica, Bilbao, Spain, 24–26 May 2018. [Google Scholar]
- Docobo Pérez, F. Tratamiento de la neumonía Experimental por Staphylococus Aureus. Estudios de Eficacia Terapéutica de Cotrimoxazol, Cloxacilina, Linezolid y Vancomicina Frente a Cepas S. Aureus Sensible y Resistente a Meticilin. Ph.D. Thesis, Seville University, Seville, Spain, 26 March 2009. [Google Scholar]
- Domínguez-Herrera, J.; López-Rojas, R.; Smani, Y.; Labrador-Herrera, G.; Pachón, J. Efficacy of ceftaroline versus vancomycin in an experimental foreign-body and systemic infection model caused by biofilm-producing methicillin-resistant Staphylococcus epidermidis. Int. J. Antimicrob. Agents 2016, 48, 661–665. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters; European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2016. [Google Scholar]
- Smani, Y.; Domínguez-Herrera, J.; Pachón, J. Rifampin Protects Human Lung Epithelial Cells Against Cytotoxicity Induced by Clinical Multi and Pandrug-resistant Acinetobacter baumannii. J. Infect. Dis. 2011, 203, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Ayerbe-Algaba, R.; Gil-Marquès, M.L.; Miró-Canturri, A.; Parra-Millán, R.; Pachón-Ibáñez, M.E.; Jiménez-Mejías, M.E.; Pachón, J.; Smani, Y. The antihelmintic oxyclozanide restores the activity of colistin against colistin-resistant Gram-negative bacilli. Int. J. Antimicrob. Agents 2019, 54, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Petica, A.; Gavriliu, S.; Lungu, M.V.; Buruntea, N.; Panzaru, C. Colloidal silver solutions with antimicrobial properties. Mater. Sci. Eng. B 2008, 152, 22–27. [Google Scholar] [CrossRef]
- Concepcion, D.D.; Verzosa, L.G.; Nuevo, J.J.M. Antimicrobial potency of colloidal silver compared with antibiotic eye drops. Philipp. J. Ophthalmol. 2007, 32, 9–11. [Google Scholar]
- Machuca, J.; Briales, A.; Labrador, G.; Díaz-De-Alba, P.; López-Rojas, R.; Docobo-Pérez, F.; Martínez-Martínez, L.; Rodríguez-Baño, J.; Pachón, M.E.; Pascual, Á.; et al. Interplay between plasmid-mediated and chromosomal-mediated fluoroquinolone resistance and bacterial fitness in Escherichia coli. J. Antimicrob. Chemother. 2014, 69, 3203–3215. [Google Scholar] [CrossRef] [PubMed]
- Goswami, M.; Mangoli, S.H.; Jawali, N. Involvement of Reactive Oxygen Species in the Action of Ciprofloxacin against Escherichia coli. Antimicrob. Agents Chemother. 2006, 50, 949–954. [Google Scholar] [CrossRef]
- Rodríguez-Rosado, A.I.; Valencia, E.Y.; Rodríguez-Rojas, A.; Costas, C.; Galhardo, R.S.; Rodríguez-Beltrán, J.; Blázquez, J. N-acetylcysteine blocks SOS induction and mutagenesis produced by fluoroquinolones in Escherichia coli. J. Antimicrob. Chemother. 2019, 74, 2188–2196. [Google Scholar] [CrossRef]
- Li, G.-Q.; Quan, F.; Qu, T.; Lu, J.; Chen, S.-L.; Cui, L.-Y.; Guo, D.-W.; Wang, Y.-C. Sublethal vancomycin-induced ROS mediating antibiotic resistance in Staphylococcus aureus. Biosci. Rep. 2015, 35, e00279. [Google Scholar] [CrossRef] [PubMed]
- Saulou-Bérion, C.; González, I.; Enjalbert, B.; Audinot, J.-N.; Fourquaux, I.; Jamme, F.; Cocaign-Bousquet, M.; Mercier-Bonin, M.; Girbal, L. Escherichia coli under Ionic Silver Stress: An Integrative Approach to Explore Transcriptional, Physiological and Biochemical Responses. PLoS ONE 2015, 10, e0145748. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Boil. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 720654. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Deshmukh, S.; Ingle, A.; Gade, A. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
| Pathogen | N | MIC50 (mg/L) | MIC90 (mg/L) |
|---|---|---|---|
| Gram-negative Acinetobacter baumannii | 45 | 4 | 4 |
| Pseudomonas aeruginosa | 25 | 2 | 4 |
| Escherichia coli | 79 | 2 | 8 |
| Klebsiella pneumoniae | 58 | 8 | 8 |
| Gram-positive | |||
| Staphylococcus aureus | 34 | 4 | 8 |
| S. epidermidis | 14 | 4 | 4 |
| Enterococcus spp. | 15 | 4 | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vila Domínguez, A.; Ayerbe Algaba, R.; Miró Canturri, A.; Rodríguez Villodres, Á.; Smani, Y. Antibacterial Activity of Colloidal Silver against Gram-Negative and Gram-Positive Bacteria. Antibiotics 2020, 9, 36. https://doi.org/10.3390/antibiotics9010036
Vila Domínguez A, Ayerbe Algaba R, Miró Canturri A, Rodríguez Villodres Á, Smani Y. Antibacterial Activity of Colloidal Silver against Gram-Negative and Gram-Positive Bacteria. Antibiotics. 2020; 9(1):36. https://doi.org/10.3390/antibiotics9010036
Chicago/Turabian StyleVila Domínguez, Andrea, Rafael Ayerbe Algaba, Andrea Miró Canturri, Ángel Rodríguez Villodres, and Younes Smani. 2020. "Antibacterial Activity of Colloidal Silver against Gram-Negative and Gram-Positive Bacteria" Antibiotics 9, no. 1: 36. https://doi.org/10.3390/antibiotics9010036
APA StyleVila Domínguez, A., Ayerbe Algaba, R., Miró Canturri, A., Rodríguez Villodres, Á., & Smani, Y. (2020). Antibacterial Activity of Colloidal Silver against Gram-Negative and Gram-Positive Bacteria. Antibiotics, 9(1), 36. https://doi.org/10.3390/antibiotics9010036






