Reduced Production of Bacterial Membrane Vesicles Predicts Mortality in ST45/USA600 Methicillin-Resistant Staphylococcus aureus Bacteremia
Abstract
1. Introduction
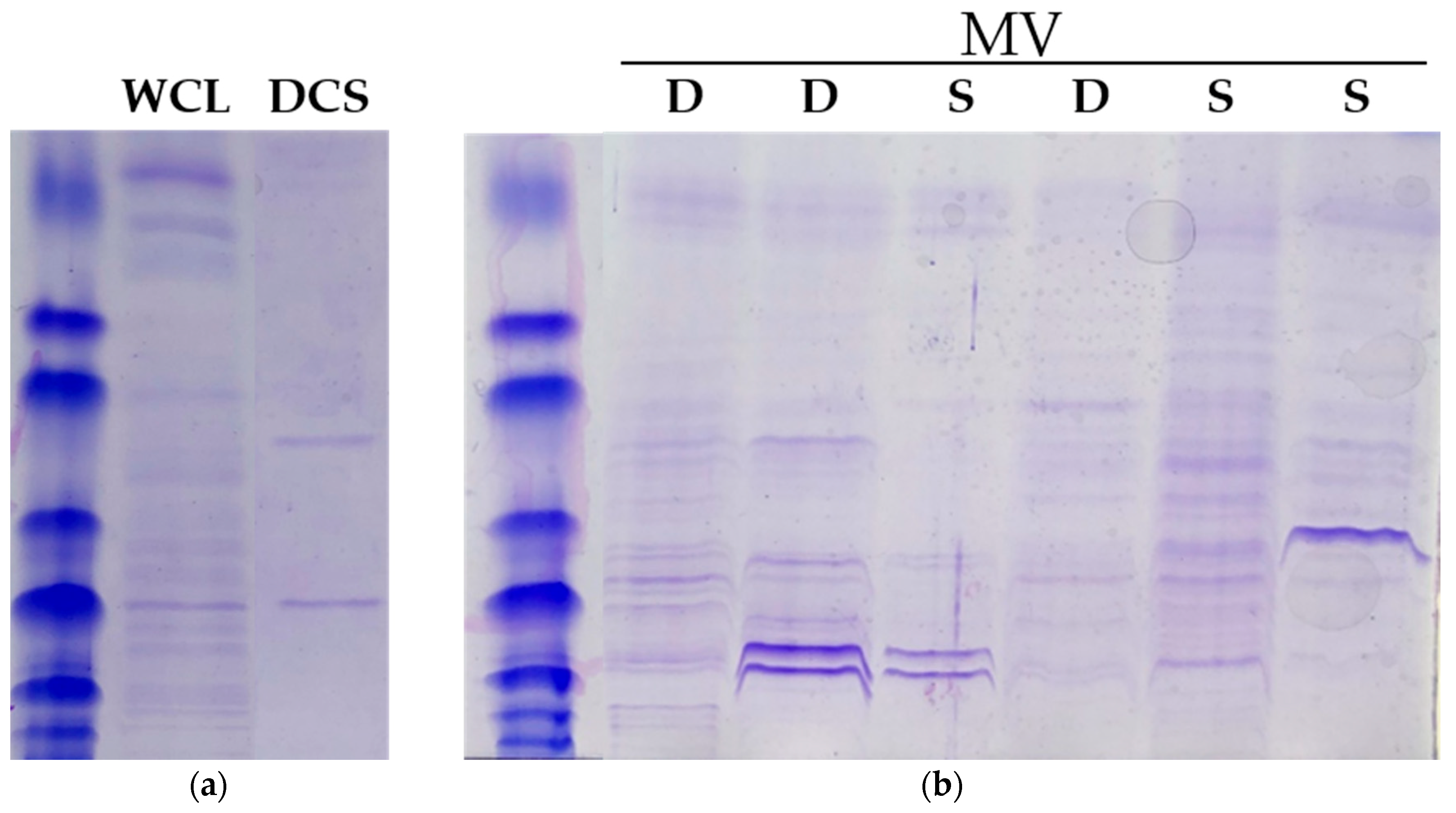
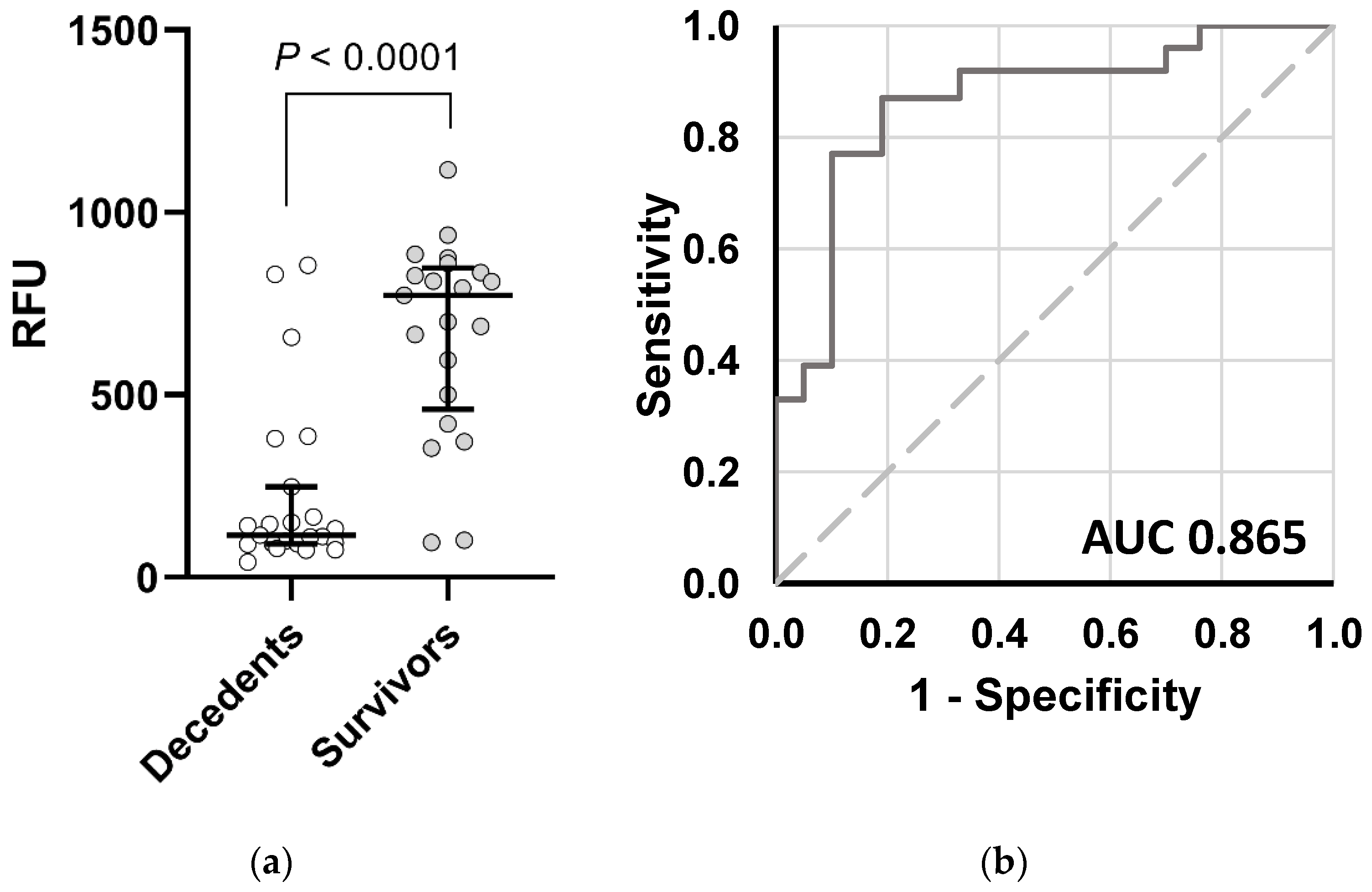
2. Results
3. Discussions
4. Materials and Methods
4.1. Study Design
4.2. Strain Characterization
4.3. Membrane Vesicle Isolation
4.4. Membrane Vesicle Detection
4.5. Protein Content Quantification
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kourtis, A.P.; Hatfield, K.; Baggs, J. Vital signs: Epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections—United States. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Geriak, M.; Haddad, F.; Rizvi, K.; Rose, W.; Kullar, R.; LaPlante, K.; Yu, M.; Vasina, L.; Ouellette, K.; Zervos, M.; et al. Clinical data on daptomycin plus ceftaroline versus standard of care monotherapy in the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 2019, 63, e02483-18. [Google Scholar] [CrossRef] [PubMed]
- Krishack, P.A.; Louviere, T.J.; Decker, T.S.; Kuzel, T.G.; Greenberg, J.A.; Camacho, D.F.; Hrusch, C.L.; Sperling, A.I.; Verhoef, P.A. Protection against Staphylococcus aureus bacteremia-induced mortality depends on ILC2s and eosinophils. JCI Insight 2019, 4, e124168. [Google Scholar] [CrossRef] [PubMed]
- Rose, W.E.; Shukla, S.K.; Berti, A.D.; Hayney, M.S.; Henriquez, K.M.; Ranzoni, A.; Cooper, M.A.; Proctor, R.A.; Nizet, V.; Sakoulas, G. Increased endovascular Staphylococcus aureus inoculum is the link between elevated serum Interleukin 10 concentrations and mortality in patients With bacteremia. Clin. Infect. Dis. 2017, 64, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Minejima, E.; Bensman, J.; She, R.C.; Mack, W.J.; Tuan Tran, M.; Ny, P.; Lou, M.; Yamaki, J.; Nieberg, P.; Ho, J.; et al. A dysregulated balance of proinflammatory and anti-inflammatory host cytokine response early during therapy predicts persistence and mortality in Staphylococcus aureus bacteremia. Crit. Care Med. 2016, 44, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, O.; Medina, E. Staphylococcus aureus strategies to evade the host acquired immune response. Int. J. Med. Microbiol. 2018, 308, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Smeltzer, M.S. Staphylococcus aureus Pathogenesis: The Importance of Reduced Cytotoxicity. Trends Microbiol. 2016, 24, 681–682. [Google Scholar] [CrossRef] [PubMed]
- Monecke, S.; Coombs, G.; Shore, A.C.; Coleman, D.C.; Akpaka, P.; Borg, M.; Chow, H.; Ip, M.; Jatzwauk, L.; Jonas, D.; et al. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS ONE 2011, 6, e17936. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Choi, D.Y.; Kim, D.K.; Kim, J.W.; Park, J.O.; Kim, S.; Kim, S.H.; Desiderio, D.M.; Kim, Y.K.; Kim, K.P.; et al. Gram-positive bacteria produce membrane vesicles: Proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 2009, 9, 5425–5436. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Defourny, K.A.Y.; Smid, E.J.; Abee, T. Gram-positive bacterial extracellular vesicles and their impact on health and disease. Front. Microbiol. 2018, 9, 1502. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Thompson, S.D.; Weidenmaier, C.; Lee, J.C. Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nat. Commun. 2018, 9, 1379. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Wadsworth International Group: Monterey, CA, USA, 1984. [Google Scholar]
- Minejima, E.; Mai, N.; Bui, N.; Mert, M.; She, R.C.; Nieberg, P.; Spellberg, B.; Wong-Beringer, A. Defining the breakpoint duration of Staphylococcus aureus bacteremia predictive of poor outcomes. Clin. Infect. Dis. 2019, ciz257. [Google Scholar] [CrossRef] [PubMed]
- Bosi, E.; Monk, J.M.; Aziz, R.K.; Fondi, M.; Nizet, V.; Palsson, B.Ø. Comparative genome-scale modelling of Staphylococcus aureus strains identifies strain-specific metabolic capabilities linked to pathogenicity. Proc. Natl. Acad. Sci. USA 2016, 113, E3801–E3809. [Google Scholar] [CrossRef] [PubMed]
- McNeely, T.B.; Shah, N.A.; Frideman, A.; Joshi, A.; Hartzel, J.S.; Keshari, R.S.; Lupu, F.; DiNubile, M.J. Mortality among recipients of the Merck V710 Staphylococcus aureus vaccine after postoperative S. aureus infections: An analysis of possible contributing host factors. Hum. Vaccin. Immunother. 2014, 10, 3513–3516. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.L.; Osaki-Kiyan, P.; Perri, M.; Donabedian, S.; Haque, N.Z.; Chen, A.; Zervos, M.J. USA600 (ST45) methicillin-resistant Staphylococcus aureus bloodstream infections in urban Detroit. J. Clin. Microbiol. 2010, 48, 2307–2310. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sakoulas, G.; Guram, K.; Reyes, K.; Nizet, V.; Zervos, M. Human cathelicidin LL-37 resistance and increased daptomycin MIC in methicillin-resistant Staphylococcus aureus strain USA600 (ST45) are associated with increased mortality in a hospital setting. J. Clin. Microbiol. 2014, 52, 2172–2174. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.R.; Hong, S.W.; Choi, E.B.; Lee, W.H.; Kim, Y.S.; Jeon, S.G.; Jang, M.H.; Gho, Y.S.; Kim, Y.K. Staphylococcus aureus-derived extracellular vesicles induce neutrophilic pulmonary inflammation via both Th1 and Th17 cell responses. Allergy 2012, 67, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Pader, V.; Hakim, S.; Painter, K.L.; Wigneshweraraj, S.; Clarke, T.B.; Edwards, A.M. Staphylococcus aureus inactivates daptomycin by releasing membrane phospholipids. Nat. Microbiol. 2016, 2, 16194. [Google Scholar] [CrossRef] [PubMed]
- Stenovec, M.; Solajer, R.; Perdih, A.; Vardjan, N.; Kreft, M.; Zorec, R. Distinct labelling of fusion events in rat lactotrophs by FM 1-43 and FM 4-64 is associated with conformational differences. Acta Physiol. 2007, 191, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Gurung, M.; Moon, D.C.; Choi, C.W.; Lee, J.H.; Bae, Y.C.; Kim, J.; Lee, Y.C.; Seol, S.Y.; Cho, D.T.; Kim, S.I.; et al. Staphylococcus aureus produces membrane-derived vesicles that induce host cell death. PLoS ONE 2011, 6, e27958. [Google Scholar] [CrossRef] [PubMed]
| Variable | Odds Ratio (95% CI) | p Value |
|---|---|---|
| MV > 301 RFU | 0.20 (0.04–0.91) | 0.037 |
| Age (years) | 1.04 (0.99–1.09) | 0.100 |
| Source Control | 0.35 (0.05–2.71) | 0.314 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, S.; Gudipati, S.; Giuliano, C.; Zervos, M.J.; Monk, J.M.; Szubin, R.; Jorgensen, S.C.J.; Sakoulas, G.; Berti, A.D. Reduced Production of Bacterial Membrane Vesicles Predicts Mortality in ST45/USA600 Methicillin-Resistant Staphylococcus aureus Bacteremia. Antibiotics 2020, 9, 2. https://doi.org/10.3390/antibiotics9010002
Dey S, Gudipati S, Giuliano C, Zervos MJ, Monk JM, Szubin R, Jorgensen SCJ, Sakoulas G, Berti AD. Reduced Production of Bacterial Membrane Vesicles Predicts Mortality in ST45/USA600 Methicillin-Resistant Staphylococcus aureus Bacteremia. Antibiotics. 2020; 9(1):2. https://doi.org/10.3390/antibiotics9010002
Chicago/Turabian StyleDey, Somrita, Smitha Gudipati, Christopher Giuliano, Marcus J. Zervos, Jonathan M. Monk, Richard Szubin, Sarah C. J. Jorgensen, George Sakoulas, and Andrew D. Berti. 2020. "Reduced Production of Bacterial Membrane Vesicles Predicts Mortality in ST45/USA600 Methicillin-Resistant Staphylococcus aureus Bacteremia" Antibiotics 9, no. 1: 2. https://doi.org/10.3390/antibiotics9010002
APA StyleDey, S., Gudipati, S., Giuliano, C., Zervos, M. J., Monk, J. M., Szubin, R., Jorgensen, S. C. J., Sakoulas, G., & Berti, A. D. (2020). Reduced Production of Bacterial Membrane Vesicles Predicts Mortality in ST45/USA600 Methicillin-Resistant Staphylococcus aureus Bacteremia. Antibiotics, 9(1), 2. https://doi.org/10.3390/antibiotics9010002






